Abstract
Background:
Extralevator abdominoperineal excision (ELAPE) has become a popular procedure for low rectal cancer as compared with abdominoperineal excision (APE). No definitive answer has been achieved whether one is superior to the other. This study aimed to evaluate the safety and efficacy of ELAPE for low rectal cancer with meta-analysis.
Methods:
The Web of Science, Cochrane Library, Embase, and PubMed databases before September 2019 were comprehensively searched to retrieve comparative trials of ELAPE and APE for low rectal cancer. Pooled analyses of the perioperative variables, surgical complications, and oncological variables were performed. Odds ratio (OR) and mean differences (MD) from each trial were pooled using random or fixed effects model depending on the heterogeneity of the included studies. A subgroup analysis or a sensitivity analysis was conducted to explore the potential source of heterogeneity when necessary.
Results:
This meta-analysis included 17 studies with 4049 patients, of whom 2248 (55.5%) underwent ELAPE and 1801 (44.5%) underwent APE. There were no statistical differences regarding the circumferential resection margin positivity (13.0% vs. 16.2%, OR = 0.69, 95% CI = 0.42–1.14, P = 0.15) and post-operative perineal wound complication rate (28.9% vs. 24.1%, OR = 1.21, 95% CI = 0.75–1.94, P = 0.43). The ELAPE was associated with lower rate of intraoperative perforation (6.6% vs. 11.3%, OR = 0.50, 95% CI = 0.39–0.64, P < 0.001) and local recurrence (8.8% vs. 20.5%, OR = 0.29, 95% CI = 0.21–0.41, P < 0.001) when compared with APE.
Conclusions:
The ELAPE was associated with a reduction in the rate of intra-operative perforation and local recurrence, without any increase in the circumferential resection margin positivity and post-operative perineal wound complication rate when compared with APE in the surgical treatment of low rectal cancer.
Keywords: Extralevator abdominoperineal excision, Abdominoperineal excision, Low rectal cancer, Surgical complications
Introduction
Abdominoperineal excision (APE) has been the standard surgery for advanced low rectal cancer for over a century. An alternative procedure, extralevator abdominoperineal excision (ELAPE), was first described by Holm et al[1] in 2007 and has gained popularity among colorectal surgeons. Several studies reported that ELAPE has better outcomes regarding the rate of intra-operative perforation (IOP), circumferential resection margin (CRM) positivity, and local recurrence (LR) as compared to APE.[2–4] This improved performance of ELAPE may result from the absence of the surgical “waist,” located where the abdominal and perineal dissections meet, that remained after APE.[1] However, other studies reported comparable outcomes for APE and ELAPE.[5,6] Thus, whether one of these methods is superior to the other remains uncertain.
Reviewing the past seven meta-analyses of ELAPE and APE studies,[7–13] we found many contradictory results with respect to oncological variables: four indicated that ELAPE was superior with respect to CRM positivity, IOP, and LR rates;[7,8,11,13] one reported that ELAPE and APE were equivalence with respect to CRM positivity and IOP rates;[9] one reported that ELAPE was superior with respect to LR and IOP rates;[10] and one reported the superiority of ELAPE with respect to the IOP rate alone.[12] However, five of these meta-analyses, conducted between 2013 and 2015, were not registered with the International Prospective Register of Systematic Review (PROSPERO) or Cochrane, failed to use the Newcastle-Ottawa Scale correctly, and simply used the random effects model without further analyzing the source of high heterogeneity.[7–11] The meta-analysis by Zhang et al[13] barely investigated oncological variables. In addition, five high-quality studies[14–18] that questioned the benefits of ELAPE as a standard operation have been published in the past 3 years.
The conflicting conclusions and shortcomings of previous meta-analyses necessitate further investigation using a larger number of patients and rigorous statistical methods. This meta-analysis aims to compare perioperative variables, surgical complications, and oncological variables between the ELAPE and APE for low rectal cancer.
Methods
This review protocol has been registered and published in PROSPERO (CRD42019118433) and was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Data sources
To acquire all cited publications, a comprehensive search of the Web of Science, Cochrane Library, Embase, and PubMed databases was conducted between 2007 and 2019. We chose to start in 2007 because ELAPE was first presented by Holm et al[1] at that time. The literature search used various combinations of the subject words and free words related to ELAPE and APE in low rectal cancer, using the following keywords: rectal neoplasm, rectum neoplasm, rectum cancer, rectal tumor, cancer of the rectum, rectal cancer, extra-levator abdominoperineal, extralevator abdominoperinea, extended abdominoperineal, extralevator abdominoperineal, cylindrical abdominoperineal, abdominoperineal prone position, standard abdominoperineal, conventional abdominoperineal, excision, or resection. Articles were also identified using the “related articles” function. The most recent study retrieved by this search was published on August 25, 2019.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) Randomized controlled trials (RCTs) and non-randomized controlled trials (non-RCTs); (2) published as a full paper about low rectal cancer in English; (3) compared ELAPE and APE in the same study, including open, laparoscopic, and robotic approaches; (4) included a minimum of 20 patients undergoing ELAPE and APE; (5) reported patients’ clinical and pathologic parameters, such as age, sex, and tumor differentiation; (6) if the same data had been published more than once, the higher quality or latest study was included; (7) evaluated at least one of the outcomes of interest mentioned below. Nonhuman studies, experimental trials, review articles, editorials, letters, and case reports were excluded.
Data extraction
Studies were finally included by reviewers independently. To resolve discrepancies, a third experienced gastrointestinal surgery professor participated in the decision-making process. The following variables were extracted from each study: first author, year of publication, country, study type, matching criteria, sample size, and outcomes of interest. For cases with missing or incomplete data, the primary authors were contacted requesting further information, but none was provided.
Outcomes of interest and definitions
-
1.
Perioperative variables: operating time, estimated blood loss, and length of hospital stay (LOS).
-
2.
Surgical complications: post-operative perineal herniation, post-operative abdominal infection, post-operative urinary dysfunction, post-operative chronic perineal pain, post-operative urinary infection, post-operative parastomal hernia, post-operative intestinal obstruction, and post-operative perineal wound complication (PWC). The PWC includes perineal wound infection, dehiscence, breakdown, wound healing problems, and sinus formation.
-
3.
Oncological variables: Rates of IOP, LR, and CRM positivity. Any tumor located less than 1 mm from the circumferential margin was defined as positive according to previous evidence.[19] LR, CRM positivity, IOP, and PWC rates were evaluated as the main outcomes of interest for this review.
Statistical analysis
For statistical analysis, this review used Review Manager Software 5.3 (The Cochrane Collaboration, London, UK). The mean difference (MD) with the 95% confidence interval (CI) was chosen as the effect measure for continuous data and odds ratio (OR) for dichotomous data. For continuous data presented as the median with quartile or range, the mean and standard deviation were estimated according to the methods described previously.[20] The Chi-square test and I2 statistics were used for assessing study heterogeneity, reflecting the total variation between the studies generated by differences between the trials rather than sample error. For I2 > 50%, which indicated heterogeneity, a random effects model was used. Otherwise, the fixed-effect model was used to analyze the outcomes of interest. For cases in which the outcomes of interest showed high heterogeneity, the reasons for the statistical heterogeneity were explored using subgroup and sensitivity analysis. Publication bias was evaluated using funnel plots. The pooled effects were determined using the Z test, and P < 0.05 was considered statistically significant. The quality of randomized controlled trials (RCTs) was assessed using the Cochrane Risk of Bias Tool.[21] The quality of non-RCTs was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I).[22]
Results
Description of eligible studies
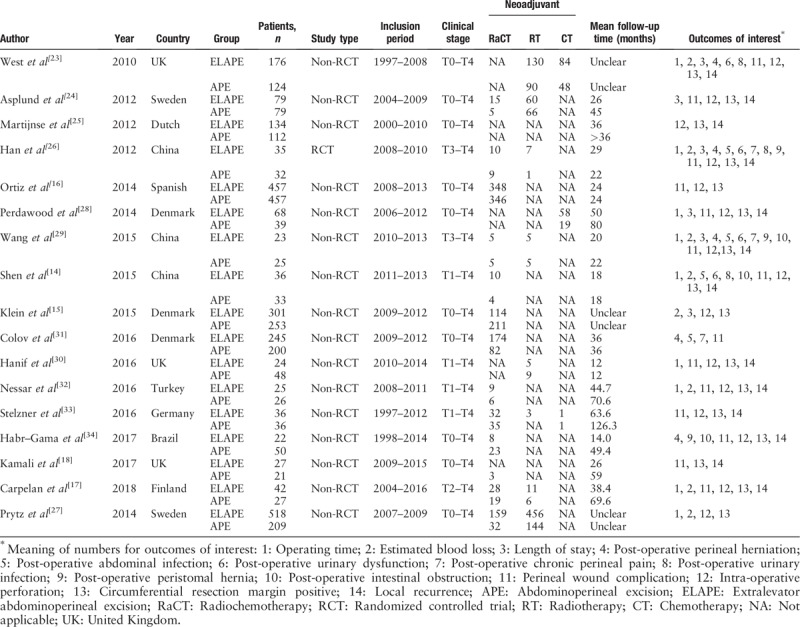
Seventeen studies[14–18,23–34] published from 2007 to 2018 fulfilled the inclusion criteria and were included in the meta-analysis [Figure 1]. The characteristics of the included studies are shown in Table 1. A total of 4049 patients (ELAPE, 2248; APE, 1801) from 17 studies were included. One RCT (from China) and 16 non-RCTs (12 from Europe, 3 from Asia, and 1 from South America) were included in the meta-analysis. Two studies by Stelzner et al[3,33] included two apparently overlapping patient cohorts. To avoid duplicate ion bias, only the latter study was included.[33]
Figure 1.
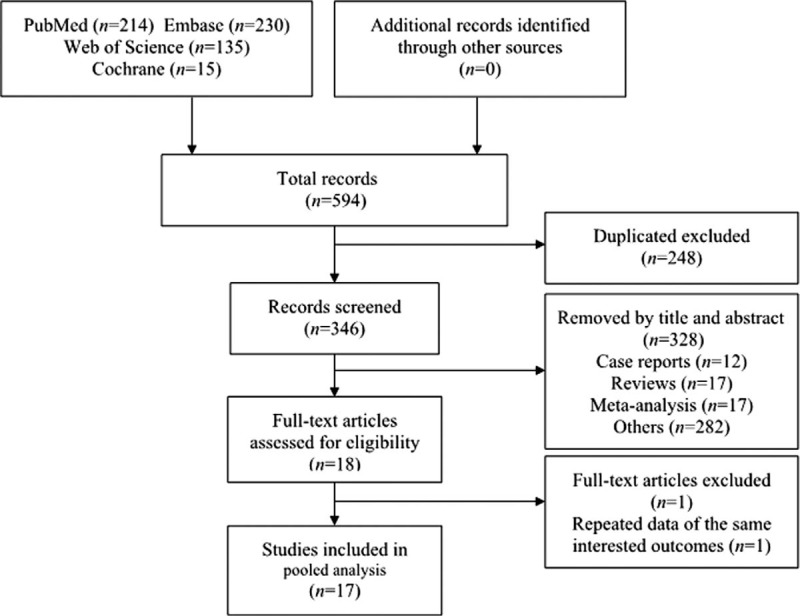
Flow diagram of the meta-analysis literature search and study selection.
Table 1.
Characteristics of included studies.
Methodological quality of included studies
Using the Cochrane Risk of Bias Tool to assess the quality of the RCT, we found that the RCT did not calculate the sample size and provided no information about the blinding method. Using the ROBINS-I, all non-RCT were assessed with low or moderate risk (Supplementary Table 1).
Meta-analysis of perioperative variables
Operating time
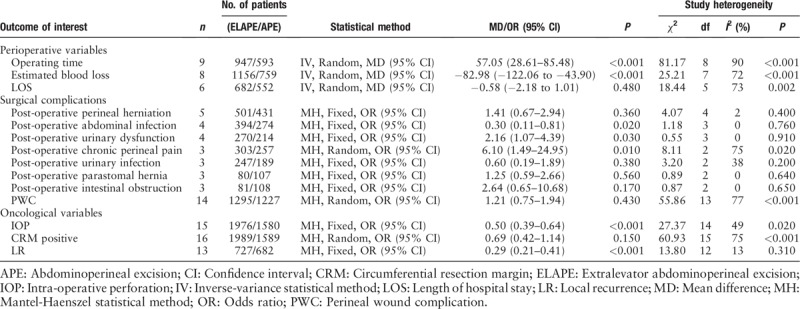
Pooled data from nine studies[14,17,23,26–30,32] that reported operating time showed significantly longer operating time for ELAPE than APE (MD = 57.05, 95% CI = 28.61–85.48, P < 0.001, Table 2) with high heterogeneity (P < 0.001, I2 = 90%). The subgroup analysis revealed ELAPE has longer operating time in Europe (P < 0.001, I2 = 87%), and no significant statistically difference in Asia (P = 0.13, I2 = 92%). When excluding one highly heterogeneous study[23] by sensitivity analysis, we still found longer operating time in ELAPE (MD = 49.63, 95% CI = 22.17–77.08, P < 0.001), indicating that the above-mentioned result was stable.
Table 2.
Results of the meta-analysis in interested outcomes.
Estimated blood loss
Pooled data from eight studies[14,15,17,23,26,27,29,32] that reported estimated blood loss showed lower blood loss for ELAPE than APE (MD = −82.98, 95% CI = −122.06 to −43.90, P < 0.001, Table 2) with high heterogeneity (P < 0.001, I2 = 72%). The subgroup analysis revealed ELAPE has lower blood loss in Europe (P = 0.002, I2 = 59%) and Asia (P = 0.008, I2 = 59%). When excluding one highly heterogeneous study[15] by sensitivity analysis, we still found lower blood loss in ELAPE (MD = −81.35, 95% CI = −131.93 to −30.76, P = 0.002), indicating that the above-mentioned result was stable.
Length of stay
Pooled data from six studies[15,23,24,26,28,29] that reported LOS showed there was no significant statistically difference between ELAPE and APE (MD = −0.58, 95% CI = −2.18 to 1.01, P = 0.48, Table 2) with high heterogeneity (P = 0.002, I2 = 73%). The subgroup analysis revealed ELAPE has shorter LOS in Asia, and no significant statistically difference in Europe (P = 0.59, I2 = 47%). When excluding one highly heterogeneous study[15] by sensitivity analysis, we found shorter LOS in ELAPE (MD = −1.34, 95% CI = −2.59 to −0.09, P = 0.04) with lower heterogeneity (P = 0.25, I2 = 26%), indicating that the above-mentioned result was unstable.
Meta-analysis of surgical complications
Post-operative perineal herniation
Pooled data from five studies[23,26,29,31,34] that reported the post-operative perineal herniation rate showed there was no significant statistically difference between ELAPE and APE (3.6% vs. 2.8%, OR = 1.41, 95% CI = 0.67–2.94, P = 0.36, Table 2) with lower heterogeneity (P = 0.40, I2 = 2%).
Post-operative abdominal infection
Pooled data from four studies[14,15,26,29] that reported the post-operative abdominal infection rate showed ELAPE was associated with lower rate of post-operative abdominal infection (1.3% vs. 5.1%, OR = 0.30, 95% CI = 0.11–0.81, P = 0.02, Table 2) with lower heterogeneity (P = 0.76, I2 = 0%).
Post-operative urinary dysfunction
Pooled data from four studies[14,23,26,29] that reported the post-operative urinary dysfunction rate showed ELAPE was associated with higher post-operative urinary dysfunction (11.1% vs. 7.0%, OR = 2.16, 95% CI = 1.07–4.39, P = 0.03, Table 2) with lower heterogeneity (P = 0.91, I2 = 0%).
Post-operative chronic perineal pain
Pooled data from the three studies[26,29,31] that reported the rate of post-operative chronic perineal pain showed ELAPE was associated with high rate of post-operative chronic perineal pain (40.6% vs. 18.3%, OR = 6.10, 95% CI = 1.49–24.95, P = 0.01, Table 2) with high heterogeneity (P = 0.02, I2 = 75%).
Post-operative urinary infection
Pooled data from the three studies[14,23,26] that reported post-operative urinary infection showed there was no significant statistically difference between ELAPE and APE (1.6% vs. 3.2%, OR = 0.60, 95% CI = 0.19–1.89, P = 0.38, Table 2) with lower heterogeneity (P = 0.20, I2 = 38%).
Post-operative parastomal hernia
Pooled data from the three studies[26,29,34] that reported the rate of post-operative parastomal hernia showed there was no significant statistically difference between ELAPE and APE (27.5% vs. 18.7%, OR = 1.25, 95% CI = 0.59–2.66, P = 0.56, Table 2) with lower heterogeneity (P = 0.64, I2 = 0%).
Post-operative intestinal obstruction
Pooled data from the three studies[14,29,34] that reported the rate of intestinal obstruction showed there was no significant statistically difference between ELAPE and APE (7.4% vs. 2.7%, OR = 2.64, 95% CI = 0.65–10.68, P = 0.17, Table 2) with lower heterogeneity (P = 0.65, I2 = 0%).
Perineal wound complication
The PWC rate was reported by 14 studies[14,16–18,23,24,26,28–34] (ELAPE group, n = 1295; APE group, n = 1227). No significant statistically difference was observed in PWC rate between ELAPE and APE (28.9% vs. 24.1%, OR = 1.21, 95% CI = 0.75–1.94, P = 0.43, Table 2) with high heterogeneity (P < 0.001, I2 = 77%, Figure 2). The subgroup analysis showed that there was no significant statistically difference in Asia (P = 0.90, I2 = 68%) and Europe (P = 0.10, I2 = 78%) [Figure 3]. When excluding two highly heterogeneous studies[23,31] by sensitivity analysis, we still found there was no significant statistically difference between ELAPE and APE (25.9% vs. 26.4%, OR = 0.97, 95% CI = 0.60–1.55, P = 0.89), indicating that the above-mentioned result was stable.
Figure 2.
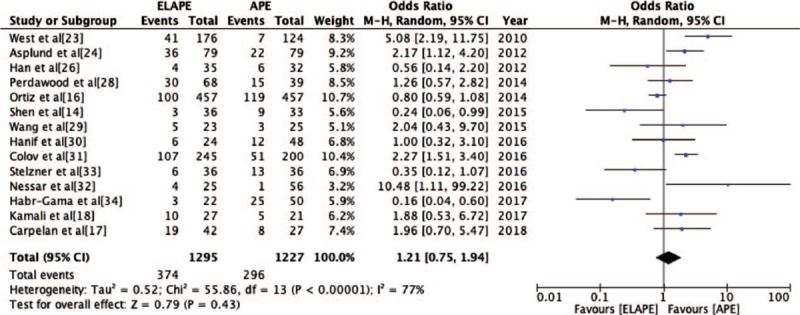
Odds Ratio for perineal wound complication from fourteen included studies. APE: Abdominoperineal excision; CI: Confidence interval; ELAPE: Extralevator abdominoperineal excision; MH: Mantel-Haenszel.
Figure 3.
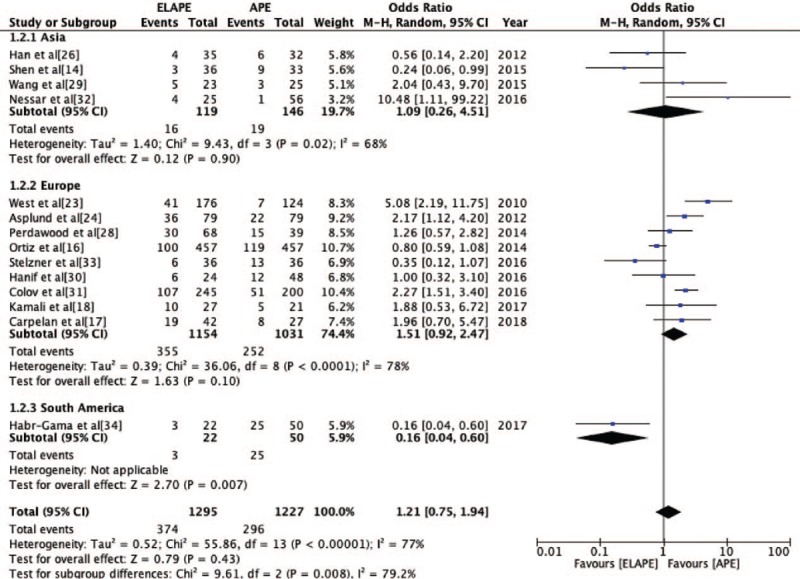
Odds Ratio for perineal wound complication grouped by Asia, Europe, and South America. APE: Abdominoperineal excision; CI: Confidence interval; ELAPE: Extralevator abdominoperineal excision; MH: Mantel-Haenszel.
Meta-analysis of oncological variables
Circumferential resection margin positivity
The CRM positivity rate was reported by 16 studies[14–18,23–30,32–34] (ELAPE group, n = 1989; APE group, n = 1589). No significant statistically difference was observed in CRM positivity rate between ELAPE and APE (13.0% vs. 16.2%, OR = 0.69, 95% CI = 0.42–1.14, P = 0.15, Table 2) with high heterogeneity (P < 0.001, I2 = 75%, Figure 4), which was consistent with the high quality meta-analysis of Negoi et al.[12] The subgroup analysis revealed there was no statistically significant difference in Europe (P = 0.53, I2 = 81%), but lower CRM positivity in Asia (P = 0.004, I2 = 0%) [Figure 5]. When excluding one high heterogeneous study[23] by sensitivity analysis, we still found there was no statistically significant difference between ELAPE and APE (12.4% vs. 13.3%, OR = 0.80, 95% CI = 0.50–1.26, P = 0.34), indicating that the above-mentioned result was stable.
Figure 4.
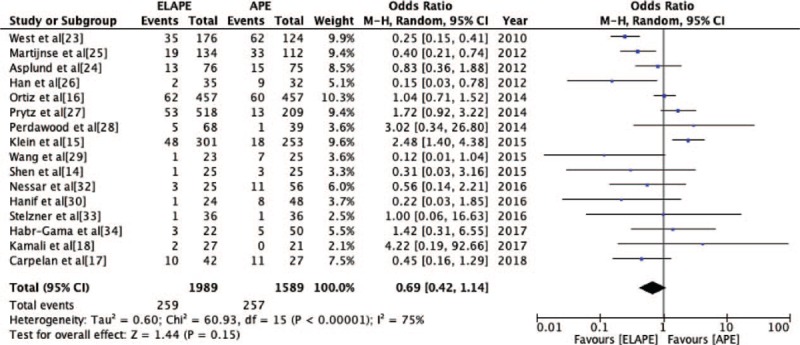
Odds Ratio for circumferential resection margin positivity from sixteen included studies. APE: Abdominoperineal excision; CI: Confidence interval; ELAPE: Extralevator abdominoperineal excision; MH: Mantel-Haenszel.
Figure 5.
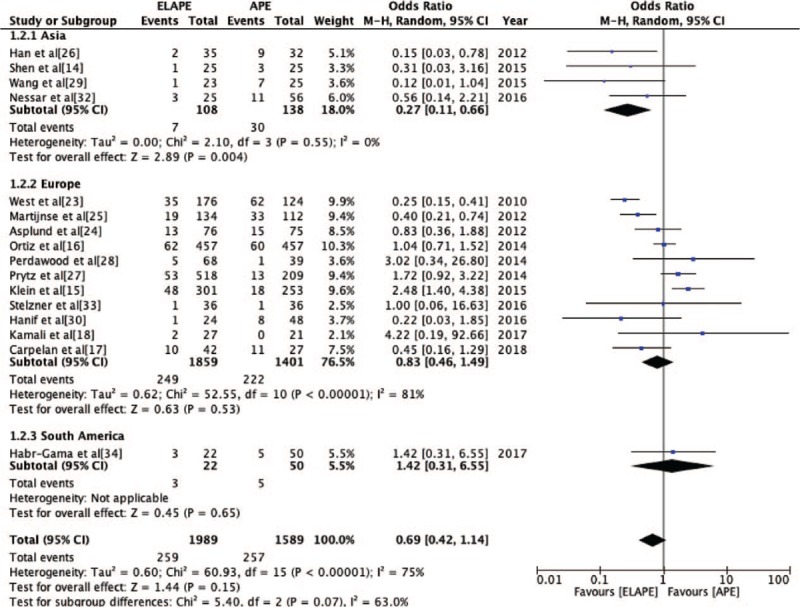
Odds Ratio for circumferential resection margin positivity grouped by Asia, Europe, and South America. APE: Abdominoperineal excision; CI: Confidence interval; ELAPE: Extralevator abdominoperineal excision; MH: Mantel-Haenszel.
Intra-operative perforation
The IOP rate was reported by 15 studies[14–17,23–30,32–34] (ELAPE group, n = 1976; APE group, n = 1580). Fixed-effect model analysis revealed significantly lower IOP rate for ELAPE than APE (6.6% vs. 11.3%, OR = 0.50, 95% CI = 0.39–0.64, P < 0.001, Table 2) with lower heterogeneity (P = 0.02, I2 = 49%, Figure 6), which was consistent with a high quality meta-analysis by Negoi et al.[12]
Figure 6.
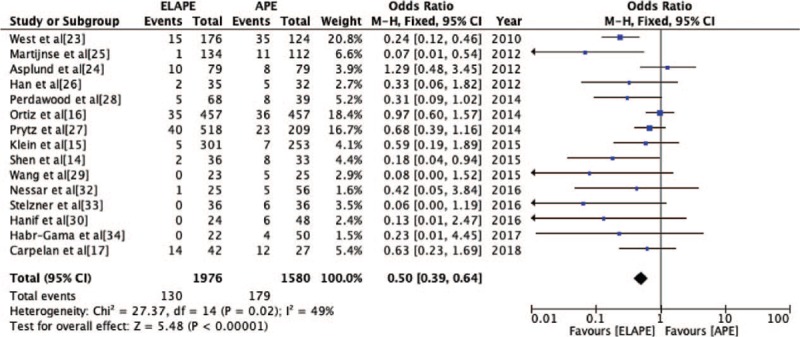
Odds Ratio for intra-operative perforation from 15 included studies. APE: Abdominoperineal excision; CI: Confidence interval; ELAPE: Extralevator abdominoperineal excision; MH: Mantel-Haenszel.
Local recurrence
The LR rate was reported by 13 studies[14,17,18,23–26,28–30,32–34] (ELAPE group, n = 727; APE group, n = 682). Fixed-effect model analysis revealed significantly lower LR rate for ELAPE than APE (8.8% vs. 20.5%, OR = 0.29, 95% CI = 0.21–0.41, P < 0.001, Table 2) with lower heterogeneity (P = 0.31, I2 = 13%, Figure 7), which was inconsistent with a high quality meta-analysis by Negoi et al.[12]
Figure 7.
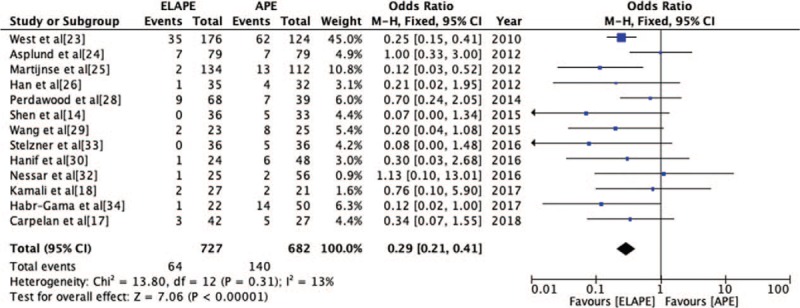
Odds Ratio for local recurrence from included 13 studies. APE: Abdominoperineal excision; CI: Confidence interval; ELAPE: Extralevator abdominoperineal excision; MH: Mantel-Haenszel.
Subgroup and sensitivity analyses
Subgroup (according to the geographical location) and sensitivity analyses were implemented to investigate operating time, estimated blood loss, LOS, PWC rate, and CRM positivity rate. Studies by Klein et al[15] and West et al[23] were the main drivers of heterogeneity in this review. Furthermore, sensitivity analysis revealed that the inclusion of the South American study[34] had little effect on the heterogeneity or stability of the variables.
Publication bias
The only RCT[26] in this study was removed. The funnel plots showed that all data within the 95% CI are distributed symmetrically for all outcomes of interest except the LR rate [Supplementary Figure 1], indicating that the publication bias was minimal.
Discussion
The present study showed that compared to APE, ELAPE had a longer operating time, lower estimated blood loss, lower incidence of abdominal infection, but a higher incidence of urinary dysfunction and chronic perineal pain. No significant difference was observed in the rate of PWC, perineal herniation, urinary infection, parastomal hernia, or intestinal obstruction between the two surgical approaches. Finally, this study showed that the IOP and LR rates were lower for ELAPE than those for APE, while the CRM positivity rate did not differ significantly between them.
We speculate that the increased operating time observed for ELAPE maybe because this technology was recently adopted and had a long learning curve for beginners. Other factors contributing to the difference in operation time and blood loss between ELAPE and APE included differences in patient positioning, removal of the coccyx, the type of perineal reconstruction, and the type of technology used (open, laparoscopic, or robotic surgery). However, the prone Jack-Knife position and the application of minimally invasive techniques can provide excellent exposure of the perineal structures and direct visualization of the operative field, which may reduce blood loss during surgery. A shorter LOS after ELAPE was observed after removing the highly heterogeneous study reported by Klein et al.[15] However, several significant limitations were present in the study of Klein et al[15] and are discussed in the following section.
Regarding surgical complications, lower rate of abdominal infection after ELAPE may attribute to the application of more minimally invasive techniques (laparoscopic or robotic surgery) in abdominal surgery. Differences in the experience of chronic perineal pain may result from the wider excision of the levator ani muscles and ischiorectal fossa fat. Han et al[26] and Wang et al[29] demonstrated that coccyx preservation significantly reduced the occurrence of chronic perineal pain in a study with a small number of patients, and Han et al[26] even proposed that coccygectomy may be the main cause of chronic perineal pain. However, it is well known that the frequency of coccygectomy is lower for the perineal method. Thus, this issue deserves further discussion. Genitourinary function is innervated by the pelvic autonomic nervous plexuses and can be influenced by injury in the region of the hypogastric plexus before it joins the parasympathetic nerves at the level of the inferior hypogastric plexus.[35,36] A recent anatomic dissection study[36] also showed that clear identification of pelvic anatomic landmarks during surgery might be useful for both achieving CRM negativity and preserving urogenital function. The intra-operative separation and dissection by ELAPE are performed using the two-plane method. The plane of the mesorectum is separated in the abdominal surgery, and the perineum is separated along the lateral plane of the levator ani muscle. Together, these procedures improve the visibility of the surgical plane and anatomy, leading to lower rates of CRM positivity, IOP, and genitourinary injury. Unfortunately, the results of this study indicated that of these three outcomes, only the IOP rate was improved by ELAPE. The higher rate of PWCs in ELAPE (ELAPE, 28.9%; APE, 24.1%) may result from the ELAPE approach and the type of closure used to repair the perineal defect after surgery (primary closure or reconstruction). One national study[31] of PWCs reported a high rate of PWC (75%) in patients who underwent APE. From an anatomical point of view, APE involves removal of structures of the mesorectum, while ELAPE uses an expanded scope of resection as compared to APE. Thus, both techniques ultimately create a large perineal defect, which can result in a high PWC rate, especially with the ELAPE approach. Primary closure was reported to have a higher rate of PWC after APE,[37] and many medical centers are placing greater efforts into the reconstruction of the pelvic floor. A high-quality meta-analysis[38] of pelvic floor reconstruction reported that the PWC rate was equivalent between 255 patients undergoing flap repair and 85 patients undergoing biological mesh repair. The latest statement[39] also reported that myocutaneous flaps and biological mesh were both effective for use in ELAPE closure. However, the evidence is insufficient to allow recommendation of one particular method of perineal closure until now. In addition, pre-operative irradiation and neo-adjuvant therapy were also defined as risk factors for PWCs.[40] A meta-analysis reported that the PWC rate after ELAPE and APE was 37.6% and 30.2%, respectively, with chemoradiation as compared to 14.8% and 15.3% without chemoradiation.[41] In view of the higher rate of PWC after ELAPE, Han et al[26] first proposed the new concept of individualized APE, which aimed to preserve normal tissue, achieve local radical excision, minimize operative trauma of the perineal wound, and minimize damage to the nerves supplying the genital organs. However, the outcomes of individualized APE should be further explored in future studies.
With respect to oncological variables, the rates of CRM positivity and IOP have been demonstrated to correlate with a poor prognosis in patients with low rectal cancer.[42,43] Because of the poor prognosis resulting from the surgical waist remaining after APE, separation of the levators close to the surgical waist in ELAPE approach. As a result, the extent of resection and the thickness of the excised tissue are undoubtedly superior with ELAPE and should theoretically reduce the rates of CRM positivity and IOP. Previous studies have shown that the IOP rate correlates significantly to the tumor level, advanced T and M stage, and quality of mesorectum excision.[16] In ELAPE, en bloc removal of the tumor and levators clearly reduced the IOP rate, as indicated by numerous studies.[14,26,29,30,32,33] However, one nationwide database study by Klein et al[15] reported opposing results. Upon careful review, obvious selection bias was present in their study. First, patients who underwent ELAPE in their study were more likely to have received neo-adjuvant therapy. Second, although ELAPE is now applied to tumors of both low and high stages, more high T-stage tumors were included in the ELAPE group in their study. Third, their lack of standardization of surgical techniques and indications was also unacceptable. Moreover, two studies from Europe[17,23] and the only RCT, from China[26] investigating advanced low rectal cancer showed the perforation is most often located anteriorly; this observation should be considered in surgical plans. Several factors, including the body mass index, tumor stage, and pre-operative therapies, also have been proven to affect the CRM positivity rate.[19,44] Neo-adjuvant therapy has been widely used and may improve the tumor stage and change the treatment strategy to some extent. A recent study reported that 10.3% of patients showed a pathological complete response to neoadjuvant therapy in a single UK tertiary center, further confirming the influence of neoadjuvant therapy.[45] Thus, the CRM positivity rate in the two groups might not be accurate, given the effects of neoadjuvant therapy. Further studies are needed to elucidate the effects of neo-adjuvant therapy ion the CRM positivity rate. Moreover, ELAPE might not be advantageous for tumors located away from the surgical waist. How et al[46] concluded that ELAPE might be more appropriate for tumors located above and below the puborectalis sling and anteriorly at the level of the prostate. Another recent study from a tertiary-care center concluded that ELAPE should be the preferred approach for low rectal tumors with involvement of the levators and that current evidence is insufficient to recommend ELAPE over APE for patients in whom the levators are not involved.[47] In the present study, we observed that the LR rate was significantly lower after ELAPE (9%) than after APE (21%). Further analysis revealed a certain bias in the funnel plot for LR rate, which may result from differences in tumor stages, pre-operative neoadjuvant therapy, and post-operative chemotherapy. Missing data also may be a contributing factor.
Compared with the previous seven meta-analyses, this study has several advantages. First, using rigorous statistical methods, this study is a registered meta-analysis with a large sample size aiming to explicitly compare the perioperative variables, surgical complications, and oncological variables between ELAPE and APE. Second, this study showed that compared to APE, ELAPE has a lower incidence of abdominal infection but a higher incidence of urinary dysfunction. The latter complication was not expected by clinicians and requires attention in surgery. Third, using subgroup and sensitivity analysis, we conclude that the higher heterogeneity observed in this study undoubtedly come from two studies in Europe,[15,23] likely resulting from their multi-center and the lack of standardization of surgical techniques and indications in one study. Therefore, the surgical indications for ELAPE deserve further discussion in the future. Meanwhile, several limitations of this meta-analysis should also be considered. First, only one small-scale RCT was included in the cohort of 17 studies, which may decrease the reliability of the results. Second, a majority of patients in the ELAPE group received neoadjuvant treatment, which could affect the long-term results. Whether this factor compared CRM positivity and the PWC rate was unknown.
In conclusion, for the surgical treatment of low rectal cancer, ELAPE was associated with decreased rates of IOP and LR, with no increase in CRM positivity or post-operative PWC rate as compared to those of APE.
Acknowledgements
The authors thank Zhi-Kun Xing and He-Jie Chen for their support in statistics and methodology. The authors also thank Jia-Xin Liu for her patience, help and support in language modification.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81672439), and the Capital's Funds for Health Improvement and Research (No. CFH 2018-2-2153), and Beijing Municipal Administration of Hospitals Incubating Program (No. PX2016018).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Qi XY, Cui M, Liu MX, Xu K, Tan F, Yao ZD, Zhang N, Yang H, Zhang CH, Xing JD, Su XQ. Extralevator abdominoperineal excision versus abdominoperineal excision for low rectal cancer: a meta-analysis. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000485
Xin-Yu Qi and Ming Cui contributed equally to this work.
References
- 1.Holm T, Ljung A, Haggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg 2007; 94:232–238.. doi: 10.1002/bjs.5489. [DOI] [PubMed] [Google Scholar]
- 2.West NP, Finan PJ, Anderin C, Lindholm J, Holm T, Quirke P. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol 2008; 26:3517–3522.. doi: 10.1200/JCO.2007. [DOI] [PubMed] [Google Scholar]
- 3.Stelzner S, Hellmich G, Schubert CA, Puffer E, Haroske G, Witzigmann H. Short-term outcome of extralevator abdominoperineal excision for rectal cancer. Int J Colorectal Dis 2011; 26:919–925.. doi: 10.1007/s00384-011-1157-0. [DOI] [PubMed] [Google Scholar]
- 4.Palmer G, Anderin C, Martling A, Holm T. Local control and survival after extralevator abdominoperineal excision for locally advanced or low rectal cancer. Colorectal Dis 2014; 16:527–532.. doi: 10.1111/codi.12610. [DOI] [PubMed] [Google Scholar]
- 5.Messenger DE, Cohen Z, Kirsch R, O’connor BI, Victor JC, Huang H, et al. Favorable pathologic and long-term outcomes from the conventional approach to abdominoperineal resection. Dis Colon Rectum 2011; 54:793–802.. doi: 10.1007/DCR.0b013e318215a1cb. [DOI] [PubMed] [Google Scholar]
- 6.Hiranyakas A, da Silva G, Wexner SD, Ho YH, Allende D, Berho M. Factors influencing circumferential resection margin in rectal cancer. Colorectal Dis 2013; 15:298–303.. doi: 10.1111/j.1463-1318.2012.03179.x. [DOI] [PubMed] [Google Scholar]
- 7.Yu HC, Peng H, He XS, Zhao RS. Comparison of short-and long-term outcomes after extralevator abdominoperineal excision and standard abdominoperineal excision for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2014; 29:183–191.. doi: 10.1007/s00384-013-1793-7. [DOI] [PubMed] [Google Scholar]
- 8.Huang A, Zhao H, Ling T, Quan Y, Zheng M, Feng B. Oncological superiority of extralevator abdominoperineal resection over conventional abdominoperineal resection: a meta-analysis. Int J Colorectal Dis 2014; 29:321–327.. doi: 10.1007/s00384-013-1794-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Sun T, Xie H, Zhang Y, Zeng H, Fu W. Extralevator abdominoperineal excision for low rectal cancer: a systematic review and meta-analysis of the short-term outcome. Colorectal Dis 2015; 17:474–481.. doi: 10.1111/codi.12921. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Xu HR, Shang Z, Chen SZ, Chen F, Deng QM, et al. Outcome of extralevator abdominoperineal excision over conventional abdominoperineal excision for low rectal tumour: a meta-analysis. Int J Clin Exp Med 2015; 8:14855–14862.. [PMC free article] [PubMed] [Google Scholar]
- 11.De Nardi P, Summo V, Vignali A, Capretti G. Standard versus extralevator abdominoperineal low rectal Cancer excision outcomes: a systematic review and meta-analysis. Ann Surg Oncol 2015; 22:2997–3006.. doi: 10.1245/s10434-015-4368-8. [DOI] [PubMed] [Google Scholar]
- 12.Negoi I, Hostiuc S, Paun S, Negoi RI, Beuran M. Extralevator vs conventional abdominoperineal resection for rectal cancer—A systematic review and meta-analysis. Am J Surg 2016; 212:511–526.. doi: 10.1016/j.amjsurg.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YF, Wang D, Zhu LZ, Wang B, Ma X, Shi B, et al. Standard versus extralevator abdominoperineal excision and oncologic outcomes for patients with distal rectal cancer: A meta-analysis. Medicine 2017; 96:52.doi: 10.1097/MD.0000000000009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Z, Ye Y, Zhang X, Xie Q, Yin M, Yang X, et al. Prospective controlled study of the safety and oncological outcomes of ELAPE procure with definitive anatomic landmarks versus conventional APE for lower rectal cancer. Eur J Surg Oncol 2015; 41:472–477.. doi: 10.1016/j.ejso.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Klein M, Fischer A, Rosenberg J, Gogenur I. Extralevatory abdominoperineal excision (ELAPE) does not result in reduced rate of tumour perforation or rate of positive circumferential resection margin. Ann Surg 2015; 261:933–938.. doi: 10.1097/SLA.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz H, Ciga MA, Armendariz P, Kreisler E, Codina-Cazador A, Gomez-Barbadillo J, et al. Multicentre propensity score-matched analysis of conventional versus extended abdominoperineal excision for low rectal cancer. Br J Surg 2014; 101:874–882.. doi: 10.1002/bjs.9522. [DOI] [PubMed] [Google Scholar]
- 17.Carpelan A, Karvonen J, Varpe P, Rantala A, Kaljonen A, Gronroos J, et al. Extralevator versus standard abdominoperineal excision in locally advanced rectal cancer: a retrospective study with long-term follow-up. Int J Colorectal Dis 2018; 33:375–381.. doi: 10.1007/s00384-018-2977-y. [DOI] [PubMed] [Google Scholar]
- 18.Kamali D, Sharpe A, Musbahi A, Reddy A. Oncological and quality of life outcomes following extralevator versus standard abdominoperineal excision for rectal cancer. Ann R Coll Surg Engl 2017; 99:402–409.. doi: 10.1308/rcsann.2017.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994; 344:707–711.. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13.doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928.doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:i4919.doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West NP, Anderin C, Smith KJ, Holm T, Quirke P. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg 2010; 97:588–599.. doi: 10. 1002/bjs. 6916. [DOI] [PubMed] [Google Scholar]
- 24.Asplund D, Haglind E, Angenete E. Outcome of extralevator abdominoperineal excision compared with standard surgery: results from a single center. Colorectal Dis 2012; 14:1191–1196.. doi: 10.1111/j.1463-1318.2012.02930.x. [DOI] [PubMed] [Google Scholar]
- 25.Martijnse IS, Dudink RL, West NP, Wascowicz D, Nieuwenhuijzen GA, van Lijnschoten I, et al. Focus on Extralevator perineal dissection in supine position for low rectal cancer has led to better quality of surgery and oncologic outcome. Ann Surg Oncol 2012; 19:786–793.. doi: 10.1245/s10434-011-2004-9. [DOI] [PubMed] [Google Scholar]
- 26.Han JG, Wang ZJ, Wei GH, Gao ZG, Yang Y, Zhao BC. Randomized clinical trial of conventional versus cylindrical abdominoperineal resection for locally advanced lower rectal cancer. Am J Surg 2012; 204:274–282.. doi: 10.1016/j.amjsurg.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Prytz M, Angenete E, Ekelund J, Haglind E. Extralevator abdominoperineal excision (ELAPE) for rectal cancer—short-term results from the Swedish Colorectal Cancer Registry. Selective use of ELAPE warranted. Int J Colorectal Dis 2014; 29:981–987.. doi: 10.1007/s00384-014-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perdawood SK, Lund T. Extralevator versus standard abdominoperineal excision for rectal cancer. Tech Coloproctol 2015; 19:145–152.. doi: 10.1007/s10151-014-1243-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang YL, Dai Y, Jiang JB, Yuan HY, Hu SY. Application of laparoscopic extralevator abdominoperineal excision in locally advanced low rectal cancer. Chin Med J 2015; 128:1340–1345.. doi: 10.4103/0366-6999.156779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanif Z, Bradley A, Hammad A, Mukherjee A. Extralevator abdominoperineal excision (Elape): a retrospective cohort study. Ann Med Surg (Lond) 2016; 10:32–35.. doi: 10.1016/j.amsu.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colov EP, Klein M, Gogenur I. Wound complications and perineal pain after extralevator versus standard abdominoperineal excision: a nationwide study. Dis Colon Rectum 2016; 59:813–821.. doi: 10.1097/DCR.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 32.Neşşar G, Demirbag AE, Celep B, Elbir OH, Kayaalp C. Extralevator abdominoperineal excision versus conventional surgery for low rectal cancer: a single surgeon experience. Ulus Cerrahi Derg 2016; 32:244–247.. doi: 10.5152/UCD.2016.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelzner S, Hellmich G, Sims A, Kittner T, Puffer E, Zimmer J, et al. Long-term outcome of extralevator abdominoperineal excision (ELAPE) for low rectal cancer. Int J Colorectal Dis 2016; 31:1729–1737.. doi: 10.1007/s00384-016-2637-z. [DOI] [PubMed] [Google Scholar]
- 34.Habr-Gama A, Sao Juliao, Mattacheo A, de Campos-Lobato LF, Aleman E, Vailati BB, et al. Extralevator abdominal perineal excision versus standard abdominal perineal excision: impact on quality of the resected specimen and postoperative morbidity. World J Surg 2017; 41:2160–2167.. doi: 10.1007/s00268-017-3963-1. [DOI] [PubMed] [Google Scholar]
- 35.Acar HI, Kuzu MA. Perineal and pelvic anatomy of extralevator abdominoperineal excision for rectal cancer: cadaveric dissection. Dis Colon Rectum 2011; 54:1179–1183.. doi: 10.1097/DCR.0b013e318224256c. [DOI] [PubMed] [Google Scholar]
- 36.Stelzner S, Holm T, Moran BJ, Heald RJ, Witzigmann H, Zorenkov D, et al. Deep pelvic anatomy revisited for a description of crucial steps in extralevator abdominoperineal excision for rectal cancer. Dis Colon Rectum 2011; 54:947–957.. doi: 10.1097/DCR.0b013e31821c4bac. [DOI] [PubMed] [Google Scholar]
- 37.Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum 2005; 48:438–443.. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 38.Foster JD, Pathak S, Smart NJ, Branagan G, Longmann RJ, Thomas MG, et al. Reconstruction of the perineum following extralevator abdominoperineal excision for carcinoma of the lower rectum: a systematic review. Colorectal Dis 2012; 14:1052–1059.. doi: 10.1111/j.1463-1318.2012.03169.x. [DOI] [PubMed] [Google Scholar]
- 39.Foster JD, Tou S, Curtis NJ, Smart NJ, Acheson A, Maxwell-Armstrong C, et al. Closure of the perineal defect after abdominoperineal excision for rectal adenocarcinoma-ACPGBI Position Statement. Colorectal Dis 2018; 20:5–23.. doi: 10.1111/codi.14348. [DOI] [PubMed] [Google Scholar]
- 40.El-Gazzaz G, Kiran RP, Lavery NI. Wound complications in rectal cancer patients undergoing primary closure of the perineal wound after abdominoperineal resection. Dis Colon Rectum 2009; 52:1962–1966.. doi: 10.1007/DCR.0b013e3181b71ef9. [DOI] [PubMed] [Google Scholar]
- 41.Musters GD, Buskens CJ, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2014; 57:1129–1139.. doi: 10.1097/DCR.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 42.Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol 2005; 23:9257–9264.. doi: 10.1200/JCO.2005.02.9231. [DOI] [PubMed] [Google Scholar]
- 43.den Dulk M, Marijnen CA, Putter H, Rutten HJ, Beets GL, Wiggers T, et al. Risk factors for adverse outcome in patients with rectal cancer treated with an abdominoperineal resection in the total mesorectal excision trial. Ann Surg 2007; 246:83–90.. doi: 10.1097/01.sla.0000259432.29056.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid TD, Chan DS, Roberts SA, Crosby TD, Williams GT, Lewis WG. Prognostic signicance of circumferential resection margin involvement following oesophagectomy for cancer and the predictive role of endoluminal ultrasonography. Br J Cancer 2012; 107:1925–1931.. doi: 10.1038/bjc.2012.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussain A, Mahmooda F, Torrance ADW, Clarke H, Howitt C, Dawson R. Oncological outcomes of abdominoperineal resection for the treatment of low rectal cancer: a retrospective review of a single UK tertiary centre experience. Ann Med Surg (Lond) 2018; 34:28–33.. doi: 10.1016/j.amsu.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.How P, West NP, Brown G. An MRI-based assessment of standard and extralevator abdominoperineal excision specimens: time for a patient tailored approach? Ann Surg Oncol 2014; 21:822–828.. doi: 10.1245/s10434-013-3378-7. [DOI] [PubMed] [Google Scholar]
- 47.Pai VD, Engineer R, Patil PS, Arya S, Desouza AL, Saklani AP. Selective extra levator versus conventional abdominoperineal resection: experience from a tertiary-care centre. Gastrointest Oncol 2016; 7:354–359.. doi: 10.21037/jgo.2015.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


