Summary
Inflammation predisposes to the development of cancer and promotes all stages of tumorigenesis. Cancer cells as well as surrounding stromal and inflammatory cells engage in well-orchestrated reciprocal interactions to form an inflammatory tumor microenvironment (TME). Cells within the TME are highly plastic, continuously changing their phenotypic and functional characteristics. Here we review the origins of inflammation in tumors, and the mechanisms whereby inflammation drives tumor initiation, growth, progression and metastasis. We discuss how tumor promoting inflammation closely resembles inflammatory processes typically found during development, immunity, maintenance of tissue homeostasis or tissue repair, and illuminate the distinctions between tissue-protective and pro-tumorigenic inflammation, including spatio-temporal considerations. Defining the cornerstone rules of engagement governing molecular and cellular mechanisms of tumor-promoting inflammation will be essential for the further development of anti-cancer therapies.
Keywords: Inflammation, tumor microenvironment, cancer, cytokine, cell plasticity, tumor progression, metastasis, mechanisms
ETOC
Grivennikov and Greten review the mechanisms underlying the initiation of pro-tumorigenic inflammatory responses, how these evolve throughout the different stages of tumor development and the plasticity of the cells within the tumor microenvironment.
Introduction
Inflammation is an ancient evolved process which involves activation, recruitment and action of cell of innate and adaptive immunity. Initially highlighted for its essential role in host defense against pathogens, inflammation is equally important for tissue repair, regeneration and remodeling and subtle forms of inflammation are essential for the regulation of tissue homeostasis (Medzhitov, 2008). During the last couple of decades the contribution of the immune system and inflammation to cancer development, progression and therapy has regained enormous interest. At present, cancer biology is constantly shifting from a “cancer cell centric” view to a more inclusive concept that places cancer cells within a network of stromal cells that are comprised of fibroblasts and vascular cells and inflammatory immune cells that all together form the tumor microenvironment (TME). Inflammation, irrespectively of its occurrence in the context of a chronic inflammatory disease or in the appearance of a tumor-elicited smoldering inflammation, has a great impact on the composition of the TME and particularly on the plasticity of both tumor and stromal cells. Thus, our current view of inflammatory processes in the immune system during carcinogenesis can be distilled to the following: anti-tumorigenic function of immunity exerts immunosurveillance and immunological sculpting of tumor heterogeneity. At the same time pro-tumorigenic inflammation promotes cancer by blocking anti-tumor immunity, shaping the TME towards a more tumor-permissive state and by exerting direct tumor-promoting signals and functions onto epithelial and cancer cells. The hope for discovery of new mechanisms underlying possible cancer cures and prevention encompassed studies of immune system towards very different directions, including cancer vaccines and armored anti-cancer immune cells, various forms of immunotherapies, anti-cancer antibodies and biological therapies. It has become clear now that the immune system can play significant pro- and anti-tumorigenic roles at all stages of the tumorigenesis (Koebel et al., 2007; McGranahan and Swanton, 2017; Rosenthal et al., 2019; Schreiber et al., 2011; Teng et al., 2008; Vesely et al., 2011; Zilionis et al., 2019). The anti-tumorigenic role of immune system is endogenous, i.e. being exerted normally in response to transformed and cancerous cells. In addition, one of the most promising recent developments in the field of cancer immunology, is the successful implementation of various cancer immunotherapies, which use various approaches to redirect or hyperactivate immune system towards the recognition, restraining and killing cancer cells. These approaches include immunological “checkpoint” blockade, immunization with cancer vaccines, neutralization of immunosuppressive cells, treatment with oncolytic viruses or employing synthetic biology with bi-specific antibodies or cells with “chimeric antigen receptors” (CAR). These exciting developments and their underlying mechanisms have been recently extensively covered by other excellent reviews (Binnewies et al., 2018; Sharma and Allison, 2015; Topalian et al., 2015) and will not be discussed here. Yet, the great promise and recent success of cancer immunotherapies also put a significant emphasis on studies of tumor microenvironment heterogeneity from the immunological standpoint of view. As a result, gene expression, cell type infiltration and other immunological signatures, sometimes at the single cell level resolution, have been defined to be informative for cancer prognosis, its sensitivity to conventional and immunotherapies and future mechanistic insights (Bindea et al., 2013; Jerby-Arnon et al., 2018; Riaz et al., 2017; Spranger and Gajewski, 2015; Sweis et al., 2016). Also, terms of immunologically “hot” or “inflamed” tumor microenvironments are being coined primarily for tumors which have high levels of infiltrating T cells and increased presence of other components required for anti-tumor immune function, whereas immunologically “cold”, “infiltration-excluded”, “T cell excluded” and most importantly “immunological desert” and “non-inflamed” terms are reserved for tumors which do not exhibit cellular and gene expression characteristics favorable for anti-tumor action primarily by T cells (Binnewies et al., 2018). Such terminology - although useful to define tumors with a potential to respond to immune-mediated treatments - does not account for the presence of another functional arm of the immune system in cancer, which is pro-tumorigenic. There, immune system plays a distinct role during tumor initiation, promotion and progression, which is often referred as “cancer-promoting inflammation”. While tumors may not characterized by a prominent T cell infiltration or their functional activation, these tumors still may present with upregulation of inflammatory mediators and recruitment of other immune cells, often with tumor promoting properties, for example macrophages, monocytes, neutrophils or innate lymphoid cells (ILC). Meanwhile, a vast body of epidemiological studies now implicates inflammation and tissue repair immune responses to enhanced tumor incidence, growth and progression. These evidences include large clinical studies on “non-specific” inhibition of inflammation with non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, reducing incidence and mortality in many cancers (Rothwell et al., 2011; Rothwell et al., 2012); or specific inhibition of cytokines such as IL-1β with canakinumab, which significantly reduces the risk of lung cancer development (Ridker et al., 2017). Also, organ and site specific chronic inflammation predisposes to cancer development at the same site (Grivennikov et al., 2010).
Here we focus on the tumor-promoting role of immune and inflammatory responses, and draw parallels between tumor-promoting inflammation and “normal” functions of inflammation in tissue regeneration and host defense. In this context we discuss potential roles and timing of inflammatory action in cancer, describe possible sources of inflammation-initiating stimuli and outline underlying mechanisms of how inflammation can promote cancer.
Parallels between “normal” inflammatory responses and “inflammation in cancer”.
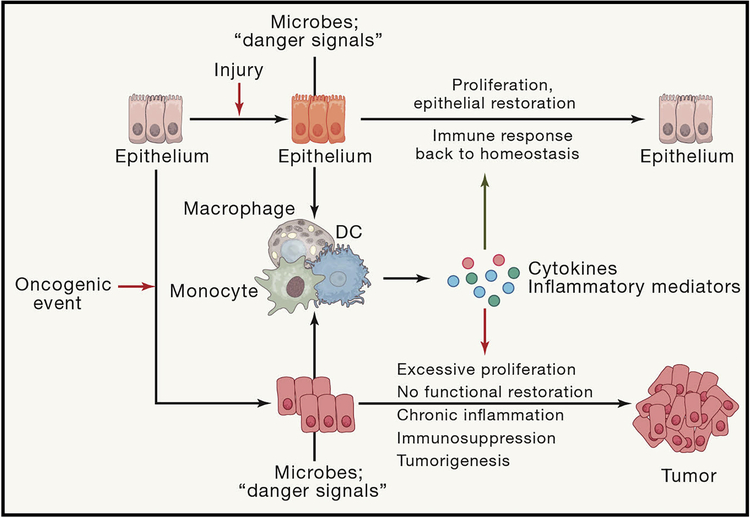
The ability to respond to infections and to perform wound healing and tissue regeneration has a much stronger positive evolutionary pressure than the avoidance of tumor development which often happens at post-reproductive age. Therefore, inflammatory mechanisms which are important for normal tissue regeneration but can also promote tumorigenesis present as an acceptable evolutionary “trade-off” (Figure 1). Low level homeostatic inflammatory response in altered tissues (Aran et al., 2016), and inflammation in the context of tissue homeostasis functions to sense and react to potential insults (stresses, tissue damage, infection, metabolic alterations and other changes to homeostasis) to restore homeostasis and to prevent loss of tissue function (Medzhitov, 2008). Tissue associated macrophages, major tissue sentinels, react to tissue alterations to remove dying cells (clearance of apoptotic cells), produce chemotactic molecules for the recruitment of other cell types (if necessary), regulate immune responses and barrier functions and support the stem cell niche. Tissue inflammatory responses may be initiated and maintained by at least three chief interdependent mechanisms. Firstly, local tissue macrophages and DC can increase their number by local proliferation if the degree of an initiating insult itself is not strong. Alike, strong perturbations of tissue homeostasis will lead to the recruitment of immune cells from bone marrow (monocytes, neutrophils and monocyte-derived cells) and secondary lymphoid tissues (lymphoid cells). Finally, recruited or locally amplified inflammatory cells further undergo local activation, differentiation and polarization, instructed by the cues from the microenvironment (Medzhitov, 2008; Okabe and Medzhitov, 2016).
Figure 1: Evolutionary and functional differences and similarities between inflammation in cancer and inflammation during infection and tissue regeneration.
Insults to epithelial tissues caused by injury or infection cause activation of myeloid cells which start to produce inflammatory cytokines to activate innate and adaptive sterilizing immunity to get rid of the pathogen and to activate epithelial cell proliferation to close down the barrier dysfunction which allowed translocation of pathogen or to repair inflicted injury. After this concerted effort insulted epithelial tissue comes back to normal state of homeostasis. However if initial disturbance of epithelial homeostasis is caused by an oncogenic event, the sterilizing immunity will not remove the intial insult and the enhanced inflammation and cytokine-driven proliferation will facilitate tumor growth rather than restoring normal epithelial homeostasis.
In the context of cancer, it is conceivable that hyperproliferation of epithelial cells also induces homeostatic responses aimed at increasing numbers of macrophages and fibroblasts, associated with epithelial tissues as basic “tissue blocks”. This is achieved by the existence of signaling circuits by various chemokines and growth factors reciprocally produced by one cellular type and consumed by another (Zhou et al., 2018). This process is likely to be uniform between tissue amplification for organism growth, limited tissue repair after wounding or infection and tumor growth. Therefore, mere epithelial and cancer growth triggers the encoded process of acquiring more macrophages and fibroblasts in the tissue. If the initial level of stress, hypoxia and other tumor specific insults is not high, then most of tumor associated macrophages can emerge from local proliferation and migration of tissue macrophages (Loyher et al., 2018; Zhu et al., 2017).
In contrast to wound healing and infections that resolve after immune cell recruitment and epithelial cell proliferation, growing tumors present with persisting oncogene-derived stress, cell death and microbial signals that altogether feed into a feed-forward loop of inflammation-induced signaling and inflammatory cell recruitment (Figure 1). As monocyte-derived cells, such as subsets of DC, monocytes themselves and neutrophils do not have their local precursors residing in the tissue, their emergence during tumor growth and progressing is invariably executed via recruitment of hematopoietic-derived (spleen, blood, bone marrow) cellular precursors. It remains to be determined whether transcriptional programs for local versus systemic myeloid cell amplification in the tumors are significantly different, but it is possible that the former is primarily driven by an increase in growth factors (i.e. CSF-1) while the latter is preferably based on chemokine induction.
Chronic inflammation, infection and autoimmunity predecing tumor formation
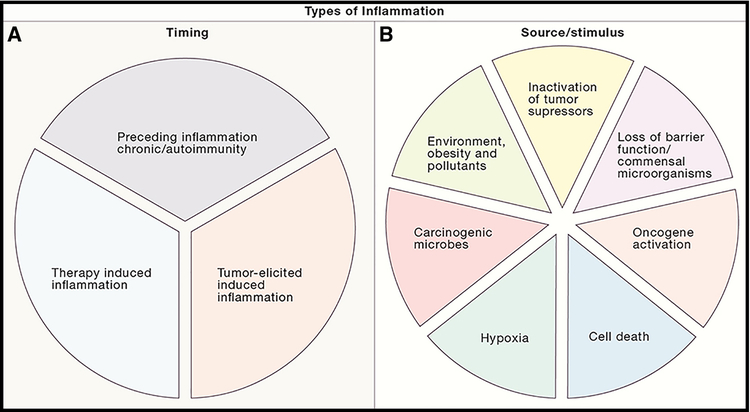
In order to deconstruct the roles and the mechanisms of action of inflammation and cancer, it is important to understand how inflammation in cancer is induced and maintained in the first place, both in terms of time and stimulus (Figure 2). Around 15–20% of all cancer cases are preceded by infection, chronic inflammation or autoimmunity at the same tissue or organ site (Grivennikov et al., 2010; Mantovani et al., 2008). In such cases, inflammation which promotes cancer is induced and exists long before tumor formation. The most prominent examples include inflammatory bowel diseases (IBD), chronic hepatitis, Helicobacter-induced gastritis or schistostoma-induced bladder inflammation increasing the risk of CRC, liver cancer, stomach cancer or bladder cancer, respectively (Trinchieri, 2012).
Figure 2: Types of inflammation in cancer: different timing and different inducers.
(A) Cancer-associated inflammation can be induced at different time points of tumor development. It can precede carcinogenesis in form of autoimmunity or infection, can be induced by malignant cells or can be triggered by anti-cancer therapy. (B) Various cell intrinsic, host dependent or environmental factors can cause tumor-associated inflammation in different tumor types.
There are also various environmental factors which predispose to and promote cancer acting in whole or in part via induction of chronic inflammation, albeit sometimes of low grade and intensity. In this case, inflammation may precede or accompany tumor development. With regard to the host, these factors may be systemic or site and organ specific. For example, inhalation of fine particles, tobacco smoke and asbestos would primarily cause lung and airway inflammation and promote lung cancer and mesothelioma (Kadariya et al., 2016; Takahashi et al., 2010). On the other hand, low grade inflammation induced by obesity, hyperglycemia and excessive lipid accumulation is generally of systemic nature, and as a result, can promote or increase risk of many different cancers, including liver, pancreatic, colon, breast and other malignancies (Quail and Dannenberg, 2019; Quail et al., 2017). Type II diabetes, which has previously been considered as an independent risk factor for cancer, may be viewed as a part of obesity-induced inflammation and obesity-related tissue injury leading to cancer sequela. With the rapid emergence of obesity epidemic in the westernized countries, the exact mechanisms of how obesity and obesity-related inflammation promote tumor progression need to be determined in order to mitigate these live threatening consequences of prevalent metabolic diseases. The action of systemic inflammation can take place even during the late stages of tumor developments, as exemplified by tobacco-smoke, obesity and bacterial products induced inflammation which activate neutrophils and their extracellular trap formation function to promote breast cancer metastasis into the lungs (Albrengues et al., 2018).
Tumor elicited inflammation
However, the development of the majority of cancers and individual tumors is not preceded by long-standing chronic inflammation. For example, while IBD predisposes to colitis associated cancer (CAC), only around 2% of CRC are preceded by intestinal inflammation (Ullman and Itzkowitz, 2011). Nevertheless, bulk transcriptional studies and other robust approaches to study the complexities of tumor microenvironment with regard to cellular heterogeneity, precise cell type identification and imaging, and cell-to-cell differential transcriptomics demonstrate enhanced expression of distinct inflammatory cytokines and chemokines in primary tumors and metastatic lesions, and qualitative and quantitative differences in inflammatory cell recruitment (Binnewies et al., 2018; Puram et al., 2017). Presence of certain chemokines, cytokines and myeloid cell subsets correlate with poor prognosis in CRC, as established in the “CRC immunoscore” by the Galon group (Mlecnik et al., 2016; Tosolini et al., 2011), which most likely will be further modified and expanded for other tumor types as well. In addition, neutralization or genetic inactivation approaches in pre-clinical animal models, demonstrates that inhibition of inflammatory responses in these seemingly “non-inflammatory” cancers stunts tumor growth and progression. The fact that cancers previously defined as “non-inflammatory” recruit immune cells and increase expression of inflammatory mediators to support tumor growth and re-shape the tumor microenvironment (TME) to their benefit, has led to the term “tumor-elicited (associated) inflammation” (TEI) (Grivennikov et al., 2010; Grivennikov et al., 2012). However, inducers of TEI in microbial-rich versus “sterile” tumors may be different (Hanahan and Coussens, 2012; Mantovani et al., 2008). For example, in CRC early oncogene-induced deterioration of protective intestinal barrier at the site of tumor formation leads to translocation of commensal bacteria and bacterial products, which are recognized by tumor-associated myeloid cells to induce IL-23 production and IL-23 dependent TEI (Grivennikov et al., 2012). In contrast, in tumors not associated with mucosal surfaces, the initial inflammatory trigger may come from sensing oncogenic transformation, metabolic alterations, cell death or hypoxia (Mantovani et al., 2008; Mantovani et al., 2019).
Therapy-induced inflammation in cancer
While not present in intact tumors, another important type of inflammation is therapy induced-inflammation, which develops in response to various anti-cancer therapies, including chemo- and radiotherapy and recently immune infiltration caused by various biologic therapies or immunotherapies. Immune system activation in the tumor upon treatment is the cornerstone for current immunotherapies (Sharma and Allison, 2015,Sharma, 2015 #16250). This process certainly can be beneficial for stimulation of anti-tumor immune responses, which will collaborate with standard therapies. In some cases, release of damage-associated molecular patterns (DAMPs) such as ATP and HMGB1 from dying tumor cells can stimulate production of IL-1α and other immunostimulatory cytokines. This along with increased release of tumor neo-antigens this may induce and sustain de novo anti-tumor T cell responses (Ghiringhelli et al., 2009) or may lead to immunosuppression (Hou et al., 2017). However, the net outcome may not be uniform across tumor types or even individual tumors (Ciampricotti et al., 2012) and will depend on how respective cytotoxic regimens or radiotherapies will affect activation and function of the cells of anti-cancer immunity. Moreover, many tumors are deficient in apoptotic cell death and therefore cell death by necrosis may be more immunostimulatory (Galluzzi et al., 2018; Weinlich et al., 2017). Importantly, in many instances partial destruction of tumors by therapies and release of dead cell material will also have immunosuppressive effects (Hou et al., 2017) and will stimulate an inflammatory response overall resembling injury to normal tissue with subsequent wound healing and tissue repair. Here, recognition of dying tumor cells would stimulate the production of cytokines and growth factors such as TNF, EGF, IL-6, Wnt ligands and other by the cells of tumor microenvironment such as myeloid cells and fibroblasts, and additional recruitment of these cells. These growth factors may serve as cell extrinsic anti-apoptotic/generally anti-cell death signals, which would decrease the efficiency of therapy being used. For example, paracrine EGF family ligand production, which can be elicited from macrophages or fibroblasts is a major factor of therapy resistance in cancer (Srivatsa et al., 2017). Other STAT3-activating cytokines such as IL-22, IL-11 and IL-6 were implicated into enforcement of stem cell phenotypes in cancer. With cancer stem cells being less proliferative and less metabolically active, they are less sensitive to many forms of chemo- and radiotherapy. Enhanced recruitment of myeloid cells and their release of the pyrimidine nucleotides confers resistance of pancreatic cancers to gemcitabine (Halbrook et al., 2019), exemplifying non-immune, metabolic role of inflammatory cells in therapy resistance. Cytokines like IL-17 also can act directly on CRC cancer cells to provide them with resistance to a first line anti-CRC therapy with 5-FU (Wang et al., 2014) and inflammatory signaling targeting remaining tumor cell is an important driver of therapy resistance (Jinushi et al., 2011; Malesci et al., 2017).
Yet, another probably underappreciated mechanism is the ability of chemotherapies to cause normal tissue damage, specifically in the intestine, translocation of inflammatory microbial products and activation of systemic inflammation, which can further promote tumors, as demonstrated for myelodysplastic syndrome (Meisel et al., 2018) and various metastatic cancers where microbial products accelerate metastatic growth (Albrengues et al., 2018; Luo et al., 2004). Altogether, therapy induced inflammation develops only after treatment, but may play an essential role in determination of therapy efficacy or resistance to therapy. In this context delineation of exact signals which induce inflammation during tumor development will undoubtedly help to fill in a broader picture how tumor evolution shapes the TME.
Sources and Stimuli for induction of inflammation during tumorigenesis
There are several mechanisms of how “ground zero” inflammatory responses may be induced and what are the relevant causes and stimuli (Figure 2B). One of the hallmarks of cancer is the loss of cell intrinsic tumor suppressor functions. One of the most commonly mutated tumor suppressors is Tp53, encoding for p53 protein. P53 protein has multifaceted functions to regulate cellular homeostasis but one of them is its transcriptional antagonism with NF-κB (Komarova et al., 2005; Schwitalla et al., 2013b), a key positive regulator of inflammation. As NF-κB activating signals are always present within the tumor microenvironment and even in a normal tissue, loss of functional p53 results in increased expression of NF-κB dependent inflammatory genes. In colorectal cancer, this inflammatory signature contributes to tumor progression and metastasis (Elyada et al., 2011; Pribluda et al., 2013; Schwitalla et al., 2013b). Loss of tumor suppressors also can inhibit proper DNA repair and accelerate DNA damage, which can trigger DNA-damage induced inflammatory pathways (Andriani et al., 2016). Moreover, activation of oncogenes, necessary for cancer development, is mechanistically linked to the increased production of cytokines and chemokines and recruitment of myeloid cells, which are either directly tumor promoting or immunosuppressive. For example, oncogenic signaling K-Ras regulates the expression of CXCL3, a key chemokine for myeloid cell recruitment (Liao et al., 2019). Moreover, K-Rras activation enhances production of cytokines and chemokines that belong to “senescence-associated secretory phenotype” (Davalos et al., 2010), including IL-1α, IL-1β, CCL2 and CXCL1. K-Ras and c-Myc activation cooperate in induction of CCL9, IL-23 and other inflammatory entities in pancreatic cancer (Kortlever et al., 2017) and overall the mechanisms where oncogene activation leads to excessive production of inflammatory cytokines and chemokines may be a unifying mechanism how inflammation is triggered in many cancers.
If tumor development and progression is initially driven by a pathogen such as H. pylori, H. hepaticus, HCV, HBV or HPV, recognition of persistent pathogens will of course promote distinct inflammatory responses. This is one of the most straightforward examples where recognition of cancer-inducing pathogens through “classical” receptors recognizing conserved molecular patterns would trigger innate inflammatory responses. Sensors like TLR2 and TLR4, STING, cGAS as well as multiple sensors associated with inflammasomes sense oncogenic bacteria and viruses (Woo et al., 2015). However, an alternative emerging paradigm is that many cancers may be promoted by commensal microbiota, either by translocation and adherence of microbes to cancer cells or by the distant release of inflammation-activating microbial metabolites. These microbes and microbial products can even travel with tumors to the site of metastasis and serve as a source of inflammation in metastasis (Bullman et al., 2017). Pancreatic cancer is often associated with chronic pancreatitis, which may be sometimes associated with infection but even in mouse models is driven by microbial induced Th17 responses (McAllister et al., 2014). Liver cancer and NASH/fibrosis which underlies HCC development are actively promoted by intestinal bacteria and their products (Dapito et al., 2012; Shalapour et al., 2017) and recent studies highlight the role of lung microbiota in induction of inflammation and tumorigenesis (Greathouse et al., 2018; Jin et al., 2019). Perhaps the best example of commensal microbiota influencing tumor growth and progression comes from colon cancer. Events following intestinal transformation lead to the deterioration of intestinal barrier, because hyperproliferating cells fail to properly differentiate and form protective tight and adherence junctions and well-developed mucus layer, isolating immune compartment from bacteria (Grivennikov et al., 2012). Broad spectrum antibiotic treatment or rendering mice germ free reduces inflammation and tumor growth, even in animal models where potential pathogens are absent. Moreover, diet-induced changes in the microbiome promote tumor progression in the presence of activating K-Ras mutations (Schulz et al., 2014). In human CRC, quite a few of bacteria have been suggested to be preferentially associated with adenomas and carcinomas, including subspecies of Escherichia coli (Arthur et al., 2012), Bacteroides fragilis (Wu et al., 2009) and Fusobacterium nucleatum (Kostic et al., 2013). A common denominator for these bacteria is their presence in tightly adherent fraction of bacteria capable of direct interaction with the surface of the tumor, either because of special ligand-receptor mode of adhesion (Rubinstein et al., 2013; Yu et al., 2017), ability to form biofilms and initiate the outgrowth of the consortia of invasive bacteria (Dejea et al., 2018; Tomkovich et al., 2019) or ability to induce low grade inflammation disrupting the barrier (Wu et al., 2009). It is reasonable to expect that the tumor promoting action of microbes in any of microbe rich cancer sites will be in part mediated by the inflammation they modulate.
While the ability to evade cell death is clearly a prominent hallmark of cancer (Hanahan and Weinberg, 2011), it is important to distinguish “who dies, when and how”. Notably, cell death is not only important in the context of therapy-induced inflammation but limited cell death of untransformed cells adjacent to tumor seeds may also be essential for tumor growth (Kuraishy et al., 2011), especially in organs like liver and skin. In part this can be explained through clearance of the space and niche and induction of compensatory proliferation of tumor clones, other mechanisms clearly involve immunological recognition of cell death and induction of pro-tumorigenic immune responses. For example, in liver cancer death of hepatocytes results in release of IL-1α and other “alarmins” to trigger expression of IL-6 and other growth factor to promote survival and growth of neighboring mutated hepatocytes (Sakurai et al., 2008). HMGB1, another alarmin released from dying cells, is important to initiate inflammatory responses and tumorigenesis (Khambu et al., 2018). Release of SAP130 cytoplasmic protein from necroptotic pancreatic cancer cells is sensed by Mincle receptor and induces CXCL1 and thus recruitment of myeloid cells which drive tumor growth and inhibit anti-tumor T cells responses (Seifert et al., 2016). The inducer of cell death, whether it will be hypoxia or metabolic induced stress in growing tumors, therapy, infection or mutagenic insult may not matter much for the consequences of cell death and inflammation. However, the type of cell death may be important, with apoptosis and autophagy being less inflammatory and necrosis and necroptosis resulting in release of DAMPs being more potent inflammatory inducers.
Although the source/inducer of inflammation in cancer may be different, it seems that the induction of inflammation is always tightly linked with the emergence of factors absolutely needed for oncogenic process-alterations in oncogenes and tumor suppressors, infections for microbial-induced cancers or deterioration of barrier function because of transformation-induced loss of tissue organization. As inflammation is wired to be induced during the responses to the loss of tissue homeostasis, the induction of inflammation is ‘pre-encoded’ in genetic and transcriptional programs required for oncogenic transformation and therefore in many conditions.
Inflammation and tumor initiation
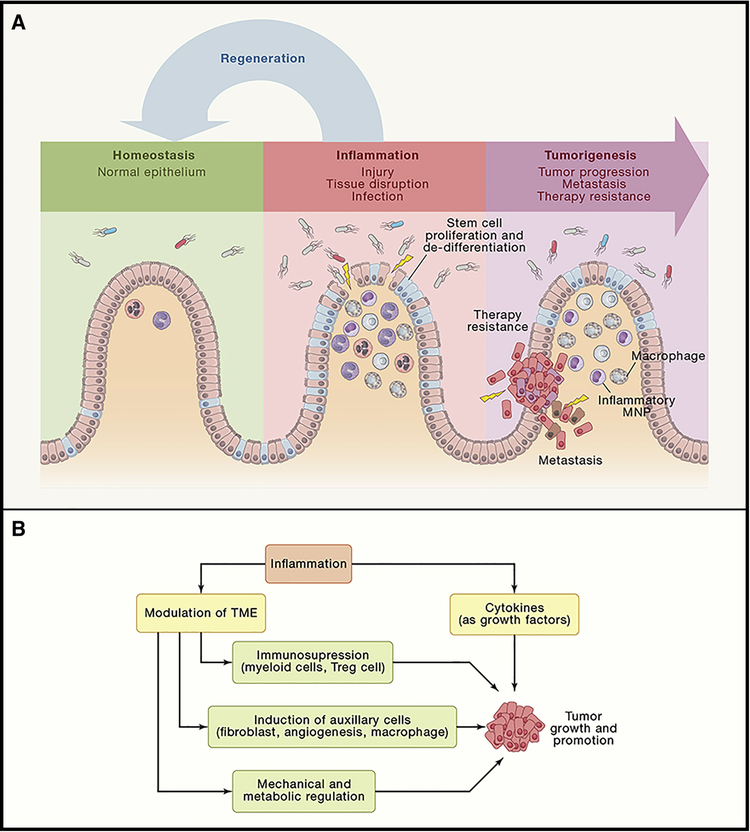
Two main interdependent events are required for successful tumor initiation. 1) One leads to accumulation of mutations and/or epigenetic alterations of genes and signaling pathways involved into tumor suppression (inactivation) and oncogenic pathways (activation). While traditionally these have been linked mostly to environmental factors (UV, carcinogens, variable radiation) and inherent errors in DNA repair and replication, inflammatory responses harbor powerful mechanisms which lead to accumulation of mutation and various epigenetic changes in adjacent epithelial cells. Macrophages and neutrophils are potent producers of reactive oxygen (ROS) and nitrogen (RNI) species which induce mutations. Therefore, induction of inflammation can lead to increased mutagenesis, predisposing to accumulation of mutations in normal tissue. Indeed, chronic intestinal inflammation leads to accumulation of mutations in Tp53 and other cancer related genes in intestinal epithelial cells (Canli et al., 2017; Chang et al., 2007; Hussain et al., 2003; Robles et al., 2016) and can trigger tumor formation even without additional extrinsic mutagens being present (Meira et al., 2008). Interestingly, potential of inflammation to induce mutations and DNA damage is accounted for by evolution, as inflammatory cytokines such as IL-22 also can induce expression of DNA damage response (DDR) genes to counteract possible genotoxic insult caused by inflammation (Gronke et al., 2019). Also, signaling by cytokines (i.e. IL-6, TNFα, IL-1β) produced by inflammatory cells activates epigenetic machinery in epithelial cells including components of DNA and histone modifications (Dnmt1, Dnmt3, DOTL1), miRNA and lncRNA modulating expression levels of oncogenes and tumor suppressors (Grivennikov, 2013). The net outcome of such epigenetic changes is proposed to be the same as inactivating mutations in tumor suppressors and activating mutations in oncogenes, and potentially can be achieved in “bi-allelic” manner at the same time. As in many cases stem cells are proposed “cells of origin” for cancer, inflammatory processes can trigger de-differentiation of post-mitotic epithelia into tumor-initiating stem like cells (Schwitalla et al., 2013a) (Figure 3A). Chronic inflammation triggering tissue damage can weaken barrier function and expose the stem cell compartment to environmental carcinogens or to bring stem cells to a close proximity of active inflammatory cells producing genotoxic compounds. In microbial rich cancer, such as colon cancer, enhanced inflammation can also shape the qualitative characteristics of epithelial-adhesive microbiota, enriching the content of species harboring genotoxic gene products, such as colibactin in some strains of E.coli (Arthur et al., 2012; Wilson et al., 2019) capable of inflicting mutations in host cells.
Figure 3: Pro-tumorigenic actions of inflammation in progression, metastasis and growth.
(A) Injury, infection or functional tissue disruption(as during malignant transformation) leads to activation of inflammatory responses which can drive the expansion and proliferation of stem cell pool, the process normally designed to restitute and normalize epithelium and its barrier function, i.e. causing normal “Regeneration” and a path to normal epithelium. However, if stem cells already harbor oncogenic mutations, and are de facto cancer stem cells, expansion of stem cell pool leads to enhanced metastasis and therapy resistance. (B) Inflammatory entities, such as cytokines and growth factors, release by immune cells within the TME can have a direct effect on pre-malignant and cancer cells by increasing their proliferation and resistance to cell death and stresses, thereby directly promoting tumor growth and progression. can be caused at different time points of cancer development. In addition, inflamamtory signals can shape TME to induce immunosupression via action of Tregs, immature myeloid cells and other supressive players; to enhance recruitment, proliferation and distinct functions of other pro-tumorigenic auxillary cells within the TME (such as fibroblasts, myeloid cells and endothelium of new blood vessels); and to alter mechanicaal and metabolic functions of TME. Altogether, these inflammation-driven changes also significantly contribute to tumor growths and progression.
2) Creation of transformed and/or malignant clones, however, should be followed by their outgrowth into a frank tumor, a process to which inflammatory mechanisms can significantly contribute. For instance, cytokine receptor signaling in mutated cells may converge at the induction of pro-survival pathways particularly mediated by NF-κB, STAT3 and other types of signaling (Dmitrieva-Posocco et al., 2019; Greten and Karin, 2005; Grivennikov et al., 2009), thereby increasing the survival probability of survival of transformed clone or enable proliferation. Specifically, it is conceivable, that such an early contribution of inflammatory signaling into tumor initiation is needed as tumor cells in limited numbers do not yet establish a full-scale TME with auxiliary stromal cells capable of producing enough tumor-supporting growth factors. The issue of inflammation-driven cell survival may be also important in context of cancer immunosurveillance and tumor elimination phase of mutated and stressed cells (Dunn et al., 2004; Schreiber et al., 2011). Signals activating STAT3 protect epithelial cells from CD8 T cytotoxic cell attack (Yu et al., 2009; Ziegler et al., 2018) and signaling by IFNκ, albeit a typically anti-tumorigenic cytokine, upregulates expression of T cell exhaustion inducing molecule programmed death (PD)-L1 on transformed epithelium, which is recognized by T cells. To the same end, inflammatory signals may increase fitness and reduce expression of “stress ligands” on cancer cells, required for proper recognition and (Iannello and Raulet, 2014; Lam et al., 2014; Shifrin et al., 2014), Also, inflammation and injury trigger cell turnover in tissues, thereby creating space for the outgrowth of malignant clones (Kuraishy et al., 2011). This is especially evident in liver and skin cancer, where death of normal cells is required for compensatory proliferation of neighboring transformed clones, thereby creating a scenario where inflammation-induced tissue injury and cell death are required for tumor outgrowth. Some of the immunological mechanisms of Crohn’s disease (CD) and ulcerative colitis (UC), as well as the expression of inflammatory mediators may be similar. However, UC which is associated with significant tissue damage and ulceration, followed by repetitive cycles of regeneration increases the risk of colorectal cancer (CRC) up to 5–8 times, while CD presented with transmural inflammation but not widespread epithelial damage, increases the risk for CRC only 1.4 times (Ullman and Itzkowitz, 2011). Therefore, while mere presence of inflammatory cytokines is needed for tumor initiation, cytokines collaborate with inflammation-induced tissue damage and regeneration.
Clones with equal genetic alterations can possess different propensity for survival and outgrowth depending on tissue microenvironment these clones are placed into, illustrating the adaptive nature of oncogenic process (DeGregori, 2017; Henry et al., 2015; Rozhok and DeGregori, 2019). Stressed and inflamed tissue maybe conducive of stimulating tumor growth while normal unaffected tissue would block it. Alike, the presence of inflammation can trigger outgrowth of dormant clones, both in the case of primary tumors and in distant metastasis. Some of the mechanisms responsible for this phenomenon may intersect with the ability of cytokines to promote survival and proliferation described above.
Inflammation and tumor promotion
Perhaps the best studied propensity of inflammation to influence tumorigenicity is its contribution to tumor promotion. From a translational stand point, insights into how inflammation drives tumor growth are important at least two-fold: inhibition of inflammation can stall tumor growth and further dedifferentiation, widening the opportunity for early detection of cancers and shedding light on how metastatic seeds outgrow once distantly established. The progress in this field has been fueled by multiple preclinical animal models of inflammation and cancer and was instrumental to reveal most of the significant mechanisms connection inflammation and cancer. Conceptually, several interdependent molecular and cellular mechanisms are at play (Figure 3B). Firstly, similar to tumor initiation, inflammatory entities can serve as direct growth factors for growing tumors. In addition, inflammatory factors are essential in shaping cell plasticity within the TME, which further affects tumor growth by at least three distinct mechanisms.
One of the first evidences on the role of inflammation in tumor promotion came from studies of inactivation of NF-κB in myeloid cells, where ablation of the IKKβ kinase led to a reduction in tumor growth in a model of colitis-associated cancer (Greten et al., 2004). Indeed, NF-κB, especially in immune cells, is a master regulator of cytokine expression whose action on epithelial and cancer cells promotes survival and proliferation and chemokine expression, which is essential for cell recruitment and re-shaping of the TME. Indeed, NF-κB dependent cytokines confer unique and non-redundant roles in tumor growth as their genetic or pharmacological inactivation reduces tumor growth acting through activation of other oncogenic pathways in epithelial and cancer cells, including STAT3, ERK, JUN and receptor Tyr-kinases (RTK) (Becker et al., 2004; Grivennikov et al., 2009; Huber et al., 2012; Popivanova et al., 2008; Putoczki et al., 2013). Similarly, signaling triggered by inflammatory cytokines including IL-6, IL-17, IL-11 can increase proliferation rates of tumor cells, especially under sub-optimal conditions present in vivo, including hypoxia, lack of nutrients, paucity of environment in production of growth factors and sculpting role of anti-tumor immunity.
Action of inflammatory cells and mediators during tumor growth also includes antagonizing potential anti-tumor immunity and promotion of tumor auxiliary functions such as stimulation of angiogenesis and recruitment of fibroblasts and other stromal cells, which exert tumor-supporting functions. Finally, through modulation of TME inflammatory signals alter mechanical and metabolic properties of stromal and tumor cells by regulating formation and consistency of extracellular matrix and availability of growth factors, regulation of consumption and availability of key metabolites, including those involved in amino acid and redox metabolism. These overall mechanisms were previously reviewed elsewhere (Grivennikov et al., 2010).
Inflammation, tumor progression and metastasis
Historically, studies on tumor initiation and growth were the first instrumental steps to demonstrate the critical role link between inflammation and cancer. However, understanding inflammatory mechanisms governing metastatic process has the most eminent impact considering that over 90% of cancer-related deaths occur because of metastatic disease. Indeed, longitudinal clinical studies that support the role of inflammation in cancer mortality (Rothwell et al., 2012) most likely underscore the role of inflammation in regulation of metastasis, rather than earlier stages of tumorigenesis. Prolonged aspirin usage reduces overall mortality, especially of gastrointestinal cancers, and distant metastasis. Experimental stimulation of resolution of inflammation or blocking inflammatory responses results in poor colonization and eradicates micrometastases (Panigrahy et al., 2019). Metastasis is a highly inefficient process with most of cells released from primary tumor eventually dying and not forming a distant metastasis. Therefore, any potential aid to get through the bottleneck would have a cardinal effect on the ‘success” of metastasis and potential patient survival. Given the apparent paucity of true metastatic models, some of the mechanisms of how inflammation may affect this process have only come to light recently.
The process of metastasis starts with the invasion on cancerous cells away from epithelial layer into the neighboring tissues and acquisition of EMT (epithelial to mesenchymal transition) phenotype, at least temporary. While such epithelium-to-’fibroblast” cell transformation may be often incomplete and partial (Varga and Greten, 2017), it renders cancerous cells mobile and allowing them to break through the basal membrane, invade the tissue and reach the lymphatics or blood vessels for further dissemination. In particular, cancer stem cells as opposed to “bulk” tumor cells are more efficient in their capacity to serve as metastatic seeds (de Sousa e Melo et al., 2017). Interestingly, cancer stem cells are also transcriptionally and functionally closer to mesenchymal cells, than regular epithelial or bulk tumor cells, probably reflecting their existence in the niche rich in mesenchymal growth and differentiation factors (Dominguez et al., 2017). Inflammation influences cancer invasion, EMT and cell migration on several levels. Cytokines, including TNF and IL-1β can affect directly expression of EMT inducing transcription factors Twist and Slug (Francart et al., 2018; Suarez-Carmona et al., 2017). Moreover, IL-11 is involved in the recruitment of TGFβ driven and producing fibroblasts, which supports tumor invasion and immune escape in the subset of colon cancer with the worst clinical prognosis (Calon et al., 2012; Calon et al., 2015). Similarly, in breast cancer, IL-11 within the tumor microenvironment acts on cancer cells to drive clonal selection of the most invasive and malignant clones (Marusyk et al., 2014). The recrutment of myeloid cells to the invasive edge of the tumor leads to production of matrix metalloproteinases (MMP) and other enzymes`, which remodel extracellular matrix and facilitate celll migration (Akkari et al., 2014; Sevenich et al., 2014). The same myeloid cells, particularly of granulocytic and monocytic lineage, sometimes referred to as myeloid derived suppressor cell (MDSC)) also contribute to the suppression of anti-tumor responses (Veglia et al., 2018; Yang et al., 2008). Increased accumulation of cytotoxic T and NK cells at the invasive margin of the primary tumor, along with decreased presence of myeloid cells as a part of the “cancer immunoscore” indeed correlates with better prognosis (Bindea et al., 2013; Mlecnik et al., 2016). Yet, it is not yet clear whether myeloid induced immunosuppression is critical at the invasion stage or at preventing metastatic outgrowth. It is conceivable that macrophages play very diverse roles in immunosuppression, chemoattraction and destruction of extracellular matrix as the invasion proceeds (Gatenbee et al., 2019). Apart from that, tissue macrophages are critical cell types during the development of several organs and can assist cell migration and establishment of tissue architecture by producing factors directly influencing motility, phenotype and positioning of epithelial and mesenchymal cells. In this regard, increased production of inflammatory entities causing enhanced recruitment of myeloid cell into the invading tumors may support tumor outgrowth via a variety of mechanisms which are evolutionary reserved for the regulation of organogenesis during ontogeny.
Cancer stem cells (CSC) are considered essential for tumor metastasis and resistance to therapy, yet it becomes increasingly clear that the number and proportion of CSC in the tumor are not constant, like for stem cells in the normal tissue. Instead, various stimuli including prominent inflammatory signaling via transcription factors NF-κB and STAT3 in cancer cells can drive their stemness, increase the proportion of CSC among the tumor cell population and thereby elevate the invasive potential (Kryczek et al., 2014; Schwitalla et al., 2013a). The same effects can originate from mesenchymal-cancer interactions (Del Pozo Martin et al., 2015; Malanchi et al., 2011) and mesenchymal and stromal cells can be regulated by inflammatory signals.
As metastatic spread typically occurs via lymphatics or blood stream, the processes of intravasation and extravasation are essential. These are mediated by the expression of adequate set of adhesion molecules and integrins, to allow heterotypic cell-cell interaction, adhesion and movement. Inflammatory cytokines are potent inducers of integrins, selectins and adhesion molecules like VCAM-1 and ICAM-1. The expression of exact and tissue specific adhesion molecules may also determine the organ-specific tropism of metastasis and therefore ‘flavors” of inflammation may potentially have an impact to which distant organ particular tumor seed or type of cancer will primarily will spread to.
The process of adhesion and extravasation can be further aided by inflammatory cells, such as monocytes and neutrophils (Kersten et al., 2017), which would precipitate the adherence of metastatic seeds, form complexes with cancer cells and mediate their adhesion and translocation throughout the vessel wall as well as establishing and maintaining the metastatic niche (Aceto et al., 2014; Szczerba et al., 2019; Wolf et al., 2012). The oligocellular complexes between cancer cells themselves, or monocyte-cancer cells, neutrophil-cancer cell also serve to protect these metastatic seeds from the immunesurveillance at the most vulnerable moment away from established immunosuppressive microenvironment of the primary tumor. It is clear, that inflammatory signals from obesity to microbes, to tumor-specific inflammatory stimuli which enhance these cell-cell interactions increase the rate of metastasis. For example, IL-17 dependent activation of neutrophils drives breast cancer metastasis through cellular interactions in the circulation and possibly via establishment of pre-metastatic niche (Coffelt et al., 2015), while lipopolysaccharide increases lung colonization and promotes tumor cell survival (Luo et al., 2004). Stimulation of inflammation by tobacco smoke, obesity or microbial compounds also increases lung metastatic burden via induction of neutrophil activation and increased neutrophil-cancer cell interactions mediated by NET’s (Albrengues et al., 2018), although it is not clear whether this process is regulated at the stage of spread or dormancy. While the process of metastatic growth from a smaller to a larger metastatic nodule is probably not much different from the growth of primary tumor and the role of inflammation there, one of the roadblocks for metastasis which remains is the ability of single cells to take off and grow in the epithelial tissue of origin different from the primary tumor. Because of that, initially after the spread and initial colonization some of the seeds remain at the dormant stage and their eventual outgrowth may be promoted by inflammatory cytokines and growth factors produced by immune cells (Krall et al., 2018). It remains to be determined across the different tumor types, whether the requirement of inflammatory and growth factors is the same for primary tumor growth and for metastatic outgrowth in different secondary organ sites.
Inflammation and cell plasticity within the tumor microenvironment
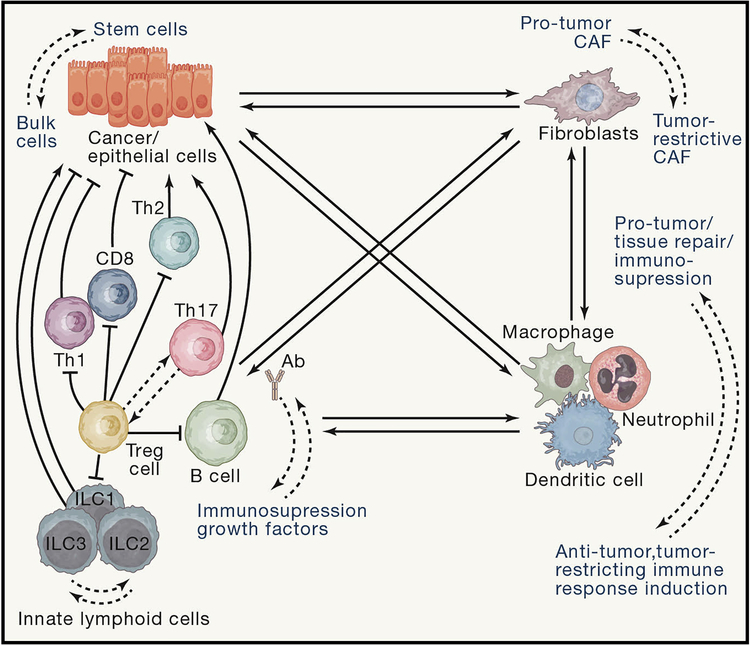
Tumor heterogeneity is an emerging theme in oncogenesis. In addition to the well-appreciated genetic and epigenetic heterogeneity of cancer cells, tumors also significantly differ in terms of quantity and phenotypic characteristics of immune and stromal cells that are being recruited causing a high degree of cellular plasticity within the TME (Figure 4). First, this plasticity exhibits itself in various types of cell polarization, driven by distinct transcriptional programs. This enables cells with the functions that are essential during the tissue regeneration or for tumor development, but not characteristic for these cells in unchallenged conditions. Secondly, spatiotemporal cellular plasticity allows transition of cells from one state to another during such processes as EMT, cell migration, MET and metastasis. It is likely that “subtypes” or ‘types” previously assigned to different cells within the tumor microenvironment as states of terminal differentiation or distinct cell types, often represent differentiation and activation states which may undergo further phenotypic and functional changes through the cell plasticity. Plasticity can be presented by different cell types during different stages of tumor development. For example, during initiation stage of intestinal cancers, stem cells are proposed to be an origin of cancer (Barker et al., 2009), however mutated epithelial and transient-amplifying cells can undergo plasticity and de-differentiation to acquire cancer stem cell phenotype in response to NF-κB activating inflammatory signaling (Schwitalla et al., 2013a). However, the best known case of cancer cell plasticity is through their ability to undergo various states of EMT and MET (Gupta et al., 2019; Pastushenko et al., 2018; Varga and Greten, 2017) and re-establishment of cancer stem cell pool during metastasis or therapy (de Sousa e Melo et al., 2017; Sanchez-Danes et al., 2018). All of these processes are strongly influenced and regulated by inflammation, cytokines and growth factors. However, not only cancer cells are subject to plasticity. Essentially all other cell types in the TME are engaged into reciprocal activation/inhibition/differentiation signals to influence and drive plasticity. For example, cancer cells instruct fibroblasts to acquire differentiation states which are tumor-promoting and immunosuppressive (Alexander and Cukierman, 2016) but activation of fibroblasts also can be driven by cytokines, such as IL-6 or IL-17, released by immune cells (Majumder et al., 2019; Wang et al., 2009). Cancer-associated fibroblasts (CAF) activated by inflammatory signals may be either pro- or anti-tumorigenic (Koliaraki et al., 2017) and depending on their proximity to cancer cells as well as their response to IL-1R engagement or TGFβ activation they may differentiate into inflammatory CAFs (iCAFs) or myofibroblasts (myCAFs) (Biffi et al., 2019; Ohlund et al., 2017). CAFs in return can provide differentiation, growth factor, survival and metabolic cues to cancer cells and maintain cancer stem cell niche, thereby promoting growth, metastasis and resistance to therapy (Calon et al., 2012; Del Pozo Martin et al., 2015; Erez et al., 2010; Marusyk et al., 2016; Shi et al., 2019). CAFs also can regulate myeloid and T cell infiltration and produce factors which regulate immune cell differentiation, plasticity and drive immunosuppressive phenotypes (Calon et al., 2015; Jiang et al., 2017; Ruhland et al., 2016). On the other hand, under different circumstances, CAFs or inflammatory signaling in CAFs may also be tumor suppressive (Ozdemir et al., 2015; Pallangyo et al., 2015). Myeloid cells also present with high degree of plasticity regulated by tumor microenvironment (Cassetta and Pollard, 2018; Yang et al., 2018; Zilionis et al., 2019). Tumor cell derived factors, such as TGFβ, other cytokines, metabolic cues or products of dead cells polarize local macrophages or incoming monocytes toward more “tissue repair” type, characterized by enhanced expression of tissue protective factors, growth factors and decreased expression of genes involved in antigen presentation and induction of antigen-dependent immune responses (Andon et al., 2017; Cassetta et al., 2019; Sica et al., 2008). Such macrophages were previously referred to as “M2 type of macrophages”, however, by now it has become evident that in the complex context of TME such clear distinction does not exist and that in vivo macrophages can express genes of both “former M1” and “former M2” types. The instructing signals for macrophage polarization also can come from infiltrating bacteria in bacteria-rich cancers (Kostic et al., 2013) or from the cells of adaptive immunity such as B cells (Affara et al., 2014; Andreu et al., 2010), Th2 cells (DeNardo et al., 2009) or T regulatory cells (Azizi et al., 2018; Bos et al., 2013). On the other hand, Th1 and CD8+ T cells, particularly via IFNγ-dependent mechanisms can drive polarization of TAMs towards the “tumor-ignorant” functional phenotype which would be devoid of secretion of growth factors and characterized by increased phagytosis, antigen presentation and cross-presentation, stimulating anti-tumor immune responses (Bos et al., 2013; Hanahan and Coussens, 2012). Importantly, different tumor entities seem to educate their individual macrophages causing cancer-specific TAM profiles (Cassetta et al., 2019). Similarly to macrophages and monocytes, the TME also imprints a significant degree of plasticity on tumor-associated neutrophils via cytokine signaling or regulation of metabolism to further shape them to be anti-tumorigenic and antigen-presenting (Eruslanov et al., 2014), directly tumor-promoting (Nywening et al., 2018; Patel et al., 2018) or immunosuppressive (Gabrilovich, 2017; Veglia et al., 2019).
Figure 4: Increased cell plasticity within the tumor microenvironment (TME).
An intricate reciprocal interplay between all cells (cancer, stromal and immune) in the TME shapes polarization of immune cells activation states ( for myeloid cells and lymphocyes) as well as of cancer-associated fibroblasts and states of differentiation of epithelial cells. These different cells types instruct reversible phenotypic and functional perturbations in neighboring cells types, executing precise multicellular responses to the exact needs of tissue (transformed tissue) by tailoring plastic changes in existing and available cell types, rather than demanding the existence of multiple new “rigid” cellular types “one new distinct cell types for each new condition”. These plastic cellular changes may have distinct, sometimes opposing, roles in the TME with regard to their net effect on tumor growth, progression or metastasis.
T cells and B cells represent the adaptive immunity arm in cancer and also possess a tremendous predisposition to plasticity within the TME. B cells recruited into tumors regulate anti-cancer immune responses, exert direct tumor promoting roles or modulate myeloid cell response, all of that dictated by the signals B cells received from TME and by the nature of ligands their BCR’s recognize (Ammirante et al., 2014; Das and Bar-Sagi, 2019; Medler et al., 2018; Pylayeva-Gupta et al., 2016). γδ T cells are important components of tumor immunesurveillance, but in the TME they can acquire tumor-promoting functions associated with cytokine expression or act as negative regulators for T cell activation in pancreatic cancer (Daley et al., 2016). αβ T cells also possess a great degree of plasticity within the TME, which is being impinged in situ and in the secondary lymphoid organs where priming of these cells occurs. Signaling by cancer cells through PD-L1-PD-1 pathway, inducible by inflammatory cytokine IFNγ can render CD8+ and CD4+ T cells exhausted (Chihara et al., 2018) and similar processes can be enforced by regulatory T cells and by metabolic products of TME, such as hypoxia, glutamine or potassium ions (Clever et al., 2016; Eil et al., 2016; Johnson et al., 2018). Microbial and inflammatory cytokine signaling can drive normally immunosuppressive and anti-inflammatory FoxP3+ Tregs to co-express transcription factor RORγt, creating a pro-inflammatory/regulatory plastic T cell lineage capable of producing tumor-promoting cytokines IL-17 and IL-22 while being generally suppressive for anti-cancer immunity (Blatner et al., 2012).
Concluding Remarks
Studies on the mechanisms of pro-tumorigenic inflammatory pathways in cancer have revealed that the pathways evolved to mediate immunity to infection and promote tissue homeostasis, are usurped by tumors towards their benefit. Induction of inflammation in the TME follows distinct timing and can happen prior to or after initiation of tumorigenesis or may become evident only at the later stages of tumorigenesis. Because of that timing, in some models of cancer, types of tumors or individual tumors, the contribution of tumor-promoting inflammation may emerge very early or remain silent until late stages of metastasis or therapy resistance. Importantly, there are several distinct stimuli that induce inflammation in tumors. Some of them, such as carcinogenic microbes, environmental pollutants (particles, smoke) and low-grade inflammation associated with obesity, as well epithelial barrier deterioration associated commensal microorganisms may serve as important targets amenable for cancer prevention via reduction of tumor-initiating inflammation by removing or neutralizing the original stimulus. This can be achieved via vaccinations, dietary interventions, understanding of antibiotics usage and better environmental protection. Other stimuli, for example associated with hypoxia, cell death or genetic and/or epigenetic modulation of tumor suppressors and oncogenes represent events that are extremely valuable to understand the biology of cancer, but probably can be targeted only in the context of cancer therapy and by modulation the signaling events and signal transduction hubs downstream of the initial stimuli. As inflammation can play instrumental roles during all stages of tumor development, future research will undoubtedly uncover molecular and cellular mechanisms and modes of operation for inflammation and immune cells especially in early tumor initiation as well as metastatic spread and metastatic outgrowth – field(s) hampered by the paucity of the faithful in vivo models, a need to work at the single cell level only on a few cells within the normal tissue (primary or metastatic sites) and timeframes for the studies of autochthonous metastasis. In many instances, animal models correctly represent TME observed in human patients (Binnewies et al., 2018; Zilionis et al., 2019), however additional challenges in the field are required to fully translate findings in animal models to human cancers, which arise and progress in individuals with great genetic variability, history of environmental exposures, dietary habits and commensal and pathogenic microbe and virus composition. The elucidation of transcriptional and other signaling programs governing cellular plasticity within the TME will further aid the studies on the complex cross-talk, interaction and functional diversification of multiple cellular types. Ultimately, this will allow creation of new fate mapping and lineage tracing reporters and identification of targets common for many types of cancer or alternatively, rapidly amenable for precision therapies based on robust molecular and genetic analyses of individual patients and their primary and metastatic tumors. As the array of modern cancer therapies continuously expands, including various therapies based directly on immune cells (checkpoint immunotherapies, vaccines, CAR T cells), it will be imperative to uncover the role of immune and inflammatory pathways in therapy resistance.
As we underlined several basic principles and mechanisms of how inflammation promotes cancer and drew parallels between the “normal” endogenous role of inflammation in immunity and tissues homeostasis, we expect that a vast body of knowledge and complexities being constantly discovered in the field can be gradually distilled into several cornerstone rules of engagement governing molecular and cellular mechanisms of tumor-promoting inflammation.
Acknowledgements
We apologize to colleagues whose important work could not be cited due to the citation limits. Supported by NIH R01CA227629 and CA218133, Pew Scholar in Biomedical Sciences, AACR-Landon Innovator and US-Israel BSF awards to S.G, NIH P30 CA-006927 to Fox Chase Cancer Center. F.R.G. is supported by institutional funds from the Georg-Speyer-Haus, by the LOEWE Center Frankfurt Cancer Institute (FCI) funded by the Hessen State Ministry for Higher Education, Research and the Arts [III L 5 - 519/03/03.001 - (0015)], and Deutsche Forschungsgemeinschaft (FOR2438: Gr1916/11-1; SFB 815, 1177 and 1292). The Institute for Tumor Biology and Experimental Therapy, Georg-Speyer-Haus is funded jointly by the German Federal Ministry of Health and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
REFERENCES
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. (2014). B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 25, 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkari L, Gocheva V, Kester JC, Hunter KE, Quick ML, Sevenich L, Wang HW, Peters C, Tang LH, Klimstra DS, et al. (2014). Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev 28, 2134–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Kuttner V, et al. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, and Cukierman E (2016). Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Current opinion in cell biology 42, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Shalapour S, Kang Y, Jamieson CA, and Karin M (2014). Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A 111, 14776–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andon FT, Digifico E, Maeda A, Erreni M, Mantovani A, Alonso MJ, and Allavena P (2017). Targeting tumor associated macrophages: The new challenge for nanomedicine. Semin Immunol 34, 103–113. [DOI] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. (2010). FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriani GA, Almeida VP, Faggioli F, Mauro M, Tsai WL, Santambrogio L, Maslov A, Gadina M, Campisi J, Vijg J, and Montagna C (2016). Whole Chromosome Instability induces senescence and promotes SASP. Scientific reports 6, 35218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran D, Lasry A, Zinger A, Biton M, Pikarsky E, Hellman A, Butte AJ, and Ben-Neriah Y (2016). Widespread parainflammation in human cancer. Genome Biol 17, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, et al. (2018). Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 174, 1293–1308 e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, and Clevers H (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. (2004). TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501. [DOI] [PubMed] [Google Scholar]
- Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, and Tuveson DA (2019). IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer discovery 9, 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. (2013). Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795. [DOI] [PubMed] [Google Scholar]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, et al. (2012). Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 4, 164ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PD, Plitas G, Rudra D, Lee SY, and Rudensky AY (2013). Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med 210, 2435–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, et al. (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, et al. (2012). Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, Sevillano M, Palomo-Ponce S, Tauriello DV, Byrom D, et al. (2015). Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet 47, 320–329. [DOI] [PubMed] [Google Scholar]
- Canli O, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, Neumann T, Horst D, Lower M, Sahin U, and Greten FR (2017). Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 32, 869–883 e865. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P, Lin EY, et al. (2019). Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 35, 588–602 e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L, and Pollard JW (2018). Targeting macrophages: therapeutic approaches in cancer. Nature reviews. Drug discovery [DOI] [PubMed]
- Chang WC, Coudry RA, Clapper ML, Zhang X, Williams KL, Spittle CS, Li T, and Cooper HS (2007). Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis 28, 2375–2381. [DOI] [PubMed] [Google Scholar]
- Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, Nyman J, Marjanovic ND, Kowalczyk MS, Wang C, et al. (2018). Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 558, 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampricotti M, Hau CS, Doornebal CW, Jonkers J, and de Visser KE (2012). Chemotherapy response of spontaneous mammary tumors is independent of the adaptive immune system. Nat Med 18, 344–346; author reply 346. [DOI] [PubMed] [Google Scholar]
- Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, et al. (2016). Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell 166, 1117–1131 e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels L, Jonkers J, and de Visser KE (2015). IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK, et al. (2016). gammadelta T Cells Support Pancreatic Oncogenesis by Restraining alphabeta T Cell Activation. Cell 166, 1485–1499 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, et al. (2012). Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, and Bar-Sagi D (2019). BTK signaling drives CD1d(hi)CD5(+) regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene 38, 3316–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Coppe JP, Campisi J, and Desprez PY (2010). Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, et al. (2017). A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 543, 676–680. [DOI] [PubMed] [Google Scholar]
- DeGregori J (2017). Connecting Cancer to Its Causes Requires Incorporation of Effects on Tissue Microenvironments. Cancer Res 77, 6065–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, et al. (2018). Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo Martin Y, Park D, Ramachandran A, Ombrato L, Calvo F, Chakravarty P, Spencer-Dene B, Derzsi S, Hill CS, Sahai E, and Malanchi I (2015). Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell reports 13, 2456–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, and Coussens LM (2009). CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva-Posocco O, Dzutsev A, Posocco DF, Hou V, Yuan W, Thovarai V, Mufazalov IA, Gunzer M, Shilovskiy IP, Khaitov MR, et al. (2019). Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity 50, 166–180 e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, David JM, and Palena C (2017). Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol 47, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, and Schreiber RD (2004). The three Es of cancer immunoediting. Annu Rev Immunol 22, 329–360. [DOI] [PubMed] [Google Scholar]
- Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, Palmer DC, Gros A, Yamamoto TN, Patel SJ, et al. (2016). Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 537, 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada E, Pribluda A, Goldstein RE, Morgenstern Y, Brachya G, Cojocaru G, Snir-Alkalay I, Burstain I, Haffner-Krausz R, Jung S, et al. (2011). CKIalpha ablation highlights a critical role for p53 in invasiveness control. Nature 470, 409–413. [DOI] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Arron ST, and Hanahan D (2010). Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 17, 135–147. [DOI] [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. (2014). Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 124, 5466–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francart ME, Lambert J, Vanwynsberghe AM, Thompson EW, Bourcy M, Polette M, and Gilles C (2018). Epithelial-mesenchymal plasticity and circulating tumor cells: Travel companions to metastases. Developmental dynamics : an official publication of the American Association of Anatomists 247, 432–450. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI (2017). Myeloid-Derived Suppressor Cells. Cancer immunology research 5, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25, 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenbee C, West J, Baker AM, Guljar N, Jones L, Graham TA, Robertson-Tessi M, and Anderson AR (2019). Macrophage-mediated immunoediting drives ductal carcinoma evolution: Space is the game changer. bioRXiv, 594598.
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. (2009). Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature medicine 15, 1170–1178. [DOI] [PubMed] [Google Scholar]
- Greathouse KL, White JR, Vargas AJ, Bliskovsky VV, Beck JA, von Muhlinen N, Polley EC, Bowman ED, Khan MA, Robles AI, et al. (2018). Interaction between the microbiome and TP53 in human lung cancer. Genome Biol 19, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, and Karin M (2004). IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296. [DOI] [PubMed] [Google Scholar]
- Greten FR, and Karin M (2005). NF-kB: Linking Inflammation and Immunity to Cancer Development and Progression. Nature Reviews Immunology 5, 749–759. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, and Karin M (2009). IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI (2013). Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 35, 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, and Karin M (2010). Immunity, inflammation, and cancer. Cell 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. (2012). Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke K, Hernandez PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, et al. (2019). Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Pastushenko I, Skibinski A, Blanpain C, and Kuperwasser C (2019). Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 24, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, Thurston G, Zhang Y, Lazarus J, Sajjakulnukit P, et al. (2019). Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer. Cell Metab 29, 1390–1399 e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, and Coussens LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322. [DOI] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Casas-Selves M, Kim J, Zaberezhnyy V, Aghili L, Daniel AE, Jimenez L, Azam T, McNamee EN, Clambey ET, et al. (2015). Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest 125, 4666–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Greten TF, and Xia Q (2017). Immunosuppressive cell death in cancer. Nat Rev Immunol 17, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr., Murphy AJ, et al. (2012). IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Hofseth LJ, and Harris CC (2003). Radical causes of cancer. Nat Rev Cancer 3, 276–285. [DOI] [PubMed] [Google Scholar]
- Iannello A, and Raulet DH (2014). Immunosurveillance of senescent cancer cells by natural killer cells. Oncoimmunology 3, e27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, Leeson R, Kanodia A, Mei S, Lin JR, et al. (2018). A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 175, 984–997 e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Hegde S, and DeNardo DG (2017). Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother 66, 1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, et al. (2019). Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell 176, 998–1013 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, and Tahara H (2011). Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proceedings of the National Academy of Sciences of the United States of America 108, 12425–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, Maseda D, Liberti MV, Paz K, Kishton RJ, et al. (2018). Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell 175, 1780–1795 e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadariya Y, Menges CW, Talarchek J, Cai KQ, Klein-Szanto AJ, Pietrofesa RA, Christofidou-Solomidou M, Cheung M, Mossman BT, Shukla A, and Testa JR (2016). Inflammation-Related IL1beta/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev Res (Phila) 9, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten K, Coffelt SB, Hoogstraat M, Verstegen NJM, Vrijland K, Ciampricotti M, Doornebal CW, Hau CS, Wellenstein MD, Salvagno C, et al. (2017). Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1beta in tumor-associated macrophages. Oncoimmunology 6, e1334744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambu B, Huda N, Chen X, Antoine DJ, Li Y, Dai G, Kohler UA, Zong WX, Waguri S, Werner S, et al. (2018). HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. J Clin Invest 128, 2419–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, and Schreiber RD (2007). Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907. [DOI] [PubMed] [Google Scholar]
- Koliaraki V, Pallangyo CK, Greten FR, and Kollias G (2017). Mesenchymal Cells in Colon Cancer. Gastroenterology 152, 964–979. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin OW, Kuprash DV, et al. (2005). p53 is a suppressor of inflammatory response in mice. FASEB J 19, 1030–1032. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, Littlewood TD, and Evan GI (2017). Cell 171, 1301–1315 e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. (2013). Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell host & microbe 14, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh HL, Dougan SK, and Weinberg RA (2018). The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S, et al. (2014). IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 40, 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishy A, Karin M, and Grivennikov SI (2011). Tumor promotion via injury- and death-induced inflammation. Immunity 35, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AR, Bert NL, Ho SS, Shen YJ, Tang LF, Xiong GM, Croxford JL, Koo CX, Ishii KJ, Akira S, et al. (2014). RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res 74, 2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, et al. (2019). KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell 35, 559–572 e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadiere B, et al. (2018). Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med 215, 2536–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Maeda S, Hsu LC, Yagita H, and Karin M (2004). Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 6, 297–305. [DOI] [PubMed] [Google Scholar]
- Majumder S, Amatya N, Revu S, Jawale CV, Wu D, Rittenhouse N, Menk A, Kupul S, Du F, Raphael I, et al. (2019). IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat Immunol 20, 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, and Huelsken J (2011). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89. [DOI] [PubMed] [Google Scholar]
- Malesci A, Bianchi P, Celesti G, Basso G, Marchesi F, Grizzi F, Di Caro G, Cavalleri T, Rimassa L, Palmqvist R, et al. (2017). Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology 6, e1342918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, and Balkwill F (2008). Cancer-related inflammation. Nature 454, 436–444. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Ponzetta A, Inforzato A, and Jaillon S (2019). Innate immunity, inflammation and tumour progression: double-edged swords. J Intern Med [DOI] [PMC free article] [PubMed]
- Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, and Polyak K (2014). Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature 514, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Tabassum DP, Janiszewska M, Place AE, Trinh A, Rozhok AI, Pyne S, Guerriero JL, Shu S, Ekram M, et al. (2016). Spatial Proximity to Fibroblasts Impacts Molecular Features and Therapeutic Sensitivity of Breast Cancer Cells Influencing Clinical Outcomes. Cancer Res 76, 6495–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, et al. (2014). Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25, 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, and Swanton C (2017). Cancer Evolution Constrained by the Immune Microenvironment. Cell 170, 825–827. [DOI] [PubMed] [Google Scholar]
- Medler TR, Murugan D, Horton W, Kumar S, Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin M, Margolin AA, et al. (2018). Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy. Cancer Cell 34, 561–578 e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R (2008). Origin and physiological roles of inflammation. Nature 454, 428–435. [DOI] [PubMed] [Google Scholar]
- Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. (2008). DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 118, 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, Pierre JF, Ernest JD, Galipeau HJ, Thuille N, et al. (2018). Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 557, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. (2016). Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 44, 698–711. [DOI] [PubMed] [Google Scholar]
- Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, et al. (2018). Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67, 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, et al. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214, 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, and Medzhitov R (2016). Tissue biology perspective on macrophages. Nat Immunol 17, 9–17. [DOI] [PubMed] [Google Scholar]
- Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. (2015). Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 28, 831–833. [DOI] [PubMed] [Google Scholar]
- Pallangyo CK, Ziegler PK, and Greten FR (2015). IKKbeta acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J Exp Med 212, 2253–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D, Gartung A, Yang J, Yang H, Gilligan MM, Sulciner ML, Bhasin SS, Bielenberg DR, Chang J, Schmidt BA, et al. (2019). Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. J Clin Invest 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, et al. (2018). Identification of the tumour transition states occurring during EMT. Nature 556, 463–468. [DOI] [PubMed] [Google Scholar]
- Patel S, Fu S, Mastio J, Dominguez GA, Purohit A, Kossenkov A, Lin C, Alicea-Torres K, Sehgal M, Nefedova Y, et al. (2018). Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol 19, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, and Mukaida N (2008). Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, Burstain I, Morgenstern Y, Brachya G, Billauer H, et al. (2013). A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 24, 242–256. [DOI] [PubMed] [Google Scholar]
- Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, et al. (2017). Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 171, 1611–1624 e1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, Nguyen PM, Preaudet A, Farid R, Edwards KM, et al. (2013). Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24, 257–271. [DOI] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, and Bar-Sagi D (2016). IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer discovery 6, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, and Dannenberg AJ (2019). The obese adipose tissue microenvironment in cancer development and progression. Nature reviews. Endocrinology 15, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, Chen IC, Wendel N, Ben-Chetrit N, Walker J, et al. (2017). Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol 19, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, et al. (2017). Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171, 934–949 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, and Group CT (2017). Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842. [DOI] [PubMed] [Google Scholar]
- Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C, Lauwers GY, Selaru FM, Popoli M, Pittman ME, et al. (2016). Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 150, 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, Lund T, Tanic M, Reading JL, Joshi K, et al. (2019). Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, and Meade TW (2011). Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, and Mehta Z (2012). Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379, 1591–1601. [DOI] [PubMed] [Google Scholar]
- Rozhok A, and DeGregori J (2019). A generalized theory of age-dependent carcinogenesis. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, and Han YW (2013). Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/beta-Catenin Signaling via its FadA Adhesin. Cell host & microbe 14, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland MK, Loza AJ, Capietto AH, Luo X, Knolhoff BL, Flanagan KC, Belt BA, Alspach E, Leahy K, Luo J, et al. (2016). Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun 7, 11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, and Karin M (2008). Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 14, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Danes A, Larsimont JC, Liagre M, Munoz-Couselo E, Lapouge G, Brisebarre A, Dubois C, Suppa M, Sukumaran V, Del Marmol V, et al. (2018). A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 562, 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, and Smyth MJ (2011). Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570. [DOI] [PubMed] [Google Scholar]
- Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, et al. (2014). High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514, 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. (2013a). Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38. [DOI] [PubMed] [Google Scholar]
- Schwitalla S, Ziegler PK, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R, Slotta-Huspenina J, et al. (2013b). Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell 23, 93–106. [DOI] [PubMed] [Google Scholar]
- Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. (2016). The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, et al. (2014). Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol 16, 876–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, et al. (2017). Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, and Allison JP (2015). The future of immune checkpoint therapy. Science 348, 56–61. [DOI] [PubMed] [Google Scholar]
- Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, et al. (2019). Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin N, Raulet DH, and Ardolino M (2014). NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol 26, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, and Mantovani A (2008). Macrophage polarization in tumour progression. Semin Cancer Biol 18, 349–355. [DOI] [PubMed] [Google Scholar]
- Spranger S, and Gajewski TF (2015). A new paradigm for tumor immune escape: beta-catenin-driven immune exclusion. Journal for immunotherapy of cancer 3, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsa S, Paul MC, Cardone C, Holcmann M, Amberg N, Pathria P, Diamanti MA, Linder M, Timelthaler G, Dienes HP, et al. (2017). EGFR in Tumor-Associated Myeloid Cells Promotes Development of Colorectal Cancer in Mice and Associates With Outcomes of Patients. Gastroenterology 153, 178–190 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Carmona M, Lesage J, Cataldo D, and Gilles C (2017). EMT and inflammation: inseparable actors of cancer progression. Mol Oncol 11, 805–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweis RF, Spranger S, Bao R, Paner GP, Stadler WM, Steinberg G, and Gajewski TF (2016). Molecular Drivers of the Non-T-cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer immunology research 4, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. (2019). Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide DH, and Karin M (2010). Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 17, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MW, Swann JB, Koebel CM, Schreiber RD, and Smyth MJ (2008). Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol 84, 988–993. [DOI] [PubMed] [Google Scholar]
- Tomkovich S, Dejea CM, Winglee K, Drewes JL, Chung L, Housseau F, Pope JL, Gauthier J, Sun X, Muhlbauer M, et al. (2019). Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J Clin Invest 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, and Pardoll DM (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, and Galon J (2011). Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer research 71, 1263–1271. [DOI] [PubMed] [Google Scholar]
- Trinchieri G (2012). Cancer and Inflammation: An Old Intuition with Rapidly Evolving New Concepts. Annual Reviews of Immunology 30. [DOI] [PubMed] [Google Scholar]
- Ullman TA, and Itzkowitz SH (2011). Intestinal inflammation and cancer. Gastroenterology 140, 1807–1816. [DOI] [PubMed] [Google Scholar]
- Varga J, and Greten FR (2017). Cell plasticity in epithelial homeostasis and tumorigenesis. Nat Cell Biol 19, 1133–1141. [DOI] [PubMed] [Google Scholar]
- Veglia F, Perego M, and Gabrilovich D (2018). Myeloid-derived suppressor cells coming of age. Nat Immunol 19, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et al. (2019). Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 569, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, and Smyth MJ (2011). Natural innate and adaptive immunity to cancer. Annual review of immunology 29, 235–271. [DOI] [PubMed] [Google Scholar]
- Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, et al. (2014). Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity 41, 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, and Yu H (2009). IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 206, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinlich R, Oberst A, Beere HM, and Green DR (2017). Necroptosis in development, inflammation and disease. Nature reviews. Molecular cell biology 18, 127–136. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carra A, Brennan CA, Chun E, Ngo L, Samson LD, et al. (2019). The human gut bacterial genotoxin colibactin alkylates DNA. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, Simonavicius N, Schneider C, Lang M, Sturzl M, et al. (2012). Endothelial CCR2 Signaling Induced by Colon Carcinoma Cells Enables Extravasation via the JAK2-Stat5 and p38MAPK Pathway. Cancer Cell 22, 91–105. [DOI] [PubMed] [Google Scholar]
- Woo SR, Corrales L, and Gajewski TF (2015). Innate immune recognition of cancer. Annu Rev Immunol 33, 445–474. [DOI] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15, 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, and Moses HL (2008). Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, McKay D, Pollard JW, and Lewis CE (2018). Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res 78, 5492–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, and Jove R (2009). STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9, 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, et al. (2017). Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 170, 548–563 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Franklin RA, Adler M, Jacox JB, Bailis W, Shyer JA, Flavell RA, Mayo A, Alon U, and Medzhitov R (2018). Circuit Design Features of a Stable Two-Cell System. Cell 172, 744–757 e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, et al. (2017). Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity 47, 323–338 e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler PK, Bollrath J, Pallangyo CK, Matsutani T, Canli O, De Oliveira T, Diamanti MA, Muller N, Gamrekelashvili J, Putoczki T, et al. (2018). Mitophagy in Intestinal Epithelial Cells Triggers Adaptive Immunity during Tumorigenesis. Cell 174, 88–101 e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, Krishnan I, Maroni G, Meyerovitz CV, Kerwin CM, et al. (2019). Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 50, 1317–1334 e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]