Abstract
Introduction:
Laser photocoagulation has been a valuable tool in the ophthalmologist’s armamentarium for decades. Conventional laser photocoagulation relies on visible retinal burns as a treatment endpoint, which is thought to result in photocoagulative necrosis of retinal tissue. Recent studies have suggested that using subthreshold (ST) laser, which does not cause detectable damage to the retina may also have therapeutic effects in a variety of retinal diseases. Areas covered: We review the proposed biological mechanisms mediating the therapeutic effects of subthreshold laser on the retina, followed by the evidence for ST laser efficacy in retinal diseases such as diabetic macular edema, central serous chorioretinopathy, age-related macular degeneration, and retinal vein occlusion.
Expert Commentary:
Multiple clinical studies demonstrate that subthreshold laser does not cause structural damage to the retina based on multimodal imaging. Evidence suggests that there is a therapeutic effect on decreasing diabetic macular edema and subretinal fluid in chronic central serous retinopathy; however, the effect may be relatively modest and is not as efficacious as first line treatments for these diseases. Given the repeatability and lack of damage to the retina by this treatment, subthreshold laser deserves further study to determine its place in the retina specialist’s armamentarium.
Keywords: subthreshold laser, micropulse laser, nanosecond laser, duty cycle, heat shock proteins, diabetic macular edema, photocoagulation
1. INTRODUCTION
1.1. Background
Phototherapy of human retinal pathology dates back to the 1940s. German ophthalmologist Gerhard Meyer-Schwickerath observed retinal damage in patients following the solar eclipse of 1945. He parlayed his findings into pioneering phototherapy of retinal detachment by focusing sunlight and later a xenon arc light source onto the retina[1]. LASER (light amplification by stimulated emission of radiation) was first reported in 1960[2] and shortly thereafter investigated in experimental animal models[3]. Following years of refinement of the optimal wavelength to achieve retinal photocoagulation, the argon laser photocoagulator was released in 1970 for mainstream ophthalmology practice[4]. One early milestone of laser phototherapy included the first large-scale clinical trial demonstrating the efficacy of laser photocoagulation in the treatment of diabetic retinopathy (DR) and diabetic macular edema (DME)[5, 6]. Several explanations account for the therapeutic mechanism of action behind this treatment: 1) pan-retinal photocoagulation destroys metabolically active cells, reducing oxygen supply-demand mismatch and stimulus for VEGF production; 2) focal laser therapy that reduces mismatch between vascular permeability and fluid extravasation out of the retina; and 3) antiangiogenic cytokine production by surviving RPE at the margins of the photocoagulative lesion.
More recently, the use of subthreshold (ST) laser therapy, which employs shorter duration of laser pulses and does not cause visible damage to the retina, has been investigated as a potential therapeutic tool for retinal disease. For the purposes of consistency with other published literature, the term subthreshold (ST) laser therapy is used to indicate laser which does not cause visible or other signs of damage to the retina. An alternative descriptor for the technology would be nondamaging or subvisible retinal laser therapy. The late 1990s and early 2000s saw the first studies of ST laser therapy investigating whether brief laser pulses to the retinal pigmented epithelium (RPE) could achieve the desired therapeutic effects while sparing nearby tissue permanent damage[7, 8, 9]. In this article, we discuss the principles and proposed biological mechanisms mediating the therapeutic effects of retinal phototherapy, followed by a review of clinical studies investigating ST laser efficacy by clinical indication. We end with discussion of future directions to investigate this laser application to treat retinal diseases.
1.2. Principles of subthreshold laser therapy
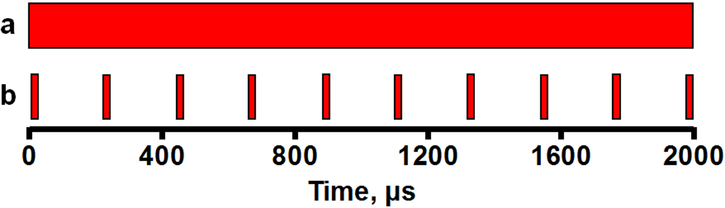
Despite the variety of applications and settings, lasers in general rely on the same principle: amplification of light by photons released by relaxation of excited electrons to their ground state. Continuous wave (CW) laser uses a steady uninterrupted light source to inflict thermal burns. The laser’s duty cycle refers to the proportion of time the laser is pulsed to total time powered on, usually reported as a percentage (Fig. 1). Pulsation describes the duration of individual episodes of laser pulses, eg 100 μs exposure time. Because the laser intensity and duty cycle may change depending on application, the laser power provides a standardized measure of the rate of energy emission. Laser wavelength can also be manipulated depending on the laser source: green (532nm), yellow (577nm), and infrared diode (810nm) are among the most commonly used. Individual wavelengths have separate absorption characteristics within the layers of the retina[4]. For applications near the fovea, 577nm light is safe due to minimal absorption by xanthophyll pigments in the inner and outer plexiform layers, while 810nm light penetrates more deeply into the choroid[10].
Figure 1.
Schematic of conventional wave versus micropulse laser duty cycle. a) Continuous wave laser uses a continuous beam of laser energy with a duty cycle of 100% (eg, laser beam present for 2000μs of a 2s period). b) Micropulse laser uses short intermittent bursts of laser energy, with individual pulses present for only a minority (eg, laser pulses for 200μs of a 2s period) of the laser on time, for duty cycle of 10%.
Adjustments to the duty cycle dictate the duration of the laser pulse and thus the effects on the tissue. If the duty cycle is lowered sufficiently (ie, a “micro” pulse of laser energy), the thermal diffusion time of tissue cooling decreases below the threshold of cell death[11]. Laser threshold refers to the laser’s tendency to cause tissue death, and current protocols generally rely on low duty cycles to achieve a ST effect. Micro[11]- and nano-pulse[12] laser settings refer to the duration of light pulse in seconds and are often ST in their effect. While the extent and intensity of ST laser treatment of retinal pathology has traditionally been left to the operating surgeon to adjust manually, often by using a supra-threshold test spot in the retinal periphery, another approach has been an automated calculation of determining the optimal ST intensity and pattern of laser application through software[13].
2. BIOLOGICAL MECHANISMS OF SUBTHRESHOLD LASER PHOTOCOAGULATION
Conventional wisdom held that clinical benefit was mediated by retinal burns via heat absorption in the RPE[3, 6], evidenced by visible scarring and damage to the photoreceptors. However, more recent evidence using laser regimens which do not cause photocoagulative damage has called into question whether lethal burns were necessary to achieve therapeutic effect[11]. In theory, shorter duration of laser pulse reduces thermal diffusion distance and thus the extent of damage to structures surrounding the RPE[11], versus the traditional continuous wave (CW) laser setting. In 1999, an early study of ST 810 nm laser found clinical efficacy for proliferative DR and macular edema (ME) despite no visible retinal scarring or damage[11], suggesting a distinct biological mechanism mediating therapy beside lethal damage, that the authors hypothesized could be due to stimulation of the RPE and a possible regression of neovascularization.
Heat shock proteins (Hsps) act as molecular chaperones against hyperthermia-induced toxic protein aggregation, produced in response to cellular injury and stress[14]; and are proposed to mediate much of the clinically desirable effect of subthreshold phototherapy. Sramek et. al found Hsp70 upregulation in mouse RPE following subthreshold laser treatment, with a temperature threshold of 49°C to achieve the effect[15]. Upregulation of Hsp70 protein was also observed in rabbit retina, which the authors argued might restore retinal fluid extravasation and decrease choroidal permeability[16]. Pre-exposure to low power laser initiated Hsp70 overexpression and protection against apoptosis associated with a subsequent high power laser insult[17]. Potential molecular protective mechanisms of Hsp family upregulation in the retina include protein chaperone activity, stabilization of the cytoskeleton, and prevention of apoptosis, especially through inhibition of cytochrome c release[18] or through a resetting phenomenon[19, 20].
Another theory postulated leukocyte recruitment activation or extracellular matrix remodeling in the choroid and retina mediated the clinical benefits of ST phototherapy. Recruitment of mouse mononuclear cells was observed in the retina and choroid, accompanied by upregulation of inflammatory cytokine and heat-shock protein genes[21]. ST phototherapy also increased trophic factor (CNTF and FGF-2) labeling in Müller cells and astrocytes, activation of microglial IL-1β release, and upregulation of Tnfα and Nos2 gene expression[22]. When stimulated with Nd:YLF laser, levels of the extracellular matrix remodeling enzyme MMP-2 increased in porcine RPE-choroidal explants, with no changes observed in ST laser trials[23]. In another study, Apoe−/− mice, which at baseline have thickened Bruch’s membrane[24], mmp2 and mmp3 genes were upregulated in response to nanosecond laser treatment[25]. Human RPE explants treated with photocoagulation showed upregulation of MMP-2 and MMP-3 secretion[26], which the authors postulated could mediate clinical benefit by degrading ECM, thus relieving inner retina hypoxia and associated pro-angiogenic signaling[26, 27]. To this end, Li et. al found ST treatment significantly downregulated pro-angiogenic and upregulated anti-angiogenic factors after ST treatment in mouse RPE in vitro [28]. Other potential retinal glial cell-derived cytokine and extracellular matrix-mediated responses to laser treatment may include changes in thrombospondin-1, pigmented epithelium derived factor (PEDF), and stromal cell derived factor-1[29, 30, 31]. Despite evidence for Hsp protective effects, inflammatory activation, and extracellular matrix remodeling, few studies have investigated potential mechanisms in vivo, and further work will elucidate their precise contributions to the therapeutic effects of ST phototherapy.
3. CLINICAL EFFICACY BY DISEASE INDICATION
3.1. Diabetic Macular Edema (DME) (Table 1)
Table 1.
Randomized clinical trials of subthreshold laser in diabetic macular edema.
| Citation | Study type | Groups | Outcome measures |
Results/conclusions |
|---|---|---|---|---|
| Laursen et. al, 2004, PMID 15317711 | Prospective, randomized | • CW 514nm: n=11 eyes • ST 810nm: n=12 eyes |
ME, VA | • No difference in outcomes |
| Figueira et. al, 2009, PMID 19054831 | Prospective, double-masked, randomized | • CW 514nm grid: n=40 eyes • ST 810nm focal: n=44 eyes |
VA, CMT, contrast sensitivity, scarring | • VA, CMT, sensitivity: No difference • Scarring: increased risk in CW |
| Vujosevic et. al 2010, PMID 20168272 | Prospective, masked, randomized | • CW 514nm: n=30 eyes • ST 810nm: n=32 eyes |
VA, FAF, CRT, microperimetry | • VA, CRT: No difference • 4°, 12° sensitivity: increased in ST • FAF: absent in ST only |
| Lavinsky et. al 2011, PMID 21345996 | Prospective, double masked, randomized | • CW 532nm: n=42 eyes • ND-ST 810nm: n=39 eyes • HD-ST 810nm: n=42 eyes |
VA, CMT | • CMT: HD-ST=CW; ND-ST inferior to both HD-ST and CW • VA: all groups statistically different (HD-ST>CW>ND-ST) |
| Venkatesh et. al 2011, PMID 21612513 | Prospective, randomized | • CW 532nm: n=23 eyes • ST 810nm: n=23 eyes |
CMT, MfERG/retinal sensitivity, VA, contrast sensitivity | • CMT, VA, contrast sensitivity: no difference between groups • MfERG: focal regions of signal void in 18/23 CW vs. 4/23 ST eyes |
| Xie et. al 2013 | Prospective, randomized | • CW 514nm: n=49 eyes • ST 810nm: n=50 eyes |
VA, CMT | • VA, CMT: no difference between groups |
| Chen et. al 2016, PMID 27096529 | Meta-analysis of randomized controlled trials comparing ST vs. CW laser in DME | • CW (various): n=195 eyes • ST 810nm: n=203 eyes |
VA, CMT | • CMT: no difference between groups • VA: ST significantly better than CW at 3, 9, 12 mos f/u |
| Wu et. al 2018 PMID 29091878 | Meta-analysis of randomized controlled trials comparing ND-ST vs. CW vs. CW+anti-VEGF | • ND-ST • CW • CW+anti-VEGF |
VA, CMT | • Ranibizumab+CW>ND-ST • CW=ND-ST • Bevacizumab+CW=ND-ST |
Abbreviations – ST, subthreshold; pts,patients; VA,visual acuity; (D)ME, (diabetic) macular edema; PDR, proliferative diabetic retinopathy; CW, continuous wave; f/u, follow-up; CMT, central macular thickness; FAF, fundus autofluorescence; CRT, central retinal thickness; ND-ST, normal density subthreshold; HD-ST, high density subthreshold; MfERG, multifocal electroretinography; VEGF, vascular endothelial growth factor
The earliest reports of application of ST laser in retinal pathology date to the late 1990s. In 1997, Friberg and Karatza investigated micropulsed 810nm diode laser in diabetic macular edema (DME). At 3 months out, 75% of first-time treated patients had clinical improvement in edema, with similar findings in ST-treated patients who had previously received CW argon photocoagulation[32]; the majority of treated patients had no changes in visual acuity[32]. In their 1999 nonrandomized study, Akduman and Olk reported reduction of diffuse DME of 74% of 50 treated eyes at 15-24 months following 1-5 treatments with ST diode 810nm laser, albeit with delayed resolution of edema[33]. Despite promising results, early clinical studies were nonrandomized, often retrospective, lacking stringent inclusion/exclusion criteria, open-label studies that lacked a control group directly comparing subthreshold laser therapy with conventional photocoagulation[8, 9, 11, 34, 35, 36].
Randomized controlled clinical trials have found at worst non-inferiority and at best superior results when comparing CW and ST treatments of DME. Three separate randomized trials comparing ST 810nm laser versus CW argon 514nm focal laser treatment found no difference in visual acuity, and either no difference in OCT thickness or perhaps a difference in ME varying the ST groups at 12 month follow-up[36, 37, 38]. In 2011 Lavinsky and colleagues conducted a randomized, double-masked, controlled trial comparing standard 532nm CW photocoagulation with standard (SD) and high-density (HD) ST micropulse 810nm laser treatment for 123 DME patients[37]. They found superior reduction of CMT (central macular thickness) in HD-ST and CW laser groups versus SD-ST and superior VA improvement in the high-density ST group[37]. An open-label nonproliferative DR study found comparable outcomes in macular thickness and VA in ST and CW treated eyes, although on multifocal ERG (MfERG) 18/23 eyes treated with CW laser versus 4/23 eyes treated with ST laser evidenced areas of signal void[38]. In 2013, a randomized clinical trial found comparable VA and edema regression outcomes in 99 eyes from 84 patients with DME randomized to receive either CW 514 nm argon or ST 810nm laser[39]. Importantly, pre-treatment macular thickness/edema influenced efficacy, as patients with moderate ME had better outcomes from ST laser versus patients with severe ME, who had no improvement in edema or VA at 6-12 months post ST laser and required rescue bevacizumab treatments[40].
More recent studies have expanded the application of ST laser treatment. When studying yellow 577nm laser versus standard diode 810nm laser comparable outcomes were observed[41, 42, 43]. One of the most recent advances in 577 nm laser therapy of DME, the automated PASCAL system, was found to be safe in an uncontrolled prospective trial of 10 eyes[13]. Indeed, the PASCAL endpoint management system affords the advantage of automatic determination of subthreshold laser energy, thus abrogating the need for titrating laser power during treatment[13].
As anti-VEGF injections have replaced focal CW photocoagulation as treatment for DME, studies have investigated how ST laser compared to anti-VEGF medications, either as monotherapy or in combination to reduce treatment burden. In a retrospective study of 38 eyes with DME treated with subthreshold 577nm laser or ranibizumab, both groups had comparable improvements in visual acuity[44]. However, ST laser was found to reduce the number of anti-VEGF ranibizumab injections needed through 12 months of follow-up[44]. A meta-analysis of 18 studies investigating anti-VEGF, CW laser, and ST laser for DME was conducted[45]. When compared by meta-analysis with change in visual acuity (logarithm of minimal angle of resolution units, logMAR), ranibizumab plus CW laser was more effective than ST laser alone, and no difference in outcome was observed between ST or CW laser alone[45]. However, ST laser alone and CW laser plus bevacizumab were no different[45].
A meta-analysis of 6 randomized controlled trials compared ST and CW laser treatment of previously untreated DME in 215 eyes with logMAR and central macular thickness as endpoints[46]. Although VA was stable in both groups, weighted mean difference in logMAR was significantly improved in the ST-treated eyes at 3, 9, and 12 but not 6 months follow-up[46]. In tracking central macular thickness, improvements were observed in both groups, with no significant differences between groups at any timepoint[46]. In summary, review of the individual reports and meta-analyses appears to point toward non-inferiority of ST efficacy in DR/DME versus CW laser, with a potentially more favorable side effect profile. However, variations in laser power and lesion density in ST laser-treated eyes may account for differential outcomes when compared with the more standardized mETDRS protocol for conventional therapy. Whether ST laser could serve as an adjunctive treatment to decrease the number of anti-VEGF injections needed to treat DME remains an open question.
3.2. Central Serous Retinopathy/Chorioretinopathy (CSCR) (Table 2)
Table 2.
Randomized clinical trials of subthreshold laser in central serous chorioretinopathy.
| Citation | Study type | Groups | Outcome measures | Results/conclusions |
|---|---|---|---|---|
| Behnia et. al 2013, PMID 23314231 | Prospective, randomized | • Observation: n=19 pts • ST 532nm: n=18 pts |
VA, contrast sensitivity | • VA: significantly better improvement in ST • Contrast sensitivity: significant difference in 3, 12 CPD in ST |
| Roisman et. al 2013, PMID 24044709 | Prospective, randomized, double blinded | • Sham: n=5 pts • ST: n=10 pts |
VA, CMT, leakage | • VA: significantly improved in ST • CMT, leakage: no difference |
| Russo et. al 2017, PMID 28283732 | Prospective, randomized | • PDT half dose: n=20 eyes • ST 689nm: n=20 eyes |
VA, CRT, CT | • No differences in outcomes |
| van Dijk et. al 2018, PMID 29776672 | Prospective, randomized | • PDT half fluence: n=89 eyes • HD-ST: n=90 eyes |
SRF, VA, retinal sensitivity, NEI-VFQ25 | • SRF, retinal sensitivity: PDT significantly better at early and late f/u • VA: PDT significantly better at early f/u only • NEI-VFQ25: no difference |
Abbreviations – ST, subthreshold; pts,patients; VA,visual acuity; CPD, cycles per degree; obs, observation; CMT, central macular thickness; PDT, photodynamic therapy; HD-ST, high-density subthreshold; SRF, subretinal fluid; f/u, follow-up
Central serous chorioretinopathy (CSCR) is characterized by appearance of subretinal fluid, which can be precipitated by steroid use. While the mechanism of this disease is unknown, some studies suggest a role for mineralocorticoid receptors in mediating choroidal hyperpermeability[47], which has been exploited with some success in trials of mineralocorticoid antagonists for CSCR[48]. Laser therapy, especially half fluence photodynamic therapy (PDT), is a recognized treatment option in recurrent or chronic CSCR. A number of pilot studies have assessed the efficacy of ST laser in treatment of chronic or recalcitrant CSCR. The earliest reported trial of ST laser treatment for CSCR found resolution of fluid leakage in 3/4 eyes by 12 weeks follow-up[34]. In 2008, a prospective nonrandomized pilot study treated 24 eyes of 22 CSCR patients with ST 810nm laser and followed patients on average 14 months, finding improvement in subretinal fluid in 3/4 of eyes by the termination of follow-up[49]. Another 2008 prospective nonrandomized study had similar findings, with 6/6 (CSCR without RPE atrophy), 8/9 (CSCR with RPE atrophy), and 5/11 (decompensated CSCR) eyes treated with ST 810nm laser found to have complete subretinal fluid reabsorption at the end of follow-up[50]. To identify sites of serous leakage as a target for the laser, indocyanine green (ICG) dye was found to be an effective companion to ST laser treatment of CSCR[51, 52].
Early controlled trials found mixed results for ST laser treatment of CSCR. Generally, ST laser showed non-inferiority to either anti-VEGF treatment or observation in VA outcomes[53, 54, 55]. Yellow 577nm ST laser also had beneficial effects in CSCR (reduction of subretinal fluid and improvement of VA), as the authors of 3 separate 2015 retrospective noncontrolled studies noted[56, 57, 58]. A prospective nonrandomized trial corroborated these results, noting significant improvements in VA, central macular thickness, and log contrast sensitivity among 15 eyes with nonresolving CSCR treated with yellow 577nm ST laser[59]. Luttrull found that increasing the density of ST laser spots may improve outcomes in CSCR[60], similar to findings in ST treatment of DME[37]. A 2017 prospective nonrandomized noncontrolled study found resolution of subretinal fluid in 36/51 eyes treated by ST 577nm yellow laser for CSCR[61].
Several retrospective studies have compared application of ST laser versus PDT to CSCR. In a study of 100 CSCR patients, the authors concluded that ST laser therapy was more effective than PDT based on significant differences in proportion of treatment responders (33/42 vs. 34/58) and mean decrease in central retinal thickness (148±163 vs. 76±104 μm)[62]. Özmert and colleagues conducted a retrospective trial comparing ST 577 nm laser with PDT at half-fluence (reduced energy density) for CSCR and found no group differences in outcomes[63]. Reduced dose PDT was compared with 689nm laser in a ST application in treatment of CSCR in a randomized prospective trial of 40 eyes. No differences were observed between the treatment modalities in final logMAR, central retinal thickness, subfoveal choroidal thickness, or duration of symptoms[64]. PDT presents numerous disadvantages that may make ST laser treatments more attractive to both clinicians and patients: side effects requiring avoidance of bright sunlight, risk of choroidal infarction and vision loss and inconvenience of dosing and administration of an IV infusion.
Recently, a large randomized prospective controlled trial, the PLACE trial (ClinicalTrials.gov #NCT01797861), commenced in 2013 with the objective of comparing high-density non-confluent laser titrated to ST effects by a test 1800mW nonmacular spot versus traditional half-dose PDT in patients suffering from chronic CSCR[65]. The authors found significantly better outcomes in PDT-treated patients versus high-density ST laser in resolution of subretinal fluid, VA improvement, and retinal sensitivity, although no differences were observed in the composite vision-related quality of life metric[66]. These results persisted in both the short (6-8 weeks post-treatment) and long (7-8 months post-treatment) terms[66] and provided the most conclusive evidence for the superiority of half-fluence PDT over ST laser treatment in CSCR.
3.3. Age-Related Macular Degeneration (Table 3)
Table 3.
Randomized clinical trials of subthreshold laser in age-related macular degeneration.
| Citation | Study type | Groups | Outcome measures | Results/conclusions |
|---|---|---|---|---|
| Olk et. al 1999, PMID 10571341 | Prospective, randomized | • CW 810nm: n=63 eyes • ST 810nm: n=57 eyes • Observation: n=109 eyes |
VA, drusen, CNV | • VA, drusen reduction: any treatment significantly better than obs at 24mos f/u • CNV: no difference among groups at 24mos f/u |
| Rodanant et. al 2002, PMID 12383815 | Prospective, randomized | • ST 810nm: n=50 eyes • Obs: n=50 eyes |
VA, drusen | • Drusen: significantly more ST eyes with drusen reduction at 18mos f/u • VA: no difference in outcomes |
| Friberg et. al, 2006, PMID 16581422 | Prospective, randomized | • ST 810nm: n=124 eyes • Obs: n=124 eyes |
VA, CNV, drusen | • VA: significant benefit to ST only at 3mos f/u • CNV: significantly elevated risk in ST • Drusen: 50% resorption of drusen area in significantly more ST eyes |
Abbreviations – ST, subthreshold; CW, continuous wave; VA, visual acuity; CNV, choroidal neovascularization; f/u, follow-up; obs, observation
Prior to the advent of anti-VEGF therapy, CW laser photocoagulation had a prominent role in the treatment of exudative age-related macular degeneration (AMD), a leading cause of adult blindness in the United States characterized by age-associated lipophilic drusen deposits under the RPE and choroidal neovascularization (CNV)[67].
3.3.1. Drusen reduction
Interestingly, a number of studies have demonstrated that laser photocoagulation could diminish drusen size in AMD patients, although not always with obvious clinical benefit to the patient. In 2000, Roider et. al provided proof-of-concept by conducting a small nonrandomized pilot study of 26 patients with retinal pathology, finding reduction of drusen in 7/10 patients treated with ST 527nm laser at 6 months follow-up[34]. In a retrospective analysis of 144 eyes of 144 dry AMD patients in Italy treated by CW argon 532nm laser or ST 810nm laser, there was no clinical benefit to ST over CW laser as measured by lack of statistical difference in visual acuity or drusen reduction[68]. The authors found that ST laser versus CW spared patients treatment-associated reduction in contrast sensitivity[68]. When functional guidance of ST 810nm laser was guided by pattern electroretinography (PERG), a readout typically used in glaucoma, PERG metrics were improved in a study of 158 eyes from 108 patients with AMD, although without VA improvement[19].
Olk and colleagues published the results of a large partially randomized pilot trial of 229 eyes from 152 patients with AMD[69]. Although they reported comparable efficacy in drusen reduction and VA improvement between CW laser and ST laser, the authors found iatrogenic choroidal neovascularization only in the CW laser and not ST laser-treated eyes[69]. A subsequent randomized clinical trial comparing ST 810nm laser versus observation in bilateral eyes of 50 patients with nonexudative AMD noted drusen reduction but no significant improvements in VA versus untreated controls[70]. Another study demonstrated no improvement of drusen area or VA in eyes of AMD patients treated with ST 532nm laser[71]. Mojana et al. found that ST 810nm laser treatment of drusen in eyes of AMD patients may be associated with chronic damage to the RPE[72].
3.3.2. Choroidal neovascularization treatment
To determine whether prophylactic ST laser could prevent progression of CNV in AMD, Friberg et. al conducted a randomized clinical trial comparing observation versus treatment in the non-CNV eyes of AMD patients whose other eye had progressed to CNV[73]. Critically, they found significantly increased risk of CNV in the treated eyes, leading the authors to recommend against prophylactic ST laser treatment of non-CNV afflicted eyes of patients suffering from AMD[73]. In the parallel arm of their study, bilateral dry AMD patients were enrolled to determine the efficacy of ST 810nm laser (experimental eye) versus observation (contralateral control eye)[74]. No benefit in risk to CNV progression was observed with treatment, but the authors did find a significant yet small improvement in VA in the laser-treated eyes[74]. In a large meta-analysis of all types of laser photocoagulation of AMD-associated drusen, photocoagulation did not reduce risk of progression to CNV[75].
3.4. Summary of ST laser for AMD
More recent work has examined the efficacy and safety of ST laser therapy, especially as a second-line treatment for AMD. In progressive AMD, drug tolerance can build to anti-VEGF therapies. A prospective nonrandomized trial of 13 eyes of 12 anti-VEGF-resistant AMD patients found that macular exudates improved in 12/13 eyes when re-sensitized to anti-VEGF therapy via a single ST 810nm laser treatment[20]. While this single study suggests that ST threshold could help in resensitizing patients to anti-VEGF therapy, no other published studies have replicated this effect. In summary, evidence for the role of ST laser treatment of AMD-associated pathology is poor at best and does not support its routine use in management. To date, the strongest evidence for ST laser finds comparable efficacy with CW laser in drusen reduction, although without a strong connection to VA improvement. One possible future direction of ST treatment of AMD is the use of nanosecond laser, which employs shorter laser pulses in a ST manner and spares retinal damage. A pilot study of AMD patients treated with nanosecond laser found no impact on retinal sensitivity, and mice treated in parallel had no evidence of damage to Bruch’s membrane or choroidal neovascularization[76]. Further work will determine whether any iteration of ST laser treatment is efficacious in AMD.
3.5. Macular Edema due to Branch Retinal Vein Occlusion
Macular edema is a known vision-threatening complication of branch retinal vein occlusion (BRVO). An early randomized study of 36 eyes found promising evidence for the role of ST 810 nm laser treatment of BRVO-associated macular edema when compared to CW krypton laser[77]. Both groups experienced similar improvements in VA, foveal thickness, and total macular volume[77]. The same group later found synergistic effects in improving mean foveal thickness and VA when ST laser was combined with intravitreal triamcinolone versus ST laser alone[78]. When the authors compared ST laser treatment of BRVO-associated ME to intravitreal bevacizumab in a prospective randomized study of 35 eyes, intravitreal bevacizumab proved superior to ST laser at 12 months follow-up as measured by improvements in central foveal thickness and VA[79]. In a retrospective nonrandomized noncontrolled study, Inagaki and colleagues found that ST laser improved macular edema in BRVO-afflicted eyes with best corrected visual acuity >20/40[80]. At this point however, no large randomized clinical trial evidence supports the routine use of ST laser phototherapy in BRVO-associated macular edema.
3.6. Additional Possible Indications for Subthreshold Laser Therapy
Applications of ST laser in rare diseases of the retina have been reported in the literature. In a small case report of ST laser as an adjuvant to first-line bevacizumab in Coat’s disease, one patient’s VA remained stable at 20/100 and submacular exudates resolved at the close of 12 months follow-up after ST laser adjuvant to bevacizumab treatment[81]. ST laser treatment has also been applied to retinal artery macroaneurysm, with fewer complications and comparable visual acuity and aneurysm central point thickness among 12 eyes randomized to receive ST laser therapy[82]. One case report successfully employed ST 577 nm laser in the treatment of chronic radiation retinopathy[83]. In a pilot study of macular telangiectasia, dose-optimized ST laser was found to decrease lacunae and improve VA in 8/10 treated eyes[16]. To our knowledge, laser strength has not yet been investigated in the following conditions often treated by CW photocoagulation: retinal capillary hemangioma, or extrafoveal retinal angiomatous proliferation lesions, polyploidal choroidal vasculopathy, retinopathy of prematurity, or retinoblastoma.
4. CONCLUSIONS
In conclusion, the application of ST laser in various retinal pathologies has demonstrated some promising effects, with efficacy noninferior to focal photocoagulation in conditions such as DME. Clinical evidence is stronger and more extensive supporting the use of laser in a ST setting than basic science evidence for its responsible biological mechanisms. Presently, thermal stimulation of RPE Hsp upregulation by the “reset” phenomenon is the leading hypothesis for subthreshold laser efficacy in retinal pathology[20]. Diabetic retinopathy and DME have the strongest body of evidence supporting the use of ST laser. While it appears that ST treatment may be noninferior to focal CW photocoagulation, no studies have compared ST to anti-VEGF treatment for DME. Additionally, whether addition of ST therapy can reduce treatment burden of anti-VEGF medications is still an open question. The largest meta-analysis confirmed statistically significant, albeit clinically minor, differences in VA improvements and similar improvements in central macular thickness for nearly 400 eyes of DME patients randomized to receive CW or ST laser therapy[46]. Smaller randomized studies found modest benefits of ST laser versus observation in treatment of CSCR[54, 55], although the results of a large multicenter randomized clinical trial demonstrated that ST laser was inferior to half-fluence PDT in treating CSCR[66]. However, this study may have been limited by the use of non-confluent laser may, resulting in undertreatment. Moreover, the location of treatment may influence efficacy for CSCR, in which ST laser may be efficacious in juxtafoveal leaks. In treatment of AMD, ST laser therapy demonstrated the theoretical benefit of reducing drusen deposition but without apparent clinical benefit[69, 70]. Although comparable to CW photocoagulation in one randomized study[77], ST laser was inferior to anti-VEGF therapy in a subsequent study[79], and anti-VEGF therapy remains first-line treatment in BRVO-associated macular edema. In general, ST laser may have its place in specific applications of vitreoretinal practice and is well-tolerated and appreciated by patients versus the more painful CW laser. More extensive long-term follow-up will reveal potential chronic adverse effects associated with ST laser treatment. Future studies will continue to experiment with alternative laser power, duty cycle, and density and size of targeted laser spots in the retina.
5. EXPERT COMMENTARY
While the prospect of a broadly applicable technique with few side effects and strong efficacy is attractive, the current state of evidence for ST laser therapy in retinal practice suggests that it falls short of such a panacea. Whether this is attributable to an incomplete understanding of its possibilities (ie, trials of alternative laser power or spot density settings) or to limited insight into its underlying biological mechanisms is uncertain. While it is clear from the literature that current algorithms for subthreshold laser do not cause any permanent structural damage to the retina, it is unclear whether subthreshold laser treatments are more efficacious than traditional ETDRS focal grid laser treatments used in previous clinical trials.
From a practical perspective, ST laser has likely not been adopted as a mainstream treatment option for retina specialists for a number of reasons. 1) The lack of well-designed clinical trials demonstrating that it is either more effective or is effective as an adjunct to the standard of care in a number of retinal diseases. 2) Laser treatments (conventional or subthreshold) are generally much less common now in the age of anti-VEGF therapy. 3) The lack of damage to the retina also prevents the retina specialist from knowing what areas have been treated, which may cause some reluctance for the retina specialist to pursue this treatment option.
6. FIVE YEAR VIEW
Additional large randomized prospective clinical trials are needed to address unanswered questions in the field. Indeed, the results of the PLACE trial helped to definitively answer the question about the efficacy of ST laser compared to half fluence PDT in CSCR. Similar types of studies are needed for other retinal indications to definitively answer the efficacy of ST laser compared to current standard of care. The prevalence of diabetic eye disease, including DR and DME, offers the greatest opportunity to conduct rigorous trials of ST laser. Intravitreal anti-VEGF injections remain the mainstay of therapy for DME, and only limited studies have compared it with ST laser[44, 45]. To this end, a number of ongoing trials should be illuminating. The DAM trial (NCT03143192) will compare the VEGF inhibitor aflibercept either alone or in combination with ST laser in 30 patients. Two separate planned trials will compare anti-VEGF antibodies alone or in combination with ST laser in 60 (NCT0229175) or 25 patients (NCT02059772). Although these trials will provide important information, small sample sizes continue to be a major limitation in this field. For AMD, a large 292 patient randomized trial is underway to study nanosecond laser (NCT01790802) in addition to smaller studies of ST laser in 20 (NCT02800356) or 21 (NCT02960828) patients.
Treatment options for retinal disease continue to expand, and ST laser may have its place as treatment for retinal disease. For ST laser to succeed as monotherapy, greater optimization of laser settings will be necessary. Compared to medical options with standardized dosing regimens, the dosing of laser treatments is more variable; as such, automation of the laser intensity and density through automated software may prevail as the predominant strategy. A tantalizing possibility for the future is to upregulate certain gene pathways through specific laser treatment algorithms. More research is needed in how laser power results in changes in gene regulation which may lead to future laser treatment which could selectively upregulate specific gene pathways. In the future, one can even imagine the delivery of genes through viral vectors to specific cell populations which may be selectively induced through specific wavelengths or power settings of the laser. This could afford the ophthalmologist on-demand temporal control of expression of therapeutic genes in cells accessible to laser activation. Laser phototherapy represented one of the first therapeutic approaches for retinal diseases and with continued research into the interaction of lasers with retinal tissues, new laser therapies, such as subthreshold laser, may emerge as additional therapeutic options for retinal disease.
7. KEY ISSUES.
Laser therapy of retinal pathology traditionally uses a continuous beam of laser energy to inflict photocoagulative necrosis. New subthreshold laser technology uses short bursts of energy to stimulate the target tissue without causing permanent tissue damage.
In vitro and animal models suggest that subthreshold laser upregulates heat shock proteins in the retina to achieve therapeutic effect.
A meta-analysis of the 6 largest RCTs found that subthreshold laser had a slight advantage over conventional laser in visual acuity outcomes in treatment of diabetic macular edema up to 12 months.
The largest RCT comparing subthreshold laser and photodynamic therapy in central serous chorioretinopathy showed significantly inferior outcomes in laser-treated eyes.
Evidence is lacking for use of subthreshold laser in age-related macular degeneration or branch retinal vein occlusion.
The chief advantages of subthreshold laser include its superior side effect profile (less pain during treatment, less retinal scarring), although this is tempered by the potential for increased treatment burden.
Variability in laser settings among different trials clouds the interpretation of subthreshold laser efficacy. Future studies will optimize laser settings, especially by automating the laser spots on the retina.
Definitive evidence comparing subthreshold laser to medical therapy (eg, anti-VEGF treatments) is needed to solidify its routine use.
Acknowledgments
Funding:
Funding has been received in the form of a grant from the National Eye Institute (1K12EY024225-01A1)
Footnotes
Declaration of interest:
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures:
A reviewer on this manuscript has disclosed that they are a paid consultant for Iridex.
REFERENCES
- 1.Meyer-Schwickerath G Light coagulation. St. Louis: C. V. Mosby; 1960. [Google Scholar]; *Earliest report of the use of light energy in ophthalmology practice.
- 2.Maiman T Stimulated Optical Radiation in Ruby. Nature. 1960;187:493–494. [Google Scholar]; *Background of laser physics.
- 3.Palanker DV, Blumenkranz MS, Marmor MF. Fifty years of ophthalmic laser therapy. Arch Ophthalmol. 2011. December;129(12):1613–9. doi: 10.1001/archophthalmol.2011.293. PubMed PMID: 22159684. [DOI] [PubMed] [Google Scholar]; **Historical evolution of laser therapy in ophthalmology.
- 4.Zweng HC, Little HL, Peabody RR. Argon laser photocoagulation of diabetic retinopathy. Arch Ophthalmol. 1971. October;86(4):395–400. PubMed PMID: 5110132. [DOI] [PubMed] [Google Scholar]
- 5.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987. July;94(7):761–74. PubMed PMID: 3658348. [DOI] [PubMed] [Google Scholar]
- 6.Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987. Winter;27(4):254–64. PubMed PMID: 3692707. [DOI] [PubMed] [Google Scholar]
- 7.Pollack JS, Kim JE, Pulido JS, et al. Tissue effects of subclinical diode laser treatment of the retina. Arch Ophthalmol. 1998. December;116(12):1633–9. PubMed PMID: 9869794. [DOI] [PubMed] [Google Scholar]
- 8.Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005. January;89(1):74–80. doi: 10.1136/bjo.2004.051540. PubMed PMID: 15615751; PubMed Central PMCID: PMCPMC1772486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luttrull JK, Musch DC, Spink CA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye (Lond). 2008. May;22(5):607–12. doi: 10.1038/sj.eye.6702725. PubMed PMID: 17293791. [DOI] [PubMed] [Google Scholar]
- 10.Scholz P, Altay L, Fauser S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv Ther. 2017. July;34(7):1528–1555. doi: 10.1007/s12325-017-0559-y. PubMed PMID: 28540655; PubMed Central PMCID: PMCPMC5504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moorman CM, Hamilton AM. Clinical applications of the MicroPulse diode laser. Eye (Lond). 1999. April;13 (Pt 2):145–50. doi: 10.1038/eye.1999.41. PubMed PMID: 10450372. [DOI] [PubMed] [Google Scholar]; **One of the earliest studies of 810nm diode laser applied to BRVO and DME using principles of micropulsed laser energy.
- 12.Casson RJ, Raymond G, Newland HS, et al. Pilot randomized trial of a nanopulse retinal laser versus conventional photocoagulation for the treatment of diabetic macular oedema. Clin Exp Ophthalmol. 2012. August;40(6):604–10. doi: 10.1111/j.1442-9071.2012.02756.x. PubMed PMID: 22300292. [DOI] [PubMed] [Google Scholar]
- 13.Hamada M, Ohkoshi K, Inagaki K, et al. Subthreshold Photocoagulation Using Endpoint Management in the PASCAL(R) System for Diffuse Diabetic Macular Edema. J Ophthalmol. 2018;2018:7465794. doi: 10.1155/2018/7465794. PubMed PMID: 29651345; PubMed Central PMCID: PMCPMC5831979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maio A Heat shock proteins: facts, thoughts, and dreams. Shock. 1999. January;11(1):1–12. PubMed PMID: 9921710. [DOI] [PubMed] [Google Scholar]; *Comprehensive background on heat shock proteins, including inducers, regulation, and cellular protection.
- 15.Sramek C, Mackanos M, Spitler R, et al. Non-damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci. 2011. March 28;52(3):1780–7. doi: 10.1167/iovs.10-5917. PubMed PMID: 21087969. [DOI] [PubMed] [Google Scholar]
- 16.Lavinsky D, Wang J, Huie P, et al. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Invest Ophthalmol Vis Sci. 2016. May 1;57(6):2488–500. doi: 10.1167/iovs.15-18981. PubMed PMID: 27159441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Zhang S, Liao H, et al. Pre-exposure to low-power diode laser irradiation promotes cytoprotection in the rat retina. Lasers Med Sci. 2015. January;30(1):127–33. doi: 10.1007/s10103-014-1619-z. PubMed PMID: 25048854. [DOI] [PubMed] [Google Scholar]
- 18.Franklin TB, Krueger-Naug AM, Clarke DB, et al. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia. 2005. August;21(5):379–92. doi: 10.1080/02656730500069955. PubMed PMID: 16048836. [DOI] [PubMed] [Google Scholar]
- 19.Luttrull JK, Margolis BW. Functionally Guided Retinal Protective Therapy for Dry Age-Related Macular and Inherited Retinal Degenerations: A Pilot Study. Invest Ophthalmol Vis Sci. 2016. January 1;57(1):265–75. doi: 10.1167/iovs.15-18163. PubMed PMID: 26818793. [DOI] [PubMed] [Google Scholar]
- 20.Luttrull JK, Chang DB, Margolis BW, et al. LASER RESENSITIZATION OF MEDICALLY UNRESPONSIVE NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: Efficacy and Implications. Retina. 2015. June;35(6):1184–94. doi: 10.1097/IAE.0000000000000458. PubMed PMID: 25650711. [DOI] [PubMed] [Google Scholar]
- 21.Caballero S, Kent DL, Sengupta N, et al. Bone Marrow-Derived Cell Recruitment to the Neurosensory Retina and Retinal Pigment Epithelial Cell Layer Following Subthreshold Retinal Phototherapy. Invest Ophthalmol Vis Sci. 2017. October 1;58(12):5164–5176. doi: 10.1167/iovs.16-20736. PubMed PMID: 29049716; ubMed Central PMCID: PMCPMC5636205. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Hematopoeitic cell recruitment to retina following laser stimulation in mouse model.
- 22.Chidlow G, Shibeeb O, Plunkett M, et al. Glial cell and inflammatory responses to retinal laser treatment: comparison of a conventional photocoagulator and a novel, 3-nanosecond pulse laser. Invest Ophthalmol Vis Sci. 2013. March 28;54(3):2319–32. doi: 10.1167/iovs.12-11204. PubMed PMID: 23439603. [DOI] [PubMed] [Google Scholar]
- 23.Treumer F, Klettner A, Baltz J, et al. Vectorial release of matrix metalloproteinases (MMPs) from porcine RPE-choroid explants following selective retina therapy (SRT): towards slowing the macular ageing process. Exp Eye Res. 2012. April;97(1):63–72. doi: 10.1016/j.exer.2012.02.011. PubMed PMID: 22387137. [DOI] [PubMed] [Google Scholar]; *Extracellular matrix response to laser stimulation.
- 24.Dithmar S, Curcio CA, Le NA, et al. Ultrastructural changes in Bruch’s membrane of apolipoprotein E-deficient mice. Invest Ophthalmol Vis Sci. 2000. July;41(8):2035–42. PubMed PMID: 10892840. [PubMed] [Google Scholar]
- 25.Jobling AI, Guymer RH, Vessey KA, et al. Nanosecond laser therapy reverses pathologic and molecular changes in age-related macular degeneration without retinal damage. FASEB J. 2015. February;29(2):696–710. doi: 10.1096/fj.14-262444. PubMed PMID: 25392267. [DOI] [PubMed] [Google Scholar]
- 26.Flaxel C, Bradle J, Acott T, et al. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina. 2007. June;27(5):629–34. doi: 10.1097/01.iae.0000249561.02567.fd. PubMed PMID: 17558327. [DOI] [PubMed] [Google Scholar]
- 27.Wilson AS, Hobbs BG, Shen WY, et al. Argon laser photocoagulation-induced modification of gene expression in the retina. Invest Ophthalmol Vis Sci. 2003. April;44(4):1426–34. PubMed PMID: 12657576. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Song Y, Chen X, et al. Biological Modulation of Mouse RPE Cells in Response to Subthreshold Diode Micropulse Laser Treatment. Cell Biochem Biophys. 2015. November;73(2):545–552. doi: 10.1007/s12013-015-0675-8. PubMed PMID: 27352351. [DOI] [PubMed] [Google Scholar]
- 29.Eichler W, Yafai Y, Keller T, et al. PEDF derived from glial Muller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004. September 10;299(1):68–78. doi: 10.1016/j.yexcr.2004.05.020. PubMed PMID: 15302574. [DOI] [PubMed] [Google Scholar]
- 30.Miura FT Y, Klettner A, Hillenkamp J, Brinkmann R, Birngruber R, Roider J, editor Vegf and Pedf Secretions Over Time Following Various Laser Irradiations on an Rpe Organ Culture 2010: Invest. Ophthalmol. Vis. Sci. [Google Scholar]
- 31.Yu AK, Merrill KD, Truong SN, et al. The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Invest Ophthalmol Vis Sci. 2013. March 1;54(3):2216–24. doi: 10.1167/iovs.12-11382. PubMed PMID: 23439599. [DOI] [PubMed] [Google Scholar]
- 32.Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997. December;104(12):2030–8. PubMed PMID: 9400762. [DOI] [PubMed] [Google Scholar]; **One of the earliest trials of 810nm diode laser in micropulse mode with explanation of micropulse laser physics.
- 33.Akduman L, Olk RJ. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME). Ophthalmic Surg Lasers. 1999. Nov-Dec;30(9):706–14. PubMed PMID: 10574491. [PubMed] [Google Scholar]
- 34.Roider J, Brinkmann R, Wirbelauer C, et al. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol. 2000. January;84(1):40–7. PubMed PMID: 10611098; PubMed Central PMCID: PMCPMC1723228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivaprasad S, Sandhu R, Tandon A, et al. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow up. Clin Exp Ophthalmol. 2007. Sep-Oct;35(7):640–4. doi: 10.1111/j.1442-9071.2007.01566.x. PubMed PMID: 17894684. [DOI] [PubMed] [Google Scholar]
- 36.Friberg TR. Infrared micropulsed laser treatment for diabetic macular edema--subthreshold versus threshold lesions. Semin Ophthalmol. 2001. March;16(1):19–24. PubMed PMID: 15487694. [DOI] [PubMed] [Google Scholar]
- 37.Lavinsky D, Cardillo JA, Melo LA Jr., et al. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011. June 17;52(7):4314–23. doi: 10.1167/iovs.10-6828. PubMed PMID: 21345996. [DOI] [PubMed] [Google Scholar]; **RCT comparing density of subthreshold laser spots in 123 DME patients which found superior results in high-density group.
- 38.Venkatesh P, Ramanjulu R, Azad R, et al. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: a prospective, randomized study using multifocal electroretinography. Photomed Laser Surg. 2011. November;29(11):727–33. doi: 10.1089/pho.2010.2830. PubMed PMID: 21612513. [DOI] [PubMed] [Google Scholar]; **RCT comparing subthreshold laser to conventional wave laser in 46 DME patients which found comparable results in both groups.
- 39.Xie TY GQ, Wang Y, et al. Randomized, controlled clinical trial comparision of SDM laser versus argon ion laser in diabetic macular edema. Int Eye Sci. 2013;13:2370–2372. [Google Scholar]; **RCT comparing subthreshold laser to conventional wave laser in 84 DME patients which found comparable results in both groups.
- 40.Mansouri A, Sampat KM, Malik KJ, et al. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye (Lond). 2014. December;28(12):1418–24. doi: 10.1038/eye.2014.264. PubMed PMID: 25359290; PubMed Central PMCID: PMCPMC4268471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolo M, Musetti D, Traverso CE. Yellow micropulse laser in diabetic macular edema: a short-term pilot study. Eur J Ophthalmol. 2014. Nov-Dec;24(6):885–9. doi: 10.5301/ejo.5000495. PubMed PMID: 24905254. [DOI] [PubMed] [Google Scholar]
- 42.Inagaki K, Ohkoshi K, Ohde S, et al. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561-577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol. 2015. January;59(1):21–8. doi: 10.1007/s10384-014-0361-1. PubMed PMID: 25392274. [DOI] [PubMed] [Google Scholar]
- 43.Vujosevic S, Martini F, Longhin E, et al. SUBTHRESHOLD MICROPULSE YELLOW LASER VERSUS SUBTHRESHOLD MICROPULSE INFRARED LASER IN CENTER-INVOLVING DIABETIC MACULAR EDEMA: Morphologic and Functional Safety. Retina. 2015. August;35(8):1594–603. doi: 10.1097/IAE.0000000000000521. PubMed PMID: 25719988. [DOI] [PubMed] [Google Scholar]
- 44.Moisseiev E, Abbassi S, Thinda S, et al. Subthreshold micropulse laser reduces anti-VEGF injection burden in patients with diabetic macular edema. Eur J Ophthalmol. 2018. January;28(1):68–73. doi: 10.5301/ejo.5001000. PubMed PMID: 28731494. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Ai P, Ai Z, et al. Subthreshold diode micropulse laser versus conventional laser photocoagulation monotherapy or combined with anti-VEGF therapy for diabetic macular edema: A Bayesian network meta-analysis. Biomed Pharmacother. 2018. January;97:293–299. doi: 10.1016/j.biopha.2017.10.078. PubMed PMID: 29091878. [DOI] [PubMed] [Google Scholar]; **Meta-analysis of all RCTs studying subthreshold laser in DME.
- 46.Chen G, Tzekov R, Li W, et al. SUBTHRESHOLD MICROPULSE DIODE LASER VERSUS CONVENTIONAL LASER PHOTOCOAGULATION FOR DIABETIC MACULAR EDEMA: A Meta-Analysis of Randomized Controlled Trials. Retina. 2016. November;36(11):2059–2065. doi: 10.1097/IAE.0000000000001053. PubMed PMID: 27096529. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012. July;122(7):2672–9. doi: 10.1172/JCI61427. PubMed PMID: 22684104; PubMed Central PMCID: PMCPMC3386817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatziralli I, Vlachodimitropoulou A, Daoula C, et al. Eplerenone in the treatment of central serous chorioretinopathy: a review of the literature. Int J Retina Vitreous. 2018;4:33. doi: 10.1186/s40942-018-0137-8. PubMed PMID: 30250750; PubMed Central PMCID: PMCPMC6145103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanzetta P, Furlan F, Morgante L, et al. Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol. 2008. Nov-Dec;18(6):934–40. PubMed PMID: 18988165. [DOI] [PubMed] [Google Scholar]
- 50.Chen SN, Hwang JF, Tseng LF, et al. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008. December;115(12):2229–34. doi: 10.1016/j.ophtha.2008.08.026. PubMed PMID: 19041477. [DOI] [PubMed] [Google Scholar]
- 51.Ricci F, Missiroli F, Cerulli L. Indocyanine green dye-enhanced micropulsed diode laser: a novel approach to subthreshold RPE treatment in a case of central serous chorioretinopathy. Eur J Ophthalmol. 2004. Jan-Feb;14(1):74–82. PubMed PMID: 15005592. [DOI] [PubMed] [Google Scholar]
- 52.Ricci F, Missiroli F, Regine F, et al. Indocyanine green enhanced subthreshold diode-laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2009. May;247(5):597–607. doi: 10.1007/s00417-008-1014-1. PubMed PMID: 19089442. [DOI] [PubMed] [Google Scholar]
- 53.Koss MJ, Beger I, Koch FH. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye (Lond). 2012. February;26(2):307–14. doi: 10.1038/eye.2011.282. PubMed PMID: 22079961; PubMed Central PMCID: PMCPMC3272193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behnia M, Khabazkhoob M, Aliakbari S, et al. Improvement in visual acuity and contrast sensitivity in patients with central serous chorioretinopathy after macular subthreshold laser therapy. Retina. 2013. February;33(2):324–8. doi: 10.1097/IAE.0b013e3182670fa3. PubMed PMID: 23314231. [DOI] [PubMed] [Google Scholar]
- 55.Roisman L, Magalhaes FP, Lavinsky D, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surg Lasers Imaging Retina. 2013. Sep-Oct;44(5):465–70. doi: 10.3928/23258160-20130909-08. PubMed PMID: 24044709. [DOI] [PubMed] [Google Scholar]
- 56.Yadav NK, Jayadev C, Mohan A, et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye (Lond). 2015. February;29(2):258–64; quiz 265. doi: 10.1038/eye.2014.315. PubMed PMID: 25613846; PubMed Central PMCID: PMCPMC4330300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JY, Park HS, Kim SY. Short-term efficacy of subthreshold micropulse yellow laser (577-nm) photocoagulation for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015. December;253(12):2129–35. doi: 10.1007/s00417-015-2965-7. PubMed PMID: 25717024. [DOI] [PubMed] [Google Scholar]
- 58.Scholz P, Ersoy L, Boon CJ, et al. Subthreshold Micropulse Laser (577 nm) Treatment in Chronic Central Serous Chorioretinopathy. Ophthalmologica. 2015;234(4):189–94. doi: 10.1159/000439600. PubMed PMID: 26406874. [DOI] [PubMed] [Google Scholar]
- 59.Abd Elhamid AH. Subthreshold micropulse yellow laser treatment for nonresolving central serous chorioretinopathy. Clin Ophthalmol. 2015;9:2277–83. doi: 10.2147/OPTH.S87499. PubMed PMID: 26664043; PubMed Central PMCID: PMCPMC4671811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luttrull JK. Low-Intensity/High-Density Subthreshold Diode Micropulse Laser for Central Serous Chorioretinopathy. Retina. 2016. September;36(9):1658–63. doi: 10.1097/IAE.0000000000001005. PubMed PMID: 27206160. [DOI] [PubMed] [Google Scholar]
- 61.Gawecki M, Jaszczuk-Maciejewska A, Jurska-Jasko A, et al. Functional and morphological outcome in patients with chronic central serous chorioretinopathy treated by subthreshold micropulse laser. Graefes Arch Clin Exp Ophthalmol. 2017. December;255(12):2299–2306. doi: 10.1007/s00417-017-3783-x. PubMed PMID: 28831603; PubMed Central PMCID: PMCPMC5696495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye (Lond). 2016. October;30(10):1371–1377. doi: 10.1038/eye.2016.142. PubMed PMID: 27391938; PubMed Central PMCID: PMCPMC5129861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozmert E, Demirel S, Yanik O, et al. Low-Fluence Photodynamic Therapy versus Subthreshold Micropulse Yellow Wavelength Laser in the Treatment of Chronic Central Serous Chorioretinopathy. J Ophthalmol. 2016;2016:3513794. doi: 10.1155/2016/3513794. PubMed PMID: 27597894; PubMed Central PMCID: PMCPMC5002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo A, Turano R, Morescalchi F, et al. Comparison of half-dose photodynamic therapy and 689 nm laser treatment in eyes with chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2017. June;255(6):1141–1148. doi: 10.1007/s00417-017-3626-9. PubMed PMID: 28283732. [DOI] [PubMed] [Google Scholar]
- 65.Breukink MB, Downes SM, Querques G, et al. Comparing half-dose photodynamic therapy with high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. Trials. 2015. September 21;16:419. doi: 10.1186/s13063-015-0939-z. PubMed PMID: 26390920; PubMed Central PMCID: PMCPMC4578347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Dijk EHC, Fauser S, Breukink MB, et al. Half-Dose Photodynamic Therapy versus High-Density Subthreshold Micropulse Laser Treatment in Patients with Chronic Central Serous Chorioretinopathy: The PLACE Trial. Ophthalmology. 2018. May 15. doi: 10.1016/j.ophtha.2018.04.021. PubMed PMID: 29776672. [DOI] [PubMed] [Google Scholar]; **Outcome of largest RCT for subthreshold laser versus photodynamic therapy in 179 central serous chorioretinopathy patients which found superior outcomes in photodynamic therapy.
- 67.Querques G, Cicinelli MV, Rabiolo A, et al. Laser photocoagulation as treatment of non-exudative age-related macular degeneration: state-of-the-art and future perspectives. Graefes Arch Clin Exp Ophthalmol. 2018. January;256(1):1–9. doi: 10.1007/s00417-017-3848-x. PubMed PMID: 29177712. [DOI] [PubMed] [Google Scholar]
- 68.Scorolli L, Corazza D, Morara M, et al. Argon laser vs. subthreshold infrared (810-nm) diode laser macular grid photocoagulation in nonexudative age-related macular degeneration. Can J Ophthalmol. 2003. October;38(6):489–95. PubMed PMID: 14620037. [DOI] [PubMed] [Google Scholar]
- 69.Olk RJ, Friberg TR, Stickney KL, et al. Therapeutic benefits of infrared (810-nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age-related macular degeneration: two-year results of a randomized pilot study. Ophthalmology. 1999. November;106(11):2082–90. doi: 10.1016/S0161-6420(99)90487-6. PubMed PMID: 10571341. [DOI] [PubMed] [Google Scholar]; *Large early RCT of subthreshold laser in 152 age-related macular degeneration patients which found drusen reduction in treated eyes.
- 70.Rodanant N, Friberg TR, Cheng L, et al. Predictors of drusen reduction after subthreshold infrared (810 nm) diode laser macular grid photocoagulation for nonexudative age-related macular degeneration. Am J Ophthalmol. 2002. October;134(4):577–85. PubMed PMID: 12383815. [DOI] [PubMed] [Google Scholar]; *RCT of subthreshold laser in 100 age-related macular degeneration patients found drusen reduction but no differences in visual acuity versus observation.
- 71.Nili-Ahmadabadi M, Espandar L, Mansoori MR, et al. Therapeutic effect of macular grid photocoagulation in treatment of nonexudative age-related macular degeneration. Arch Iran Med. 2007. January;10(1):14–9. doi: 07101/AIM.005. PubMed PMID: 17198447. [PubMed] [Google Scholar]
- 72.Mojana F, Brar M, Cheng L, et al. Long-term SD-OCT/SLO imaging of neuroretina and retinal pigment epithelium after subthreshold infrared laser treatment of drusen. Retina. 2011. February;31(2):235–42. doi: 10.1097/IAE.0b013e3181ec80ad. PubMed PMID: 21157398; PubMed Central PMCID: PMCPMC3530923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friberg TR, Musch DC, Lim JI, et al. Prophylactic treatment of age-related macular degeneration report number 1: 810-nanometer laser to eyes with drusen. Unilaterally eligible patients. Ophthalmology. 2006. April;113(4):622 e1. doi: 10.1016/j.ophtha.2005.10.066. PubMed PMID: 16581422. [DOI] [PubMed] [Google Scholar]
- 74.Friberg TR, Brennen PM, Freeman WR, et al. Prophylactic treatment of age-related macular degeneration report number 2: 810-nanometer laser to eyes with drusen: bilaterally eligible patients. Ophthalmic Surg Lasers Imaging. 2009. Nov-Dec;40(6):530–8. doi: 10.3928/15428877-20091030-01. PubMed PMID: 19928717. [DOI] [PubMed] [Google Scholar]; *Trials of subthreshold laser in age-related macular degeneration show that prophylactic laser treatment of drusen is not effective against choroidal neovascularization.
- 75.Virgili G, Michelessi M, Parodi MB, et al. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev. 2015. October 23(10):CD006537. doi: 10.1002/14651858.CD006537.pub3. PubMed PMID: 26493180; PubMed Central PMCID: PMCPMC4733883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vessey KA, Ho T, Jobling AI, et al. Nanosecond Laser Treatment for Age-Related Macular Degeneration Does Not Induce Focal Vision Loss or New Vessel Growth in the Retina. Invest Ophthalmol Vis Sci. 2018. February 1;59(2):731–745. doi: 10.1167/iovs.17-23098. PubMed PMID: 29392319. [DOI] [PubMed] [Google Scholar]
- 77.Parodi MB, Spasse S, Iacono P, et al. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology. 2006. December;113(12):2237–42. doi: 10.1016/j.ophtha.2006.05.056. PubMed PMID: 16996596. [DOI] [PubMed] [Google Scholar]
- 78.Parodi MB, Iacono P, Ravalico G. Intravitreal triamcinolone acetonide combined with subthreshold grid laser treatment for macular oedema in branch retinal vein occlusion: a pilot study. Br J Ophthalmol. 2008. August;92(8):1046–50. doi: 10.1136/bjo.2007.128025. PubMed PMID: 18556425. [DOI] [PubMed] [Google Scholar]
- 79.Parodi MB, Iacono P, Bandello F. Subthreshold grid laser versus intravitreal bevacizumab as second-line therapy for macular edema in branch retinal vein occlusion recurring after conventional grid laser treatment. Graefes Arch Clin Exp Ophthalmol. 2015. October;253(10):1647–51. doi: 10.1007/s00417-014-2845-6. PubMed PMID: 25382074. [DOI] [PubMed] [Google Scholar]
- 80.Inagaki K, Ohkoshi K, Ohde S, et al. Subthreshold Micropulse Photocoagulation for Persistent Macular Edema Secondary to Branch Retinal Vein Occlusion including Best-Corrected Visual Acuity Greater Than 20/40. J Ophthalmol. 2014;2014:251257. doi: 10.1155/2014/251257. PubMed PMID: 25276413; PubMed Central PMCID: PMCPMC4167817. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Nonrandomized trial of subthreshold laser in 32 patients with branch retinal vein occlusion-associated macular edema.
- 81.Lin CJ, Hwang JF, Chen YT, et al. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina. 2010. April;30(4):617–22. doi: 10.1097/IAE.0b013e3181c2e0b7. PubMed PMID: 19996822. [DOI] [PubMed] [Google Scholar]
- 82.Battaglia Parodi M, Iacono P, Pierro L, et al. Subthreshold laser treatment versus threshold laser treatment for symptomatic retinal arterial macroaneurysm. Invest Ophthalmol Vis Sci. 2012. April 2;53(4):1783–6. doi: 10.1167/iovs.11-8772. PubMed PMID: 22395893. [DOI] [PubMed] [Google Scholar]
- 83.Wong JG, Nguyen TTH. Yellow Pattern 577-nm Micropulse Laser: Treatment of Macular Edema from Radiation Retinopathy - A Case Report. Case Rep Ophthalmol. 2017. Jan-Apr;8(1):81–86. doi: 10.1159/000456028. PubMed PMID: 28413404; PubMed Central PMCID: PMCPMC5346916. [DOI] [PMC free article] [PubMed] [Google Scholar]