Abstract
Our study was designed to construct nomograms to predict the overall survival (OS) and cancer-specific survival (CSS) of lip carcinoma patients.
A search of the Surveillance, Epidemiology, and End Results (SEER) database provided us with detailed clinical data of the 1780 lip carcinoma patients. On the basis of the credible random split-sample method, the 1780 patients were placed into 2 groups, with 890 patients in the modeling group and 890 patients in the counterpart's group (proportion = 1:1). By employing Kaplan–Meier univariate and Cox multivariate survival analyses based on the modeling cohort, the nomograms were developed and then used to divide the modeling cohort into low-risk cohort and high-risk cohort. The survival rates of the 2 groups were calculated. Internal and external evaluation of nomogram accuracy was performed by the concordance index (C-index) and calibration curves.
With regard to 5- and 8-year OS and CSS, the C-indexes of internal validation were 0.762 and 0.787, whereas those of external validation reached 0.772 and 0.818, respectively. All the C-indexes were higher than 0.7. The survival curves of the low-risk cohort were obviously better than those of the high-risk cohort.
Credible nomograms have been established based on the SEER large-sample population research. We believe these nomograms can contribute to the design of treatment plans and evaluations of individual prognosis.
Keywords: cancer-specific survival; lip carcinoma; nomogram; overall survival; Surveillance, Epidemiology, and End Results
1. Introduction
Lip carcinoma is one of the most common head and neck malignant tumors, with an incidence of 1.8 per 100,000 individuals.[1,2] The incidence of local lymph node involvement in patients with lip carcinoma ranges from 5% to 20%; once local lymph nodes are invaded, the 5-year survival rate is only 50%.[3,4] Currently, surgical resection is the predominant treatment for lip carcinoma. However, due to the tumor location at the junction of the oral cutaneous and the mucosa, lip defects after surgery may affect eating, pronunciation, and appearance. Furthermore, secondary surgery may be inevitably required to reconstruct the tissue defects, adversely affecting the patients’ physical and mental health.
Clinicians often face a dilemma when making decisions regarding which is the optimized therapeutics for patients with lip carcinoma: conservative operations performed to the greatest possible extent to preserve function, or extensive excision to extend survival.[5] Therefore, personalized therapy based on individual prognostic evaluations is essential for patients. Currently, the American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) classifications 8th edition is the single clinical practice guideline for assessing prognosis of lip carcinoma patients.[6,7] However, the survival of lip carcinoma patients is affected by several other elements, including age, gender, race, radiation, and surgery rather than merely the TNM stage.[8,9,10]
Therefore, it is indispensable to determine how both cancer- and noncancer-related risk factors influence the probability of death so as to help clinicians tailor personalized treatment. At present, a method based on Kaplan–Meier and multivariate Cox proportional hazard models to investigate each independent risk factor and construct a survival nomogram of cancer patients is widely in use to assess the prognosis of carcinoma, including hepatocellular carcinoma,[11] gastric cancer,[12] nasopharyngeal cancer,[13] and breast cancer.[14] Most significantly, National Comprehensive Cancer Network (NCCN) clinical guidelines embrace nomograms for early detection of prostate cancers.[15]
In our study, we aimed to generate survival nomograms for lip carcinoma patients so that clinicians can be equipped with a quantitative tool to evaluate the 5- and 8-year overall survival (OS) and cancer-specific survival (CSS) for better risk stratification and clinical decision making.
2. Materials and methods
2.1. Demographic and clinicopathologic information
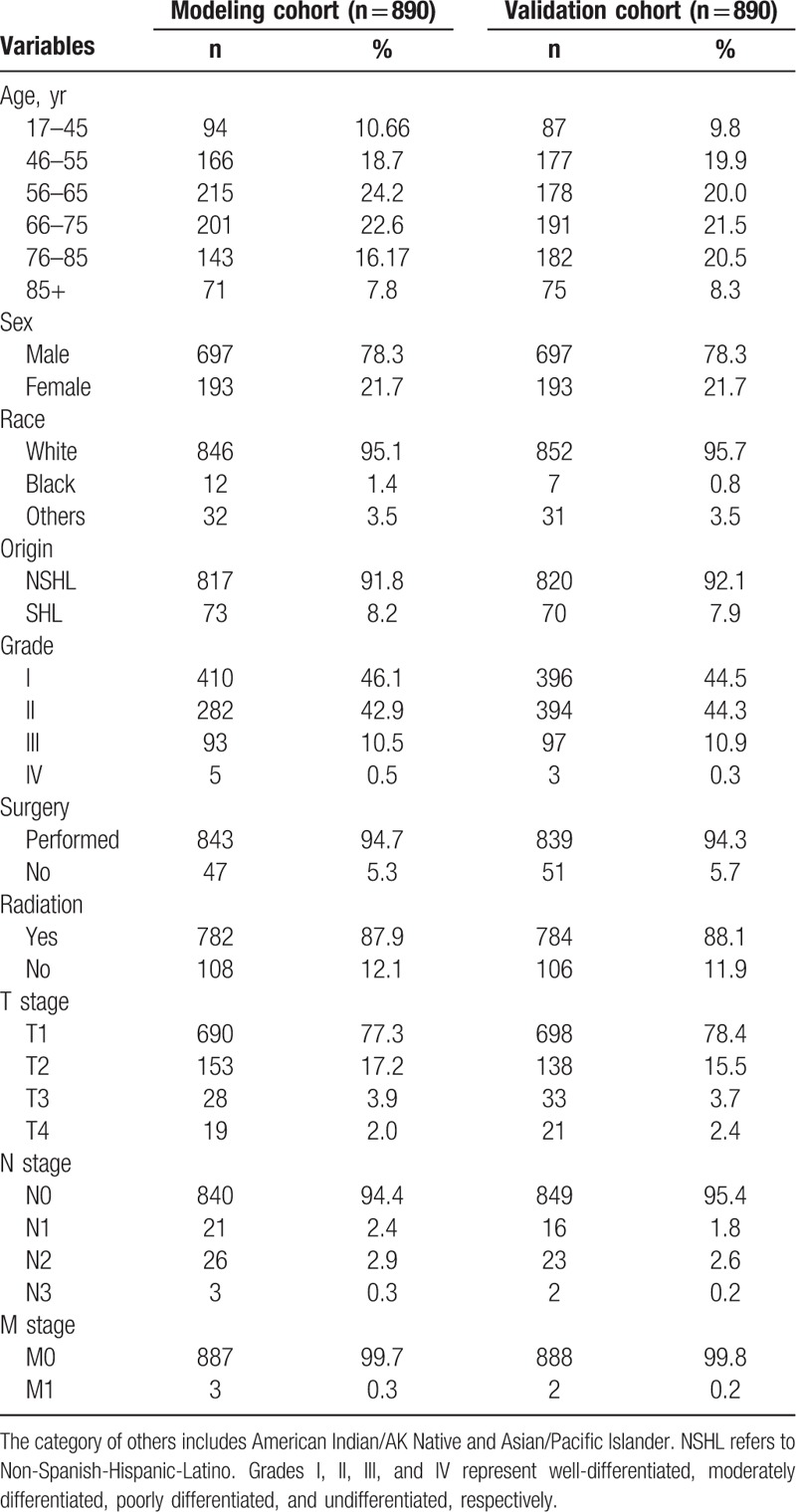
The clinicopathologic information for all 1780 patients with lip carcinoma from 2004 to 2013 came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute: Surveillance, Epidemiology, and End Results Program (http://seer.cancer.gov). Patients’ clinicopathologic information were recorded in detail (Table 1). Among the patients studied, the minimum age was 17 years while the maximum age was 104 years. The patients’ race origins covered white, black, and others (American Indian/AK Native, Asian/Pacific Islander). The study was approved by the Ethical Review Committee of the Chinese PLA General Hospital in 2017 (Approval Number: S2017-064-01).
Table 1.
Patients’ clinic pathologic data.
2.2. Survival analysis
All survival information was collected from SEER database referred to OS and CSS, respectively. OS and CSS analysis were then conducted via the Kaplan–Meier and Cox proportional hazards models. To verify the internal and external nomogram accuracy of the model, the concordance index (C-index), and calibration plots were used.[16] And we selected a split ratio 1:1 in this study. The analysis method was in accordance with a previous study.[17] A 2-sided P-value was used for statistical analysis, with P < .05 considered as statistically significant.
2.3. Nomogram establishment
The SPSS 21.0 was used to analyze the data of lip carcinoma patients to obtain independent prognostic risk factors affecting OS and CSS. The nomograms were established with the “cmprsk package” of R software (Version 3.2.4).
2.4. Nomogram verification
Internal and external verification of the nomogram accuracy was performed by 1000-time bootstrapping and 10-fold cross-validation. C-index and calibration plots, acquired by “rcorrcens” and “calibrate” commands in R software, were used to assess the fitting degree.[16] The 2 lines constituting the calibration plot were the 45° reference line and the actual line and the degree of nomogram accuracy was determined by the interval between the 2 lines.
In addition to survival prediction, each patient's score based on the nomogram was calculated. According to their nomogram-based scores, the patients were divided into 2 groups. Survival in these 2 groups was compared by using the Kaplan–Meier method.
3. Results
3.1. Patient clinicopathologic information
The random split-sample method was used to divide the 1780 lip carcinoma patients into modeling and validation cohorts. Of the 1780 patients, 1394 (78.3%) were males, 1698 (95.4%) were white, and 1637 (91.9%) were Non-Spanish-Hispanic-Latino. The proportions of T1 and T2 were 77.9% (1388/1780) and 16.3% (291/1780), respectively. The percentage of T3-T4 was 5.7% (101/1780). The proportions of N0 and M0 tumors were 94.9% (1689/1780) and 99.7% (1775/1780), respectively. The data for all patients are displayed in Table 1.
3.2. Survival analysis and nomogram development
The median follow-up period for lip carcinoma was 42 months (0–119 months). Based on the SEER database, we obtained data of the OS and cancer-specific death of 1780 lip carcinoma patients. In the modeling group, 401 (22.5%) patients were deceased at the time of the last follow-up: 114 (6.4%) patients died of lip carcinoma and 287 (16.1%) patients died of other causes. Univariate and multivariate analyses for OS and CSS were performed with SPSS 21.0 (Tables 2 and 3). The independent prognostic risk factors affecting OS and CSS were obtained, showing statistical significance (P < .05).
Table 2.
Univariate and multivariate analyses of overall survival in nomogram cohort.

Table 3.
Univariate and multivariate analyses of cancer-specific survival in nomogram cohort.

Figure 1 shows the nomogram based on all of the above factors. SPSS software was used to analyze CSS. Univariate and multivariate survival analyses identified race, pathologic grade, surgery, T stage, and M stage as independent risk factors affecting prognosis. Furthermore, another nomogram was constructed to predict CSS for 5 and 8 years (Fig. 2). In Figure 2, a C-index value of higher than 0.7 can predict that OS and CSS conform to the actual OS and CSS. The C-index values of OS and CSS in internal validation were 0.762 and 0.787 while those in external validation increased slightly to 0.772 and 0.818. Furthermore, the internal and external calibration curves were close to the dotted line with a slope of 45°, the ideal line (Figs. 3 and 4).
Figure 1.
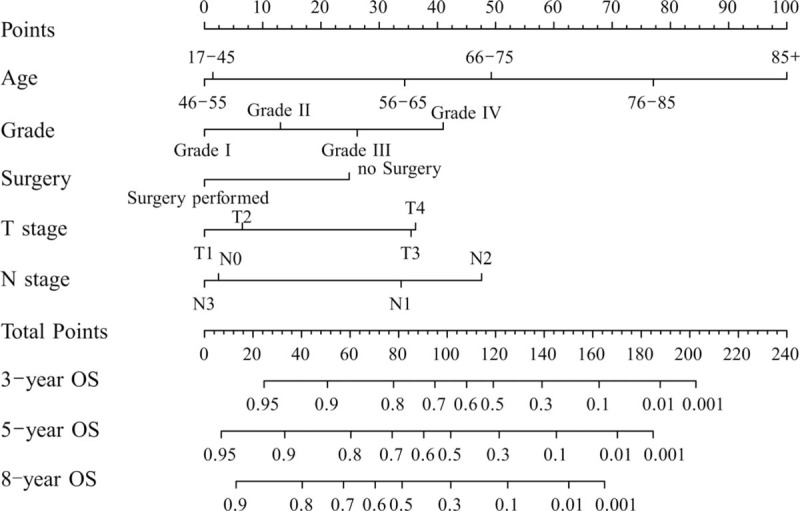
Nomogram used to predict the overall survival (OS) rate. Grades I, II, III, IV represents well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated, respectively.
Figure 2.
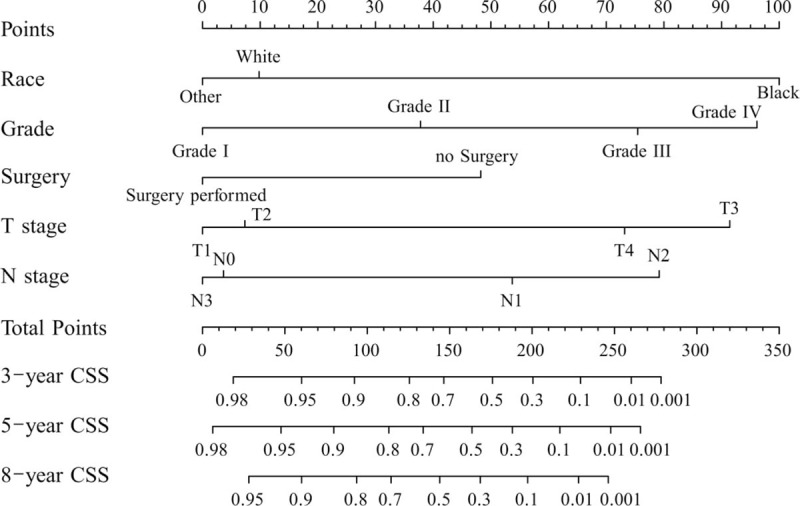
Nomogram used to predict 5- and 8-year cancer-specific survival (CSS). The category of others includes American Indian/AK Native and Asian/Pacific Islander. NSHL refers to Non-Spanish-Hispanic-Latino. Grades I, II, III, and IV represents well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated, respectively. Bootstrap resampling and 10-fold cross-validation were used to perform nomogram validation and the Harrell C-index and calibration curves were used to evaluate the internal and external nomogram accuracy.
Figure 3.

Internal calibration nomogram showing 5- and 8-year overall survival (OS) (A, B); external calibration nomogram showing 5- and 8-year OS (C, D). The 45° line is an indication that the actual survival (Y-axis) and nomogram-forecast survival (X-axis) forms an ideal match. The perpendicular line shows 95% confidence interval.
Figure 4.

Internal calibration nomogram showing 5- and 8-year cancer-specific survival (CSS) (A, B); external calibration nomogram showing 5- and 8-year CSS (C, D).
3.3. OS and CSS curves
With our OS and CSS nomograms, it was possible to calculate each patient's total score by adding up score for every factor. According to the OS nomogram of the modeling group, the total score of each patient was calculated, with the cut-off value of 76.5. The validation group was thus divided into the high-risk cohort (≥76.5) and the low-risk cohort (<76.5). The survival curve was drawn according to the Kaplan–Meier method. The log-rank test showed that the survival time of the 2 groups was significantly different (P < .001). The OS rate of the high-risk cohort was significantly lower than that of the low-risk cohort. According to the CSS nomogram, the cut-off value was 103. The validation group was also divided into a high-risk cohort (≥103) and a low-risk cohort (<103). The CSS rate of the high-risk cohort was lower than that of the low-risk cohort (P < .001) (Fig. 5).
Figure 5.

Kaplan–Meier curves for high- and low-risk group patients based on nomogram scores (A for overall survival, B for cancer-specific survival).
4. Discussion
Lip carcinoma is one of the most common head and neck malignant tumors.[10] Responsible for causing facial defects and affecting the quality of patients’ life, lip carcinoma has imposed great burdens on public medical and health care services and increasingly become a serious public health problem in many countries.[18] It has been found that males were 13 times more likely than females to suffer from lip carcinoma.[19,20] This finding has been confirmed by the data in our study. Although the OS rate of lip carcinoma patients has increased, the incidence of local lymph node involvement in patients with lip carcinoma remains between 5% and 20%. In cases of local lymph node involvement, the 5-year survival rate was only 50%.[3,4] The incidence of lip carcinoma varies significantly in countries and regions. The prognosis of lip carcinoma also varies by occupation. For instance, among the people engaged in agricultural labor, the morbidity of lip carcinoma is high and the prognosis is poor.[21] However, to date, only the 8th AJCC staging system has been used to assess the prognosis of patients with lip carcinoma, even though it is not sufficiently comprehensive.[22] Therefore, a personalized approach to prognosis assessment is critical for caregivers and patients, especially in less developed countries and areas with large agricultural population, which means that building a reliable nomogram prediction model should be a priority.
According to the univariate survival analysis, for the OS, seven independent variables (race, grade, surgery, radiation, T stage, N stage, and M stage) showed significant difference. After the Enter method was used for Cox multivariate survival analysis, the significant independent variables became age, grade, surgery, T stage, and N stage. For the CSS, on the basis of the results of univariate survival analysis, 7 independent variables (race, grade, surgery, radiation, T stage, and N stage) showed significant difference. After Cox multivariate survival analysis, the independent risk factors became race, grade, surgery, T stage, and N stage. Univariate factors without statistical differences were not included in the nomograms (Figs. 1 and 2). The estimated OS and CSS calculated via the Kaplan–Meier method is consistent with a previous report.[17] Our findings that the OS of blacks was lower than that of whites also agrees with data from 1975 to 2000 patients collected by Ries et al.[23] The reasons for this disparity in survival are unknown. Many scholars believe that it may be related to differences in biologic factors, behavioural risk factors, economic factors, social factors, and the acquisition or utilization of cancer treatment.[24] The group of 46 to 55 years old had an advantage in OS over the group of 17 to 45; however, for those over 56 years old, OS decreased with age. In the Cox multivariate analysis of CSS, there was no significant difference in terms of the age factor; therefore, the age variable was not included in the nomogram. In a multicenter study by Dhanuthai et al,[25] the age distribution of oral cancer peaked at 50 to 59 years, coinciding with our statistical data regarding lip carcinoma. However, the results of the nomogram construction showed 2 outliers. The 1st outlier appeared in the N3 stage: N3 stage patients showed better OS and CSS. The reason may be that there were only 3 patients in the N3 stage and the high survival rate of the 3 patients resulted in the abnormal phenomenon. Second, the M-stage factor was not included in the construction of the nomograms. The reason was only 3 patients were in the M1 stage. In future study, the sample size should be increased to balance this bias.
The C-index and calibration curves were employed to validate the internal nomogram precision (modeling cohort) and external nomogram accuracy (validation cohort). The internal validation's C-indexes targeting 5- and 8-year OS and CSS were 0.762 and 0.787, while those of the external validation were 0.772 and 0.818. All C-index values were higher than 0.7. Besides, the calibration curves demonstrated an excellent coherence with the 45° reference line. In addition to survival probability, the cut-off was obtained after ROC analysis, which was in accordance with values in a previous report.[26] Moreover, the high-risk cohort showed lower OS and CSS than did the low-risk cohort, and the log-rank test showed statistical significance. Many studies have applied this multivariate-based score system to evaluate prognosis.[27,28,29]
Based on these nomograms, we can predict the prognosis of lip carcinoma patients in a simple and efficient way. First, based on the clinicopathologic elements, vertical lines can be drawn to correspond to the points on the axes. With acquisition of the total points, 5- and 8-year OS and CSS can be obtained to predict the prognostic value.[30] The prediction of the nomogram regarding prognosis is more accurate and better than that of the AJCC staging manual, which can be of benefit to surgeons and patients. Take, for example, 2 equal stages of T2N1M0 lip carcinoma patients: 1 case is a 55-year-old black man with grade II after surgery; the other is a 70-year-old white female with grade III, without surgery. The 2 scores of OS were 54 and 153 points, and OS scores of 5 years were 82% and 4%, respectively. Correspondingly, their CSS scores were 198 and 193 points, and CSS scores of 5 years were 31% and 32%, respectively. Clearly, when AJCC staging was used for prognosis assessment, the 2 patients did not show any distinction; however, when the prognostic evaluation was performed using nomograms, the disparity of OS and CSS was more accurately displayed. This is of great guiding significance for surgeons and patients. Above all, accurate and personalized predictions of prognosis are the reason we devote ourselves to nomogram models.
Undeniably our study has its limitations. The factors investigated in our study included only age, gender, race, origin, grade, surgery, radiation, and TNM stage. However, several scholars have suggested that the survival of lip carcinoma patients may be influenced by other relevant pathogenetic mechanisms, such as smoking, premalignant lesions, several viruses, immunosuppression, and chronic trauma.[31,32,33] It should also be emphasized that greater exposure to sunlight makes the lower lip 12 times more likely to develop carcinoma.[34,35] That is to say, more factors should be considered to establish a more accurate and credible nomogram model to predict the prognosis of lip carcinoma.
5. Conclusion
The nomogram model successfully constructed and validated in our study can be used to predict OS and CSS in patients with lip carcinoma. We believe that these models can provide a reference for surgeons to conduct individual prognostic assessments.
Acknowledgment
The authors are very grateful to Yi Shuai for his efforts in offering scientific research guidance.
Author contributions
Conceptualization: Rui Zhao, Tingting Jia, Bo Qia, Shuang Qu, Haizhong Zhang.
Data curation: Tingting Jia, Jiawu Liang, Liang Zhu.
Formal analysis: Hang Feng, Yipeng Ren.
Funding acquisition: Haizhong Zhang.
Investigation: Rui Zhao, Tingting Jia, Bo Qia, Shuang Qu, Liang Zhu, Fengze Wang.
Methodology: Tingting Jia, Shuang Qu, Lejun Xing, Yipeng Ren.
Project administration: Yipeng Ren, Fengze Wang, Haizhong Zhang.
Resources: Bo Qia, Shuang Qu, Liang Zhu, Lejun Xing.
Software: Jiawu Liang, Shuang Qu, Liang Zhu, Lejun Xing, Yipeng Ren.
Supervision: Hang Feng, Lejun Xing, Fengze Wang, Haizhong Zhang.
Validation: Rui Zhao, Jiawu Liang, Fengze Wang.
Writing – original draft: Rui Zhao, Tingting Jia, Bo Qia, Jiawu Liang, Hang Feng.
Writing – review & editing: Fengze Wang, Haizhong Zhang.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, C-index = concordance index, CSS = cancer-specific survival, NCCN = National Comprehensive Cancer Network, OS = overall survival, SEER = Surveillance, Epidemiology, and End Results, TNM = tumor node metastasis.
RZ, TTJ, and BQ contributed equally to this work.
This research was supported by National Key R&D Program of China (Grant no: 2017YFB1304300).
The authors have no conflicts of interest to disclose.
References
- [1]. Guyon A, Bosc R, Lange F, et al. Retrospective outcome analysis of 39 patients who underwent lip surgery for cutaneous carcinoma. J Maxillofac Oral Surg 2016;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Rowe D, Gallagher RP, Warshawski L, et al. Females vastly outnumber males in basal cell carcinoma of the upper lip. A peculiar subset of high risk young females is described. J Dermatol Surg Oncol 1994;20:754–6. [DOI] [PubMed] [Google Scholar]
- [3]. Agostini T, Spinelli G, Arcuri F, et al. Metastatic squamous cell carcinoma of the lower lip: analysis of the 5-year survival rate. Arch Craniofac Surg 2017;18:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Gooris PJ, Vermey A, de Visscher JG, et al. Supraomohyoid neck dissection in the management of cervical lymph node metastases of squamous cell carcinoma of the lower lip. Head Neck 2002;24:678–83. [DOI] [PubMed] [Google Scholar]
- [5]. Kristensen RN, Stemann AP, Lock-Andersen J. Lip carcinoma: clinical presentation, surgical treatment, and outcome: a series of 108 cases from Denmark. J Plast Surg Hand Surg 2017;51:342–7. [DOI] [PubMed] [Google Scholar]
- [6]. Pfister DG, Spencer S, Brizel DM, et al. Head and neck cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Moeckelmann N, Ebrahimi A, Tou YK, et al. Prognostic implications of the 8th edition American Joint Committee on Cancer (AJCC) staging system in oral cavity squamous cell carcinoma. Oral Oncol 2018;85:82–6. [DOI] [PubMed] [Google Scholar]
- [8]. Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol 2009;45:454–60. [DOI] [PubMed] [Google Scholar]
- [9]. Weinberger PM, Merkley M, Lee JR, et al. Use of combination proteomic analysis to demonstrate molecular similarity of head and neck squamous cell carcinoma arising from different subsites. Arch Otolaryngol Head Neck Surg 2009;135:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009;45:309–16. [DOI] [PubMed] [Google Scholar]
- [11]. Li J, Liu Y, Yan Z, et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer 2014;110:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Liu J, Geng Q, Liu Z, et al. Development and external validation of a prognostic nomogram for gastric cancer using the national cancer registry. Oncotarget 2016;7:35853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Cho JK, Lee GJ, Yi KI, et al. Development and external validation of nomograms predictive of response to radiation therapy and overall survival in nasopharyngeal cancer patients. Eur J Cancer 2015;51:1303–11. [DOI] [PubMed] [Google Scholar]
- [14]. Wen J, Feng Y, He X, et al. Development and validation of a prognostic nomogram based on the log odds of positive lymph nodes (LODDS) for breast cancer. Oncotarget 2016;7:21046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw 2003;5:714–36. [PubMed] [Google Scholar]
- [16]. Rao SJ. Regression Modeling strategies: with applications to linear models, logistic regression, and survival analysis. Publ Am Stat Assoc 2005;98:257–8. [Google Scholar]
- [17]. Zumsteg ZS, Cookwiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol 2016;2:1617–23. [DOI] [PubMed] [Google Scholar]
- [18]. Souza RL, Fonseca-Fonseca T, Oliveira-Santos CC, et al. Lip squamous cell carcinoma in a Brazilian population: epidemiological study and clinicopathological associations. Med Oral Patol Oral Cir Bucal 2011;16:e757–62. [DOI] [PubMed] [Google Scholar]
- [19]. Kocatürk S, Ozdemir N, Erkam U, et al. Evaluation of occult lymph node metastasis in lower lip cancers and approach to N(0) neck metastasis [in Turkish]. Kulak Burun Bogaz Ihtis Derg 2002;9:41–5. [PubMed] [Google Scholar]
- [20]. Altinyollar H, Bulut H, Berberoglu U. Is suprahyoid dissection a diagnostic operation in lower lip carcinoma? J Exp Clin Cancer Res 2002;21:29–30. [PubMed] [Google Scholar]
- [21]. Alves AM, Correa MB, Silva K, et al. Demographic and clinical profile of oral squamous cell carcinoma from a service-based population. Braz Dent J 2017;28:301–6. [DOI] [PubMed] [Google Scholar]
- [22]. Lydiatt W, Sullivan O, Patel S B. Major changes in head and neck staging for 2018. Am Soc Clin Oncol Educ Book 2018;38:505–14. [DOI] [PubMed] [Google Scholar]
- [23]. Ries LA GEM, Kosary CL, Hankey BF, et al. (Eds.) SEER Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute; 2003. Available at: http://seer.cancer.gov/csr/1975_2000 Accessed June 2004. [Google Scholar]
- [24]. Tomar SL, Loree M, Logan H. Racial differences in oral and pharyngeal cancer treatment and survival in Florida. Cancer Causes Control 2004;15:601–9. [DOI] [PubMed] [Google Scholar]
- [25]. Dhanuthai K, Rojanawatsirivej S, Thosaporn W, et al. Oral cancer: a multicenter study. Med Oral Patol Oral Cir Bucal 2018;23:e23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Zhang B, Yuan Z, Zhao L, et al. Nomograms for predicting progression and efficacy of post-operation radiotherapy in IIIA-pN2 non-small cell lung cancer patients. Oncotarget 2017;8:37208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Bobdey S, Balasubramaniam G, Mishra P. Nomogram prediction for survival of patients with oral cavity squamous cell carcinoma. Head Neck 2016;38:1826–31. [DOI] [PubMed] [Google Scholar]
- [28]. Ribeiro KC, Kowalski LP, Latorre MR. Impact of comorbidity, symptoms, and patients’ characteristics on the prognosis of oral carcinomas. Arch Otolaryngol Head Neck Surg 2000;126:1079–85. [DOI] [PubMed] [Google Scholar]
- [29]. Luo XL, He W, Huang H, et al. Design of a prognostic score model for nasopharyngeal carcinoma. Head Neck 2015;37:624–9. [DOI] [PubMed] [Google Scholar]
- [30]. Wang F, Zhang H, Wen J, et al. Nomograms forecasting long-term overall and cancer-specific survival of patients with oral squamous cell carcinoma. Cancer Med 2018;7:943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am 2001;34:647–66. [DOI] [PubMed] [Google Scholar]
- [32]. Boda D, Neagu M, Constantin C, et al. HPV strain distribution in patients with genital warts in a female population sample. Oncol Lett 2016;12:1779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Lupu M, Caruntu A, Caruntu C, et al. Non-invasive imaging of actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin Oncol 2018;8:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Veness MJ, Ong C, Cakir B, et al. Squamous cell carcinoma of the lip. Patterns of relapse and outcome: reporting the Westmead Hospital experience, 1980–1997. J Med Imaging Rad Oncol 2015;45:195–9. [DOI] [PubMed] [Google Scholar]
- [35]. Koç C, Akyol M U, Celikkanat S, et al. Role of suprahyoid neck dissection in the treatment of squamous cell carcinoma of the lower lip. Ann Otol Rhinol Laryngol 1997;106:787–9. [DOI] [PubMed] [Google Scholar]


