Supplemental Digital Content is available in the text
Keywords: meta-analysis, selenium, sepsis
Abstract
Background:
To understand the clinical outcomes of selenium therapy in patients with sepsis syndrome, we conducted a meta-analysis of randomized controlled trials (RCT).
Methods:
A total of 13 RCTs comparing selenium and placebo for patients with sepsis were reviewed systematically.
Results:
However, we could not detect the association of selenium treatment with a decreased mortality at different time course (relative risk [RR] [95% confidence interval, CI]: 0.94 [0.82–1.06] at day 28; 0.73 [0.36–1.47] at day 90; 1.16 [0.78–1.71] at 6 months; respectively). Selenium supplementation did not show favorable efficacy in the incidence of renal failure, secondary infection or duration of mechanical ventilation (RR [95% CI]: 0.65 [0.41–1.03]; 0.96 [0.87–1.06]; standard mean difference [SMD] [95% CI]: 0.17 [−0.30–0.63]; respectively). Interestingly, we found that selenium therapy was benefit for sepsis patients with reduced duration of vasopressor therapy, staying time in intensive care unit and hospital, and incidence of ventilator-associated pneumonia (SMD [95% CI]: −0.75 [−1.37 to −0.13]; −0.15 [CI: −0.25 to −0.04]; −1.22 [−2.44 to −0.01]; RR [95% CI]: 0.61 [0.42–0.89]; respectively).
Conclusion:
Based on our findings, intravenous selenium supplementation could not be suggested for routine use.
1. Introduction
Sepsis is a life-threatening complication of an infection. During sepsis, the excessive reactive oxygen molecules and/or depletion antioxidants contribute to the dysfunction of the cells and sepsis-related deaths.[1] Some processes are involved in the pathophysiology of sepsis and oxidative stress is suggested to play a vital role.[1,32,33] Selenium is reported as an important trace element to this antioxidation process.[2–4] Selenium-containing proteins, such as thioredoxin reductases and glutathione peroxidases, are essential antioxidant enzymes to remove harmful reactive oxygen species.[3,5,6] In recent research, selenium may be involved in the immune response and regulation of thyroid hormones.[7,8] In addition, the selenium deficiency is often observed in a patient with sepsis, especially those accompanied with diets of inferior quality, including chronic disease, critical illness, and gastrointestinal illness.[9,33] Selenium is seemed to be great important in some conditions of the systemic inflammatory response, and highly oxidative stress may play an essential role in the progress of complications.[3,4,10–12]
In the current decades, series clinical trials have evaluated the effect of seleno-compounds in patients with systemic inflammation.[13–16] They included selenious acid and sodium selenite and their combination with another antioxidant therapy was also observed. Previous evidence suggests that in patients with sepsis syndromes, a decreased selenium level may be associated with increased mortality and incidence of organ failure, with enhanced levels of reactive oxygen markers.[7,13–14] However, published systematic reviews revealed an inconsistent conclusion about the efficacy of selenium supplementation on mortality in sepsis patients.[17–20] Therefore, we retrieved the systematic review to investigate the clinical outcomes of selenium supplementation in the populations of the sepsis syndrome.
2. Material and methods
2.1. Identification of trials
We performed a literature search in MEDLINE (1950 to June 2018), EMBASE (1974 to June, 2018) and the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 6, 2018), using the following terms: “critical illness,” “sepsis,” “sepsis syndrome,” “septic,” “severe sepsis,” “systemic inflammatory response syndrome,” “sepsis shock,” “selenium,” “selenium compounds,” “sodium selenite.” We also screened the reference of included trials and related reviews and searched on ClinicalTrials.gov to include additional studies. A detail study protocol was uploaded on PROSPERO (CRD42018102706).
2.2. Selection criteria
Among the retrieved results, only reporting clinical trials (RCT) involving humans were included. Other inclusion criteria included: study population of sepsis, severe sepsis, sepsis shock or systemic inflammatory response syndrome (SIRS); intravenous selenium supplementation (either with or without loading dose) with a placebo control; mortality at day 28, mortality at day 90 and mortality at 6 months; incidence of renal failure, duration of vasopressor therapy, duration of mechanical ventilation, staying course of intensive care unit (ICU) and hospital, secondary infection, incidence of ventilator-associated pneumonia (VAP) and serious adverse events. If the studies with other antioxidant agents’ treatment, the studies about patients with burns, trauma, or undergoing major surgery, or the studies only with abstract would be excluded. No language restrictions were applied.
2.3. Data extraction and risk of bias assessment
All eligible reported were screened by 2 investigators (SL and LT) independently and abstracts, data integrity, references, and methodological quality were evaluated using a data abstraction form. If data were incomplete data, emails were sent to the authors for supplemental information. The risk of bias and methodological quality were assessed using the Cochrane risk bias tool. Any disagreement was resolved through consensus or referred to a third investigator (QL).
2.4. Statistical analysis
For dichotomous outcomes, the Mantel–Haenszel method was used to estimate the relative risk (RR) with 95% confidence interval (CI). For continuous outcomes, the inverse variance was employed to estimate standard mean difference (SMD) with 95% CI. Statistical heterogeneity was quantified by Mantel–Haenzel chi-square test and the I2 statistic. Significant heterogeneity was suggested as P < .10 or I2 > 50%. The subgroup analysis was disaggregated by loading dose or not, the published years of included trials, type of participants of individual trials or set of eligible trials. Publication bias was evaluated by funnel plots, and asymmetry of funnel plot was measured by the Egger test.[21,22] Data analyses were conducted using Review Manager Software (RevMan 5.3) and Stata (version 14, Stata Corp, College Station, TX).
3. Results
3.1. Description of studies
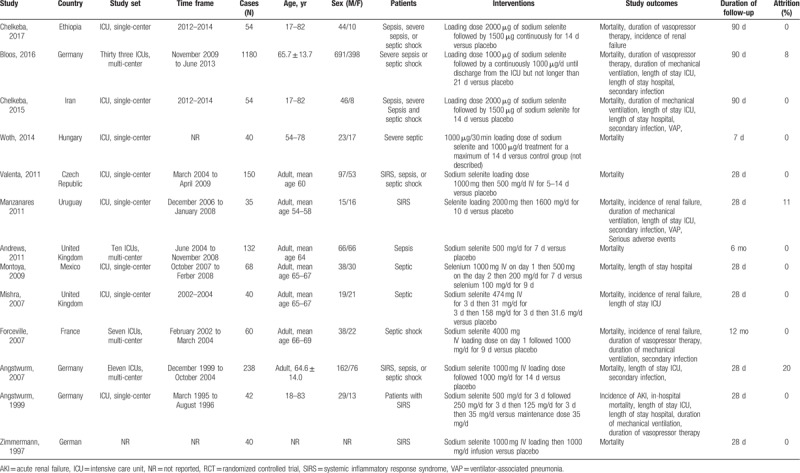
We retrieved a total of 291 potentially relevant reports by our electronic searches, which included 237 unique studies after deduplicating (Fig. 1). Based on the included criteria, 224 articles were excluded after the review process. Ultimately, 13 articles with 1922 individuals published from 1997 to 2017 were selected for inclusion in our meta-analysis.[13–16,23–31] The treatment duration of studies varied from 7 days to 21 days and a loading dose of selenium was administered in 9 trials (Table 1). Four studies were conducted at a multicenter and 9 were single-center studies. The patients with septic shock and SIRS were studied in 1 and 3 studies, respectively. The others included patients with sepsis, severe sepsis or septic shock. The age of patients studied ranged from 17 years old to 83 years old and majorities (60%) were male. The sample size ranged from 35 to 1180 (mean, 161). Regarding the quality of studies, 50% of the studies were patient-blinded, 50% had incomplete outcome data, 10% were outcome-blinded, and 25% were allocation-concealed. A high risk of bias of studied included was shown (Fig. S1 and Fig. S2).
Figure 1.
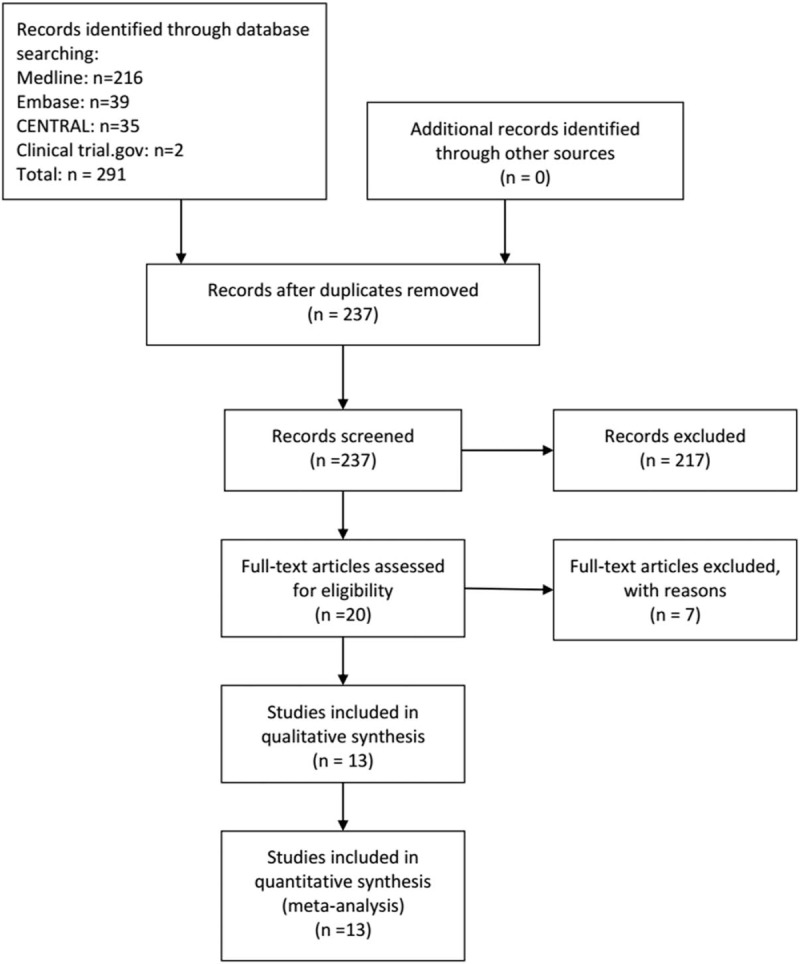
Selection process of trials.
Table 1.
Descriptive characteristics of studies included in the meta-analysis.
3.2. Mortality
Thirteen RCTs with a total population of 1922 patients with sepsis reported data on mortality at day 28,[13–16,24–31] 2 studies with 1143 patients of sepsis reported data on mortality at day 90[13,23] and 1 studies with 50 patients of sepsis reported data on mortality at 6 months.[30]
At day 28, selenium treatment was not associated with a decreased mortality when compared to placebo (RR = 0.94, 95% CI: 0.82–1.06) (Fig. 2). As no significant heterogeneity in the eligible trials, the fixed-effect model was used (I2 = 0%, P = .47). No matter loading dose selenium used or not, there was no significant difference on mortality at day 28 between subgroups (RR = 0.94, 95% CI: 0.81–1.08; RR = 0.94, 95% CI: 0.69–1.27, respectively) used fixed-effect model (I2 = 12%, P = .33; I2 = 0%, P = .45, respectively) (Fig. S3).
Figure 2.
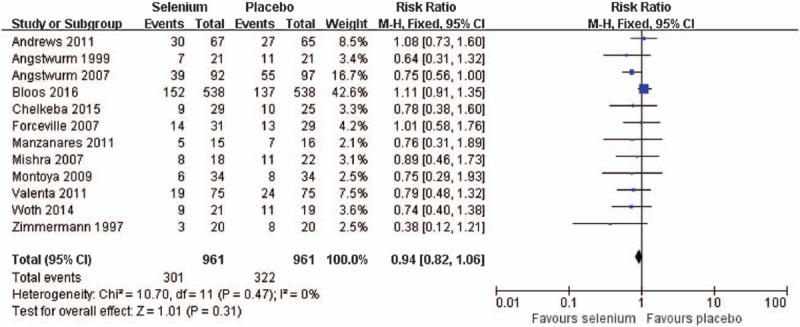
Effect of selenium versus placebo on the mortality at day 28.
At day 90, pooled analyses showed similar mortality between the selenium and control group (RR = 0.73, 95% CI: 0.36–1.47) (Fig. S4). The random-effect model was used due to significant heterogeneity in the eligible trials (I2 = 83%, P = .01).
At 6 months, meta-analyses showed no significant effect of the selenium treatment, when compared with placebo (RR = 1.16, 95% CI: 0.78–1.71) with a fixed-effect model (Fig. S5).
3.3. Other clinical outcomes
Five trials[16,23,27,29,30] with 227 patients with sepsis reported data on the incidence of renal failure, and selenium supplementation were not associated with reduced incidence of renal failure compared with control group (RR = 0.65, 95% CI: 0.41–1.03), used a fixed effect model (I2 = 0%, P = .59) (Fig. 3).
Figure 3.
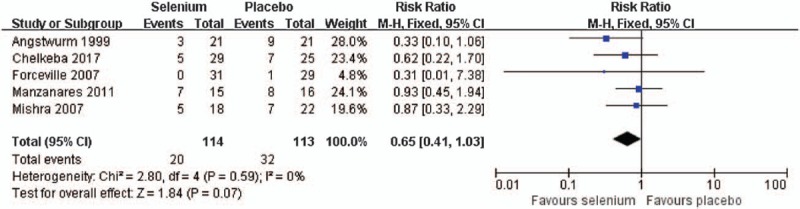
Effect of selenium versus placebo on the incidence of renal failure.
A pooled analysis of 4 trials[13,16,23,30] with 1246 sepsis patients shown that selenium treatment was associated with reduced duration of vasopressor therapy (SMD = −0.75, 95% CI: −1.37 to −0.13; Fig. 4) with the random effect model (I2 = 87%, P < .0001). When data from 5 trials about mechanical ventilation[13,16,24,27,30] were included, selenium supplementation showed similar effect with placebo (SMD = 0.17, 95% CI: −0.30 to 0.63) using a random effect model (I2 = 80%, P = .0005) (Fig. S6).
Figure 4.
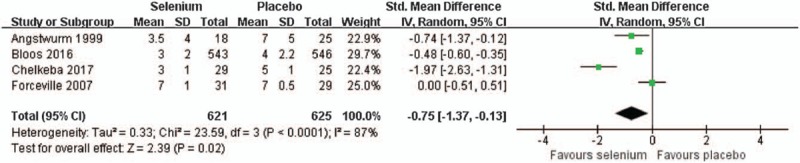
Effect of selenium versus placebo on the duration of vasopressor therapy.
The aggregated results of the 6 trials[13,15,16,24,27,29] with 1445 patients shown that selenium supplementation was associated with reduced the staying time in ICU (SMD = −0.15, 95% CI: −0.25 to −0.04) with a fixed effect model (I2 = 57%, P = .04) (Fig. S7). Four trials[13,16,24,28] with 1253 patients on staying time in hospital, and selenium supplementation was not associated with reduced this time (SMD = −1.22, 95% CI: −2.44 to −0.01) (Fig. S8) and the random effect model was used (I2 = 96%, P < .0001).
A meta-analysis of 5 trials[13,15,24,27,30] with 1472 patients shown that selenium treatment was not associated with reduced secondary infection compared with placebo (RR = 0.96, 95% CI: 0.87–1.06), used the fixed effect model (I2 = 38%, P = .17) (Fig. S9).
The aggregated results of the 2 trials[24,27] with 85 patients shown that selenium supplementation was associated with reduced incidence of VAP compared with placebo (RR = 0.61, 95% CI: 0.42–0.89), and the fixed effect model was used (I2 = 0%, P = .53) (Fig. S10).
Two RCTs[27,30] with a total population of 91 patients with sepsis reported data on serious adverse events, included respiratory failure, refractory shock, and acute kidney injury. The aggregated results shown, between the selenium and control group, that there were no significant different on incidence of respiratory failure, refractory shock or acute kidney injury (RR = 3.45, 95% CI: 0.75–15.90; RR = 0.85, 95% CI: 0.34–2.14; RR = 0.84, 95% CI: 0.41–1.72; respectively) (Fig. S11). The fixed effect model was used (I2 = 0%, P = .62; I2 = 0%, P = .77; I2 = 0%, P = .52; respectively).
3.4. Sensitivity analyses and Publication bias
Based on the published years of included trials, we conducted a subgroup analysis and showed that before 2001 selenium supplementation was associated with reduced mortality at day 28 (RR = 0.36, 95% CI: 0.14–0.95), while this associate could not be observed from 2012 to 2011 and from 2012 to recently (RR = 0.76, 95% CI: 0.55–1.04; RR = 1.09, 95% CI: 0.84–1.40; respectively) (Fig. S12). Moreover, selenium treatment decreased mortality at day 28 in the subgroup of patients with SIRS than placebo (RR = 0.43, 95% CI: 0.19–0.96). But, no significant inter-group difference in mortality was found among the subgroup patients with sepsis shock or with sepsis, severe sepsis and sepsis shock at day 28 (RR = 0.95, 95% CI: 0.77–1.16; RR = 1.01, 95% CI: 0.37–2.80; respectively) (Fig. S13). In addition, pooled analysis of trials of single-center shown that selenium treatment was associated with reduced mortality at day 28 compared with placebo (RR = 0.62, 95% CI: 0.41–0.92), but not in multi-center trials (RR = 1.02, 95% CI: 0.82–1.28) (Fig. S14).
No evidence of potential publication bias was shown by Egger test and Begg test (P = .133 and P = .837, respectively, Fig. 5).
Figure 5.
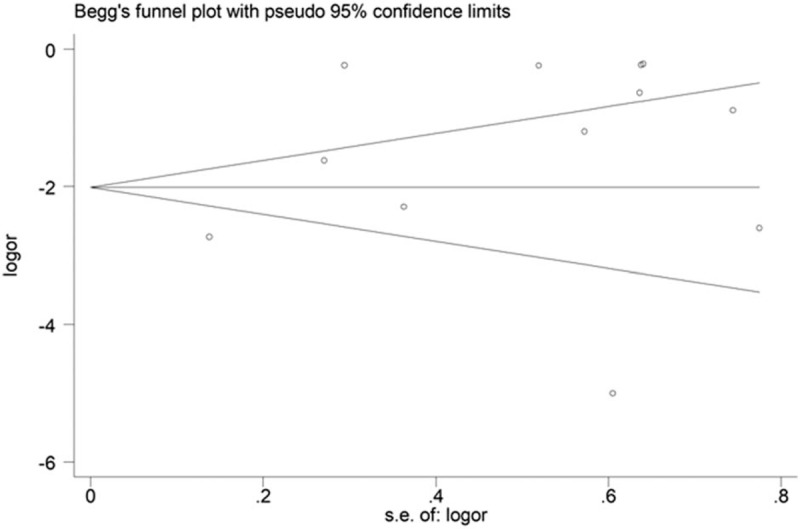
Begg funnel plot.
4. Discussion
Our analysis included 13 trials, with 1922 individuals with sepsis and randomly assigned to selenium or placebo group. Generally, selenium supplementation shows no effect on decreased mortality of adult's patients with sepsis. Interestingly, some subgroups, such as the study published before 2001, SIRS patients, single-center studies, show that selenium can reduce mortality in patients with sepsis. Additionally, patients may benefit from selenium treatment with less duration of vasopressor therapy and shorter staying time in ICU and hospital, but not in the incidence of renal failure and serious adverse events, duration of mechanical ventilation or secondary infection. In addition, our results show that the safety of selenium is acceptable.
In contrast to previous systematic reviews, this meta-analysis included 4 additional RCTs and 1 of them was a multi-center large sample trial. We analyzed overall mortality based on the follow-up period, and reversed the secondary clinical outcomes including safety data. But, a previous systematic review showed that selenium treatment reduced in day 28 mortality, and less secondary clinical outcomes were defined.[19] Another published meta-analysis focused population of critically ill patients included 21 RCTs with 4044 patients. It showed that selenium does not improve clinical outcomes.[17] The applicability of our results is only for adults with sepsis, and there is inadequate evidence to support this intervention for routine use. It is well known that the RCT is the strictest method because it is usually used to determine the causal relationship between treatment and outcome. The randomization process is the strength of RCT. None of the included studies was reported with overall low bias risk. Nine of the 13 studies were at high bias risk in the generation of random sequence. Only 4 studies registered in the database and only 2 trials published the study protocol, and we had deemed 9 of 13 studies at high reporting bias risk. Therefore, we should interpret the results with prudence as the overall high bias risk of included studies. From what has been discussed above, the evidence quality of this study was low.
Some limitations of existing evidence should be acknowledged. First, this report included patients with sepsis syndrome at few countries and regions, and with different study set, with different baseline characteristics; all of which could have resulted in the heterogeneity. Second, methodologic quality was low or unclear in most of the eligible studies. The published years of included trials or the study setting of single-center or multi-center revealed different results, suggesting that the possible benefit is unclear among different study time or different setting, even though difference of this subgroup was statistically significant. Thirdly, the diagnosis of all trials is based on sepsis 1.0 or 2.0. It is worth to expect whether there will be a different clinical outcome based on the new diagnostic criteria of sepsis. Fourth, most of the eligible trials were small sample-size studies, and few trials reported more outcomes in relation to a clinical endpoint. Although it is needed to carry out high-quality RCTs recruiting a larger number of patients with sepsis syndrome, and reporting more outcomes in connection with a clinical endpoint, for made stronger conclusions, performing RCTs in critically ill patients may be faced with ethical challenges. Moreover, on account of the limited availability of data, relevant adverse effects associated with treatment could not be fully evaluated. Additionally, there is a need to conduct longer follow-up trial to evaluate the influence on mortality and other clinical outcomes. Last, most of the eligible trials did not access the cost-effectiveness of selenium supplementation, which is important to identify feasibility, specifically in developing countries.
5. Conclusion
In conclusion, our analyses shown the effect of selenium supplementation on duration of vasopressor therapy, staying time in ICU and hospital, incidence of VAP, but we failed to find the effect on mortality, either with loading does or not, and on a reduction in the incidence of renal failure, duration of mechanical ventilation, and incidence of secondary infection. And, there was not obvious harmful effect of the treatment among sepsis patients. Thus, intravenous selenium supplementation could not be suggested to routine use for serious sepsis patients. Moreover, well-designed, large-sample studies of selenium therapy, with low bias risk, according to the CONSORT statement, should be conducted. Detail clinical outcomes, longer period follow-up period, and life quality of patient and cost-effectiveness should be assessed.
Author contributions
Every author was responsible for the content of this manuscript and approved to submit. The study was designed by SL and LT. The search strategies were conducted by QZ. The selection of study, assessment of validity, and extraction of data were done by PG and XA. TT and LH entered and analyzed the data.
Conceptualization: Pengfei Guo.
Data curation: Tian Tang.
Formal analysis: Shaojun Li, Pengfei Guo.
Funding acquisition: Liping Tan.
Investigation: Tian Tang, Lan Hu.
Project administration: Liping Tan.
Software: Shaojun Li, Qing Zou.
Validation: Qing Zou.
Writing – original draft: Shaojun Li.
Writing – review and editing: Xiaoxiao Ao.
Liping Tan orcid: 0000-0003-1804-2746.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, ICU = intensive care unit, RCT = randomized controlled trials, RR = relative risk, SIRS = systemic inflammatory response syndrome, SMD = standard mean difference, VAP = ventilator-associated pneumonia.
This research was supported by the projects of basic and frontier research, Chongqing Science and Technology Commission (Fund number: cstc2014jcyjA10032).
The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Mantzarlis K, Tsolaki V. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev 2017;2017:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Uhle F, Chousterman BG, Grützmann R, et al. Pathogenic, immunologic, and clinical aspects of sepsis–update 2016. Expert Rev Anti Infect Ther 2016;14:917–27. [DOI] [PubMed] [Google Scholar]
- [3].Somlaw N, Lakananurak N, Dissayabutra T, et al. Antioxidant vitamins and trace elements in critical illness. PloS One 2016;31:457–74. [DOI] [PubMed] [Google Scholar]
- [4].Van Zanten AR. Glutamine and antioxidants: status of their use in critical illness. Curr Opin Clin Nutr Metab Care 2015;18:179–86. [DOI] [PubMed] [Google Scholar]
- [5].Geoghegan M, McAuley D, Eaton S, et al. Selenium in critical illness. Curr Opin Crit Care 2006;12:136–41. [DOI] [PubMed] [Google Scholar]
- [6].Angstwurm MW, Gaertner R. Practicalities of selenium supplementation in critically ill patients. Curr Opin Clin Nutr Metab Care 2006;9:233–8. [DOI] [PubMed] [Google Scholar]
- [7].Vavrova L, Rychlikova J, Mrackova M, et al. Increased inflammatory markers with altered antioxidant status persist after clinical recovery from severe sepsis: a correlation with low HDL cholesterol and albumin. Clin Exp Med 2016;16:557–69. [DOI] [PubMed] [Google Scholar]
- [8].Annetta MG, Pittiruti M, Vecchiarelli P, et al. Immunonutrients in critically ill patients: an analysis of the most recent literature. Minerva Anestesiol 2016;82:320–31. [PubMed] [Google Scholar]
- [9].Duntas LH, Benvenga S. Selenium: an element for life. Endocrine 2015;48:756–75. [DOI] [PubMed] [Google Scholar]
- [10].Schomburg L. Selenium in sepsis–substitution, supplementation or pro-oxidative bolus? Crit Care 2014;18:444–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manzanares W, Langlois PL, Hardy G. Update on antioxidant micronutrients in the critically ill. Curr Opin Clin Nutr Metab Care 2013;16:719–25. [DOI] [PubMed] [Google Scholar]
- [12].Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2012;16:705–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bloos F, Trips E, Nierhaus A, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med 2016;176:1266–76. [DOI] [PubMed] [Google Scholar]
- [14].Andrews PJ, Avenell A, Noble DW, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:1–8. [DOI] [PubMed] [Google Scholar]
- [15].Angstwurm MW, Engelmann L, Zimmermann T, et al. Selenium in intensive care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 2007;35:118–26. [DOI] [PubMed] [Google Scholar]
- [16].Angstwurm MW, Schottdorf J, Schopohl J, et al. Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 1999;27:1807–13. [DOI] [PubMed] [Google Scholar]
- [17].Manzanares W, Lemieux M, Elke G, et al. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care 2016;20:356–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Landucci F, Mancinelli P, De Gaudio AR, et al. Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care 2014;29:150–6. [DOI] [PubMed] [Google Scholar]
- [19].Kong Z, Wang F, Ji S, et al. Selenium supplementation for sepsis: a meta-analysis of randomized controlled trials. Am J Emerg Med 2013;31:1170–5. [DOI] [PubMed] [Google Scholar]
- [20].Huang TS, Shyu YC, Chen HY, et al. Effect of parenteral selenium supplementation in critically ill patients: a systematic review and meta-analysis. PloS One 2013;8:e54431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287–98. [DOI] [PubMed] [Google Scholar]
- [22].Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ 1998;316:469–71. [PMC free article] [PubMed] [Google Scholar]
- [23].Chelkeba L, Ahmadi A, Abdollahi M, et al. The effect of high-dose parenteral sodium selenite in critically ill patients following sepsis: A clinical and mechanistic study. Indian J Crit Care Med 2017;21:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chelkeba L, Ahmadi A, Abdollahi M, et al. The effect of parenteral selenium on outcomes of mechanically ventilated patients following sepsis: a prospective randomized clinical trial. Ann Intensive Care 2015;5:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Woth G, Nagy B, Merei A, et al. The effect of Na-selenite treatment on the oxidative stress-antioxidants balance of multiple organ failure. J Crit Care 2014;29: 883.e7-11. [DOI] [PubMed] [Google Scholar]
- [26].Valenta J, Brodska H, Drabek T, et al. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med 2011;37:808–15. [DOI] [PubMed] [Google Scholar]
- [27].Manzanares W, Biestro A, Torre MH, et al. High-dose selenium reduces ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation. Intensive Care Med 2011;37:1120–7. [DOI] [PubMed] [Google Scholar]
- [28].Montoya C, Hernández A, Antonio J. Efecto antiinflamatorio del selenio en pacientes sépticos. Rev Asoc Mex Med Crit y Ter Int 2009;23:199–205. [Google Scholar]
- [29].Mishra V, Baines M, Perry SE, et al. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr 2007;26:41–50. [DOI] [PubMed] [Google Scholar]
- [30].Forceville X, Laviolle B, Annane D, et al. Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care 2007;11:R73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zimmermann T, Albrecht S, Kuhne H, et al. Selenium administration in patients with sepsis syndrome. A prospective randomized study. Med Klin 1997;92Suppl 3:3–4. [DOI] [PubMed] [Google Scholar]
- [32].Yorulmaz H, Ozkok E, Kaptan E, et al. Therapeutic effects of simvastatin on Galectin-3 and oxidative stress parameters in endotoxemic lung tissue. Biosci Rep 2018;38:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Belsky JB, Wira CR, Jacob V, et al. A review of micronutrients in sepsis: the role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr Res Rev 2018;31:281–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


