Supplemental Digital Content is available in the text.
Background.
Normothermic machine perfusion (NMP) may allow resuscitation and improved assessment of kidneys before transplantation. Using discarded human kidneys, we investigated the mechanistic basis and translational potential of NMP compared with cold static storage (CS).
Methods.
Discarded deceased donor kidneys (n = 15) underwent 1-hour NMP following CS. Renal perfusion, biochemical, and histologic parameters were recorded. NMP was directly compared with CS in paired donor kidneys using simulated transplantation with allogeneic whole blood, followed by assessment of the aforementioned parameters, in addition to RNA sequencing.
Results.
Kidneys were successfully perfused, with improved renal blood flows and resistance over the course of perfusion, and evidence of urine output (median 21 mL), in all but one kidney. NMP completely resolved nonperfused regions in discarded donation after circulatory death kidneys. In paired kidneys (n = 4 pairs), transcriptomic analyses showed induction of stress and inflammatory pathways in NMP kidneys, with upregulation of pathways promoting cell survival and proliferation. Furthermore, the NMP pairs had significantly better renal perfusion (1.5–2 fold improvement) and functional parameters, and amelioration of cell death, oxidative stress, and complement activation.
Conclusions.
In this pilot preclinical study using simulated transplantation of paired kidneys, NMP of discarded marginal kidneys demonstrated some significant mechanistic benefits in comparison to CS alone. NMP may have potential to reduce organ discards and enhance early graft function in such kidneys.
Normothermic machine perfusion (NMP) is a technique that has recently been reapplied to deceased donor kidney preservation before transplantation, with the potential to enhance kidney transplant outcomes.1-4 Hypothermic machine perfusion (HMP) has been the dominant form of MP in kidney transplantation; however, it has not gained universal acceptance because its benefits are primarily in reducing delayed graft function, with equivocal, if any, impact on graft survival.5-8 Furthermore, there is limited evidence that HMP can increase utilization of marginal organs or significantly impact graft outcomes. NMP, however, has the dual potential of improving organ function post transplantation, and also allows for accurate functional assessment in a near-physiologic state, helping to improve kidney utilization and outcomes from marginal grafts.3,4,9-11 Furthermore, NMP enables the directed delivery of therapeutics to the kidney during perfusion while metabolic processes are active.12
Preliminary evidence suggests the superiority of NMP over the current gold standard of cold static storage (CS) alone, and a randomized control trial (RCT) is currently underway to compare both techniques.1,13 Nevertheless, many questions remain unanswered before the widespread uptake of NMP. Little is known about how NMP may improve graft outcomes, with sparse evidence limited to porcine studies.14,15 Understanding molecular benefits of NMP is crucial to more clearly inform clinicians as how best to utilize the technology. Current clinical evidence exists only for 1 hour of preimplantation NMP,1,2,16 but experimental data indicate that longer periods of NMP (>8–24 h) may be more beneficial to subsequent transplant function.3,17,18 Brief (1–3 h) preimplantation NMP, however, remains most attractive, as it is much more clinically feasible.
The primary aim of this study was to investigate the comparative efficacy of brief NMP to CS alone, using paired human kidneys, with a particular focus on the mechanistic changes that underlie any potential advantages offered by NMP. We also examined the following parameters that have not been clearly investigated using human kidneys—(1) biochemical, acid-base, and perfusion-related trends during NMP; (2) passenger leukocyte load of donor kidneys and the use of NMP to induce extravasation of these leukocytes; and (3) the comparative efficacy of NMP with autologous or banked (allogeneic) blood.
MATERIALS AND METHODS
A detailed description of study methods can be found in Methods S1 (SDC, http://links.lww.com/TXD/A226).
Ethics
Ethics approval for this project was obtained from the Western Sydney Local Health District human research ethics committee.
Inclusion and Exclusion Criteria
Kidneys were obtained for the purposes of this research from any deceased donor if—(1) they were deemed unsuitable for transplantation for any reason during or after procurement or (2) the kidneys had been deemed medically unsuitable before retrieval in the context of a planned liver-only donor. Kidneys were only excluded from subsequent NMP when autologous or allogeneic blood was not available for perfusion.
Kidney Procurement and Donor Details
Retrieval was undertaken in a standard fashion, after aortic cannulation and cold perfusion with Soltran (kidney-only donor) and/or University of Wisconsin (UW) solution (liver/pancreas donors). If autologous blood was to be utilized for subsequent NMP, it was collected via the inferior vena cava immediately upon commencement of cold perfusion. Kidneys were stored in the final flush solution (UW or Soltran), surrounded by 0.9% sodium chloride ice slush, before transportation to our center. Kidneys remained on ice slush until the commencement of NMP.
Donor/retrieval details that were recorded included age, sex, comorbidities, donation pathway (donation after brain death [DBD] or donation after circulatory death [DCD]), ABO blood group, kidney donor profile index (KDPI),19,20 donor cause of death, intended and actual organs retrieved, reason for kidney discard/nonutilization, cross-clamp time, warm ischemic time (WIT), cold ischemic time (CIT), and kidney anatomy.
Blood Preparation
Packed red blood cells (PRBCs) were isolated from autologous donor whole blood as detailed in Methods S1 (SDC, http://links.lww.com/TXD/A226). O+ or O- PRBCs (1 unit) were utilized for NMP experiments employing allogeneic (banked) blood. All subsequent simulated transplantation experiments were conducted using whole blood-bank blood (O+ or O-). The total volume of each whole blood unit was approximately 500 mL, with 250 mL of this used for each paired kidney (see below).
Ex Vivo Perfusion Set-Up
The NMP system was assembled as previously described; a schematic diagram of the perfusion set-up can also be found in this reference.21 A continuous supply of oxygen, delivered as 95% oxygen/5% carbon dioxide, was delivered to the oxygenator at a flow rate of 1.5 L/min. Creatinine was added to the circuit (Merck, Darmstadt, Germany) to enable subsequent quantification of creatinine clearance (CrCl). The kidney was placed in a customized, 3D-printed perfusion chamber, with the renal vein left open.22 Urine output (UO) was replaced with Hartmann’s solution. NMP was undertaken at a temperature of 37°C, with flow rates adjusted to maintain at a mean arterial pressure (MAP) of 75–85 mm Hg.
To provide a direct comparison between CS and NMP in the absence of the ability to transplant these kidneys, ex vivo reperfusion with whole blood was undertaken in paired kidneys to simulate transplantation (MAP 85–95 mm Hg and temperature 37°C).15,23,24 Perfusion parameters, additives, and constituents in both the NMP and ex vivo whole blood reperfusion system are detailed in Methods S1 (SDC, http://links.lww.com/TXD/A226). Perfusion parameters (pressure and flow) and UO were sequentially recorded during NMP and whole blood reperfusion.
Perfusion Experiments
Single kidneys (n = 7) underwent NMP for 1–3 hours. These kidneys were used to (1) establish NMP system feasibility, functionality, and safety; (2) compare NMP using autologous and banked blood; and (3) investigate leukocyte extravasation from the graft during NMP (see Figure 1).
Paired kidneys (n = 8; ie, 4 kidney pairs) were randomly allocated to either “CS” or “NMP” groups. “CS” kidneys underwent standard CS, a subsequent 30 minutes simulated second WIT (SWIT) at room temperature, and then ex vivo whole blood reperfusion for 60 minutes to simulate the immediate post transplant reperfusion period. “NMP” kidneys underwent CS, followed by 1 hour of NMP, a simulated SWIT of 30 minutes, and finally ex vivo whole blood reperfusion for 60 minutes.
FIGURE 1.
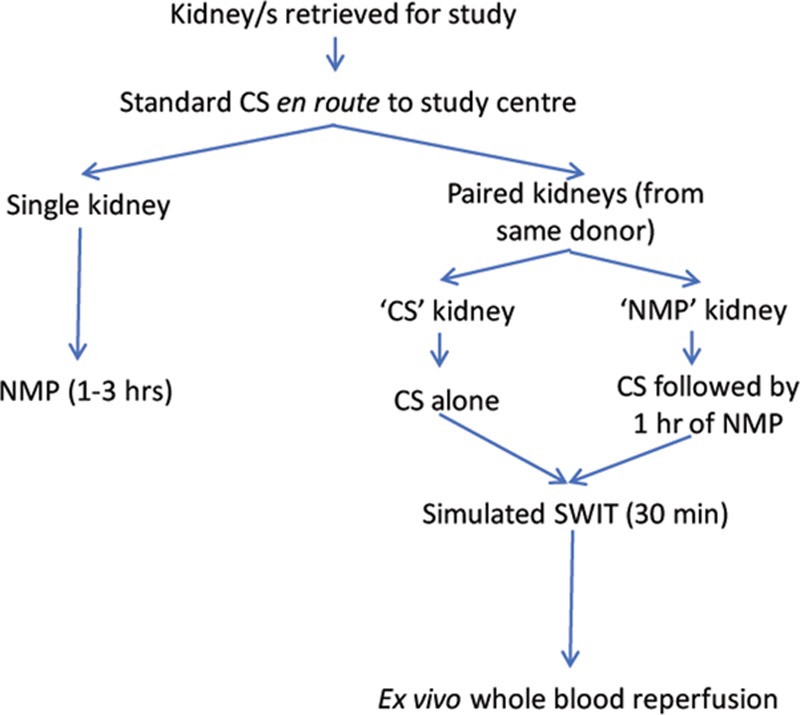
Experimental flow diagram. Experimental pathways followed after kidney retrieval. Please note one set of paired kidneys (from donor 3) did not undergo ex vivo whole blood reperfusion as whole blood was not available. CS, cold static storage; NMP, normothermic machine perfusion; SWIT, second warm ischemic time.
Samples
Kidney biopsy time points, in addition to analyses of blood and urine samples during NMP and/or ex vivo whole blood reperfusion, are detailed in Methods S1 (SDC, http://links.lww.com/TXD/A226).
Measurements and Analyses
Renal blood flow (RBF) and intra-renal resistance (IRR = MAP/RBF)1 were recorded throughout perfusion and normalized to a kidney weight of 250 g. UO (mL) was recorded hourly. CrCl (mL/min/100 g/h), fractional excretion of sodium (%; FeNa), and renal oxygen consumption (mm Hg × mL/min/g) during NMP and ex vivo whole blood reperfusion were calculated as described previously.23
Renal Histopathology
All biopsies underwent Periodic acid-Schiff staining according to standard methods. Each pre and post-NMP and post ex vivo whole blood reperfusion was assigned a Remuzzi25 score by a blinded renal histopathologist.
Immunofluorescence
Renal tubular epithelial cell death was compared by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method (Sigma-Aldrich/Merck, MO, USA) between paired kidneys (NMP versus CS pairs). Renal tissue oxidative stress was quantified and compared in paired NMP/CS samples using dihydroethidium (DHE) (Thermo Fisher Scientific), an indicator of tissue superoxide levels. Complement (C9) staining was also performed in paired samples using C9 primary antibody (Abcam, Cambridge, United Kingdom) and goat anti-rabbit secondary antibody conjugated to Alexa Fluor 647 (Invitrogen, CA, USA); see Methods S1 (SDC, http://links.lww.com/TXD/A226).
Flow Cytometry Analysis for Leukocyte Effluent From the Graft During NMP
Blood samples were taken from the circuit at different time points (n = 3 kidneys) to analyze leukocyte extravasation from the graft. Samples were taken from the PRBC blood bag, and then at “start” NMP (5 min after the commencement of NMP), 1-hour post commencement of NMP, and 1.5 and 2 hours post commencement of NMP. Samples were prepared as described previously.26 Relevant markers and antibodies used for flow cytometry evaluation of absolute numbers of granulocytes, monocytes, natural killer (NK) cells, B cells, T cells, natural killer T cells, and dendritic cells are detailed in Methods S1 (SDC, http://links.lww.com/TXD/A226).
RNA Expression by Next-Generation Sequencing
Targeted whole transcriptome RNA expression27,28 was analyzed using paired kidneys undergoing NMP or CS alone, followed by ex vivo whole blood reperfusion. Kidney biopsies from each group were taken at end-CS, end-NMP (if applicable), and end-ex vivo reperfusion. Further details can be found in Methods S1 (SDC, http://links.lww.com/TXD/A226).
Statistical Analyses
Unless otherwise indicated, data are presented in the format mean ± 1 standard deviation. Continuous parametric variables were compared using the unpaired Student’s t test, while nonparametric continuous variables have been compared using the Mann-Whitney U test. The paired t test was used for comparison of baseline and end-NMP data for each individual kidney or functional data for each paired kidney at the end of ex vivo reperfusion. RBF and IRR graphs were compared by first calculating the area under the curve (AUC) for each parameter plotted on the graph. GraphPad Prism v. 7.02 was used for all of these statistical analyses. For all data comparisons, a P < 0.05 was considered as statistically significant.
Differential gene expression analysis was performed using voom.29 For all comparisons, changes in gene expression were deemed significant if they had a Benjamini-Hochberg adjusted P < 0.05. Pathway analysis was performed using a hypergeometric test to test if any Gene Ontology or Reactome categories were enriched for differentially expressed genes.30-32 Wilcoxon rank-sum tests with directional alternative hypotheses were used on the test statistics to test if any of the pathways were significantly up or downregulated. Further pathway analyses were conducted through the use of Ingenuity Pathway Analysis (IPA) (Qiagen Inc.).33
RESULTS
Renal Histology, Hemodynamics, and UO During Normothermic Machine Perfusion
Fifteen kidneys were obtained from 10 human donors. Donor and perfusion characteristics are summarized in Table 1; Table S1 (SDC, http://links.lww.com/TXD/A226) demonstrates each kidney before and during perfusion. Eleven kidneys underwent NMP for 1–3 hours as defined in the methods. Four of these 11 kidneys underwent NMP followed by simulated transplantation using ex vivo reperfusion with whole (allogeneic) blood, while their direct pairs had CS alone followed by ex vivo whole blood reperfusion. There were no significant histopathologic changes induced during NMP (in comparison to the end-CS biopsy of the same kidney), as assessed by light microscopy (Table S2, SDC, http://links.lww.com/TXD/A226).
TABLE 1.
Donor and perfusion characteristics

During NMP, hemodynamic changes demonstrated a rise in RBF and decline in IRR over time, with the exception of one kidney (DCD-D3) (Figure 2A). Median RBF and IRR after 1 hour was 260 mL/min/250 g (range, 172–359) and 0.29 mm Hg/mL/min/250 g (range, 0.23–0.45), respectively. The median hourly UO was 21 mL (range, 0–46 mL); only one kidney did not produce any urine (DCD-D4) (Figure 2B). Although there was no UO recorded from the DCD-D4 kidney during NMP, perfusate creatinine levels dropped over the course of perfusion (997 μmol/L at baseline to 633 μmol/L after 60 min NMP and 585 μmol/L after 90 min NMP). This indicates either (1) an abnormality related to ureteric function/vermiculation (unlikely as the ureter was not significantly distended during perfusion) or (2) urine leak into the perfusion chamber from an unidentified site (more likely). Figure 2B provides a graphical depiction of UO, CrCl, and FeNa. UO was generally positively correlated with CrCl and inversely related to FeNa, although these relationships were not absolute (Figure 2B).
FIGURE 2.
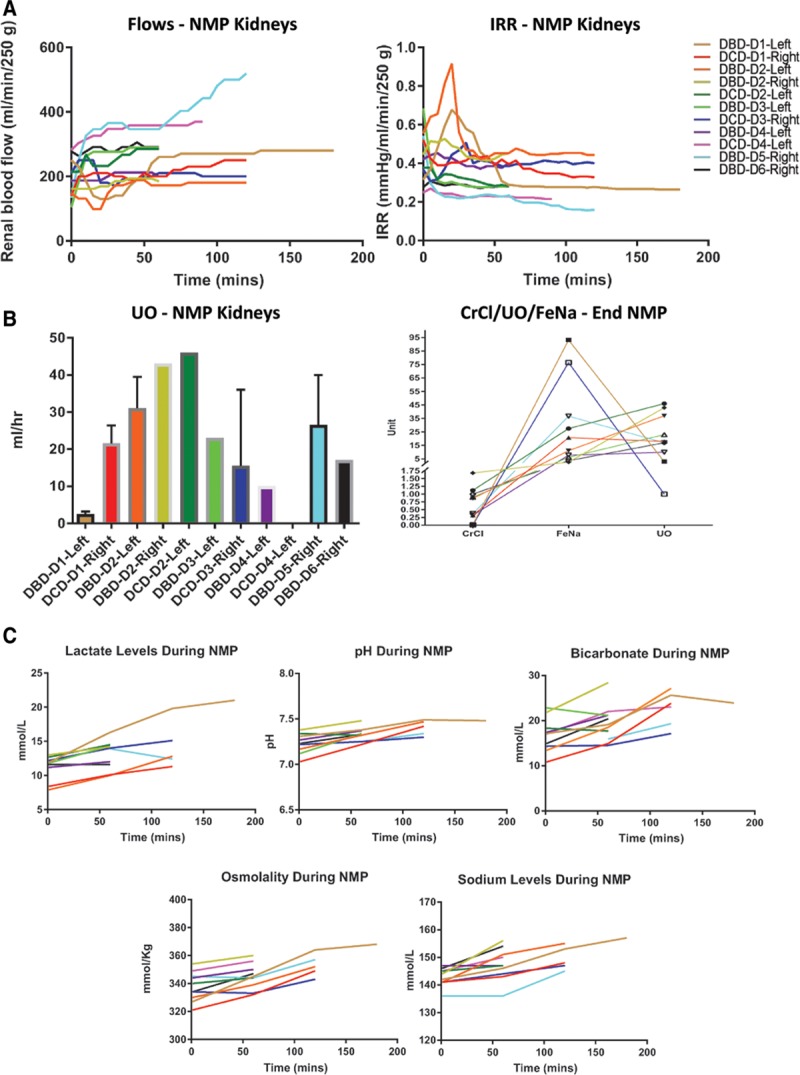
Perfusion, functional, and biochemical changes during normothermic machine perfusion (NMP). A, Renal blood flow and intra-renal resistance (IRR) during NMP, with a mean arterial pressure maintained between 75–85 mm Hg. B, Left panel—Urine output (UO) per h of NMP for each donor kidney. Right panel—Relationship between UO, creatinine clearance (CrCl), and fractional excretion of sodium (FeNa) in each donor kidney. C, Perfusate acid-base balance (pH, lactate, and bicarbonate) and electrolyte (sodium and osmolality) concentrations over the course of NMP, plotted for each individual kidney. DBD, donation after brain death; DCD, donation after circulatory death.
Impact of Ischemic Time on Perfusion Parameters During NMP in DCD Compared With DBD Kidneys
NMP is likely to have differential impacts on DCD and DBD kidneys depending on the severity of the initial ischemic insult. It is useful to gain an understanding of RBF and IRR during NMP and establish potential correlations between donor types, perfusion parameters, and ischemic times to provide more objective graft functional assessment. Fig. S1 (SDC, http://links.lww.com/TXD/A226) plots each donor kidney’s RBF and IRR, split by donor type (DCD or DBD) and arranged according to ischemic time. DCD kidneys with a lower WIT/CIT demonstrated an elevated RBF (median, 328 mL/min/250 g; range, 286–370) and lower IRR (median, 0.26 mm Hg/mL/min/250 g) in comparison to the 2 kidneys with a higher WIT and CIT (median RBF 205 mL/min/250 g [range, 200–210] and median IRR 0.41 mm Hg/mL/min/250 g [range, 0.39–0.42]; Fig. S1A, SDC, http://links.lww.com/TXD/A226). In contrast, DBD kidneys showed no obvious correlation between CIT and RBF or IRR; indeed, the 2 kidneys with the greatest CITs had comparatively better perfusion parameters during NMP (Fig. S1B, SDC, http://links.lww.com/TXD/A226). A correlation between perfusion parameters and KDPI could not be established in either donor subset.
Perfusate Lactate Trends During Brief (1–3 Hours) Renal NMP
Lactate clearance is a biomarker that defines effective perfusion and/or suitability for transplantation during NMP of other organs such as the liver and heart.34-36 Lactate levels did not decline after brief NMP in this series but increased significantly from baseline (11.2 mmol/L) until 60 minutes (13.1 mmol/L; P = 0.002; Figure 2C). However, a rising lactate was not indicative of acidemia in the perfusate, and a general uptrend was observed with respect to perfusate pH (7.23–7.33; P = 0.003) and bicarbonate levels (16.8–19.4 mmol/L; P = 0.009; Figure 2C). Furthermore, there was an observed increase in perfusate sodium levels after 60 minutes of NMP (143–148 mmol/L; P = 0.007), and a corresponding elevation in osmolality (338–345 mmol/kg; P = 0.004) (Figure 2C).
Leukocyte Mobilization From the Graft into the Circuit During NMP
Perfusion fluid samples were taken before and at defined intervals after the commencement of NMP from donors 8–10, and then analyzed using flow cytometry. A significant efflux of leukocytes (CD45+) was detected in all tested samples within 2–3 minutes of commencement of NMP (“start NMP” samples). Dendritic cells (DC-SIGN+ cells) were not detectable by our methods at any time-point. However, large populations of granulocytes (CD45loSSChi) were detected, along with smaller populations of CD14+ monocytes, CD3+ T cells, and CD19+ B-cells, and CD56+ NK cells (Figure 3).
FIGURE 3.
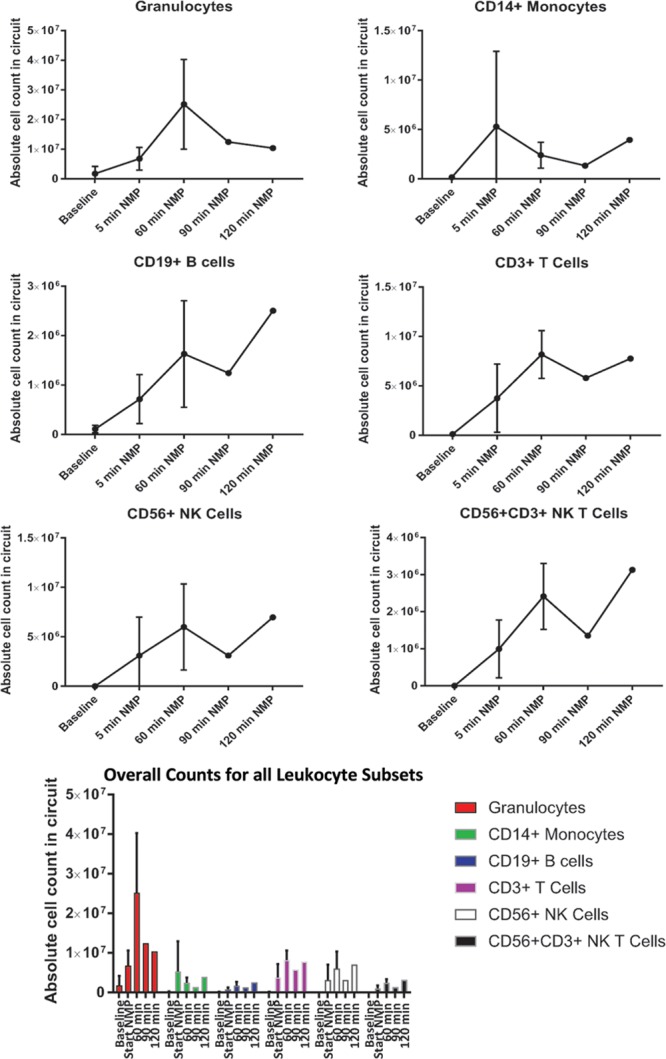
Leukocyte efflux from the donor kidney during normothermic machine perfusion (NMP). Absolute cell counts for granulocytes, monocytes, NK cells, and lymphocytes were calculated by flow cytometry using the preperfusion sample as a baseline, followed by NMP arterial line sampling at selected time points. n = 3 kidneys. NK, natural killer.
NMP Under Special Circumstances—Kidneys With Multiple Vessels, and Kidneys Discarded Due to Poor In Situ Perfusion at Retrieval
NMP can be safely and effectively performed in kidneys with more than one artery, including more than one artery on a patch and/or separate upper or lower pole arteries (Figure 4A and B). Back-table arterial reconstruction is not required, and perfusion is facilitated by the use of Y-connectors attached to separate cannulae.
FIGURE 4.

Normothermic machine perfusion (NMP) is possible in the presence of anatomical variation, and useful in poorly perfused kidneys. A and B, NMP is feasible and safe for kidneys with multiple renal arteries, achieves good renal blood flows, and intra-renal resistance. Kidneys shown are from (A) donor 7 (DBD-D4) and (B) donor 8 (DBD-D5). C, NMP is ideal for the assessment of kidneys discarded for poor in situ perfusion. Left panel—Kidney (donor 2; DCD-D1) visualized at end-cold storage, discarded due to a nonperfused lower pole and patchy middle region. Right panel—The lower pole of the same kidney is pictured 5 min after the commencement of NMP, showing complete resolution of the previously nonperfused area. DBD, donation after brain death; DCD, donation after circulatory death.
NMP can also be utilized to assess and/or predict adequacy of renal perfusion in kidneys discarded due to poor in situ perfusion post retrieval (Figure 4C). Two kidneys in this series (from donor 2 and donor 9) were discarded due to poor perfusion at retrieval in the context of DCD donation. In both cases, NMP “cleared” the nonperfused region(s) within 10 minutes of commencement, thereby rendering these kidneys potentially transplantable.
Use of Donor Autologous PRBCs for NMP
Banked blood transfusions (allo-) may increase the risk of allosensitization in kidney transplant recipients; the feasibility of using autologous blood for NMP was therefore explored.37,38 Autologous whole blood was collected from 3 donors, and PRBCs were subsequently isolated for the perfusion of 4 kidneys; all other kidneys underwent NMP using banked blood group O blood (Table 1). There were no significant baseline differences with respect to KDPI (median 89 versus 83; P = 0.783) and CIT (median 8.9 versus 11.7 h; P = 0.226) in kidneys perfused with autologous or banked blood, respectively. Baseline blood parameters for both autologous and banked blood are outlined in Table S3 (SDC, http://links.lww.com/TXD/A226). Hemoglobin levels (43.8 versus 65.3 g/L; P = 0.094) and hematocrit (14.3% versus 20.7%; P = 0.150) were lower in the autologous blood group, and white cell count was higher (0.15 × 109/L versus 0.06 × 109/L; P = 0.071), despite using identical volumes of PRBCs, but this did not reach statistical significance; platelet counts were however statistically lower in the banked blood group (45.0 × 109/L versus 0.9 × 109/L; P < 0.001). Perfusate potassium levels showed a trend towards an increase in the banked blood group (5.4 versus 7.6 mmol/L; P = 0.107); there were no differences in sodium (142.0 versus 143.9 mmol/L; P = 0.404) and bicarbonate (13.0 versus 14.3 mmol/L; P = 0.491) concentrations.
Fig. S2 (SDC, http://links.lww.com/TXD/A226) compares NMP parameters between both groups after 60 minutes of NMP. Although AUC for RBF was significantly higher (P = 0.005) and IRR significantly lower (P = 0.001) in the banked blood perfusion group, these differences are unlikely to be clinically meaningful. There were no significant differences in glomerular (CrCl 0.7 versus 0.5 mL/min/100 g/h; P = 0.610) or tubular function (FeNa 31.9 versus 26.4%; P = 0.806), or renal oxygen consumption (298.0 versus 381.7 mL/min/g; P = 0.330) in the autologous compared with banked blood groups, respectively (Fig. S2, SDC, http://links.lww.com/TXD/A226). Perfusate lactate dehydrogenase levels were not significantly different (P = 0.125), while aspartate aminotransferase levels were significantly lower in the group perfused with banked blood (121.8 versus 49.2 U/L; P = 0.039).
Gene Expression Changes Involving Inflammatory, Stress, Cell Death, and Survival-Related Pathways During NMP
Targeted whole transcriptome RNA expression was performed, comparing paired kidneys treated with CS alone or NMP after an equivalent initial period of CS (n = 3 pairs—pair 1–3, identified in Table 1). Samples were taken at the end of CS (“end-CS”), after NMP (if applicable; “end-NMP”), and after simulated transplantation (“end-ex vivo”). Simulated transplantation allowed for a direct comparison between CS and NMP kidneys and involved ex vivo reperfusion with whole blood at a MAP of 80–90 mm Hg, without the addition of any protective mediators. The SWIT (“anastomoses”) was approximated by leaving each kidney at room temperature for 30 minutes before reperfusion.
The principal component analysis (PCA) plot revealed unique population clusters, defined by donor kidney pairs (Fig. S3, SDC, http://links.lww.com/TXD/A226). Within each pair, end-CS samples were clustered close together, while the end-ex vivo samples were distinctly different (Fig. S3, SDC, http://links.lww.com/TXD/A226). Importantly, at baseline (ie, end-CS) within the kidney pairs, there were no differentially expressed genes between the NMP and CS groups.
One hour of NMP induced multiple gene expression changes. In comparison to biopsies taken at the end of CS, NMP in the same kidneys modified expression of 200 genes (n = 196 were significantly upregulated, and n = 4 were down-regulated) (Figure 5A). A total of 115 pathways were significantly enriched for differentially expressed genes. The most differentially up and down-regulated genes and pathways are also indicated in Figure 5A, while Table S4 (SDC, http://links.lww.com/TXD/A226) outlines expression patterns for all genes/pathways.
FIGURE 5.
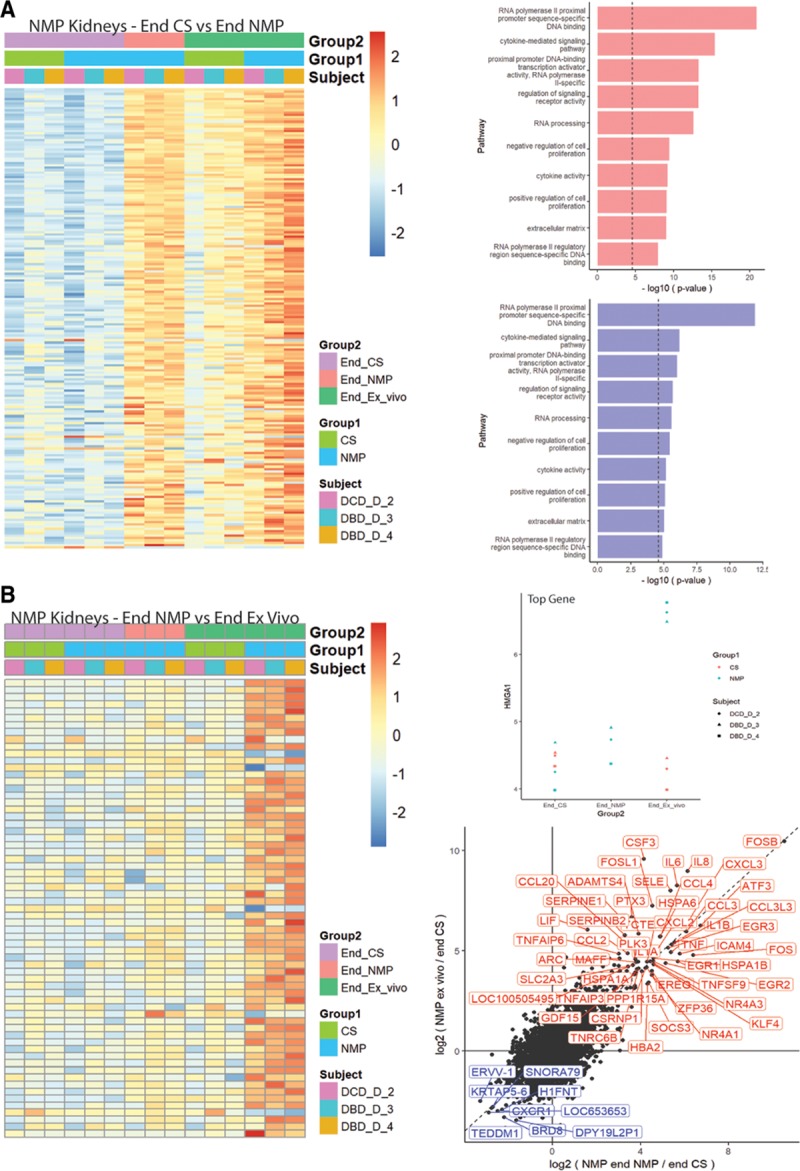
Comparison of whole transcriptome gene expression following cold static storage (CS) or normothermic machine perfusion (NMP). Sequential biopsies were taken immediately before the commencement of NMP (end-CS), after 1 h of NMP (end-NMP), and after 1 h of simulated transplantation (end-ex vivo). A, Left panel—Gene expression changes (heatmap) after NMP in comparison to the end-CS period (for the same kidneys). Right panels—Pathway analyses, displaying the most up- (top) and down-regulated (bottom) pathways after NMP. B, Left panel—Heatmap showing differentially expressed genes in the NMP group of kidneys after ex vivo whole blood reperfusion (in comparison to the end-NMP samples from the same kidneys). Right panels—(top) HMGA1 is the most differentially expressed gene between end-NMP and end-ex vivo samples. Bottom, Scatter plot showing the most up- and down-regulated genes after ex vivo reperfusion (simulated transplantation) in comparison to end-NMP samples from the same kidneys. DBD, donation after brain death; DCD, donation after circulatory death.
Ex vivo whole blood reperfusion (simulated transplantation) produced distinctly different gene expression changes (65 genes) in comparison to those induced by NMP (Figure 5B, and Table S5, SDC, http://links.lww.com/TXD/A226). This therefore indicates the technique does not merely recapitulate changes induced by the NMP process. These gene expression changes were not evident in the CS group of kidneys after ex vivo whole blood reperfusion, as shown in the scatter plots displayed in Figures 5 and 6.
FIGURE 6.
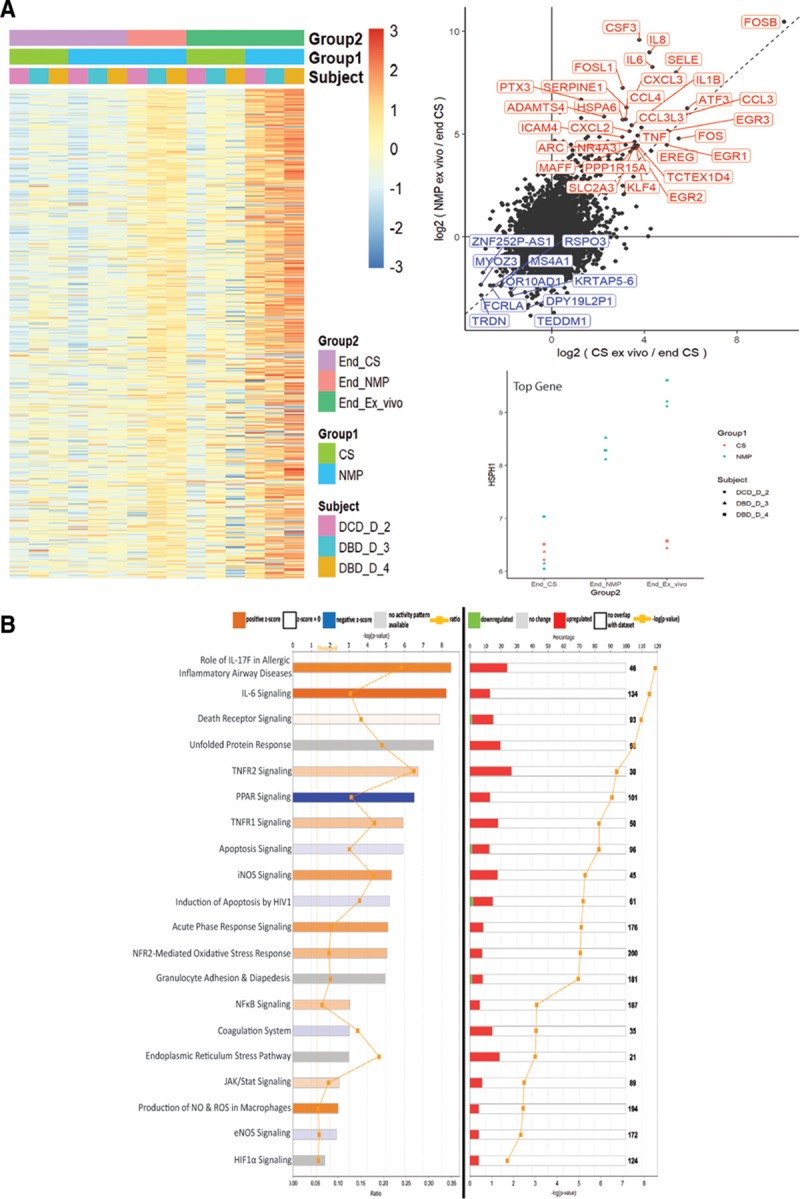
Changes in whole transcriptome gene expression following normothermic machine perfusion (NMP). Comparisons were conducted after a simulated second warm ischemic period of 30 min, followed by reperfusion of each kidney with whole allogeneic blood at a mean arterial pressure of 85 mm Hg and temperature of 37°C. A, Left panel—Gene expression heatmap comparing paired kidneys having cold static storage (CS) or NMP, after ex vivo whole blood reperfusion. Right panels—(top) Scatter plot outlining the most significantly up- and down-regulated genes in the NMP group in comparison to CS paired kidneys. Bottom, Most differentially expressed gene between NMP and CS kidneys (HSPH1), with an obvious difference in expression at the end-ex vivo time point for all 3 kidney pairs. B, Ingenuity Pathway Analysis showing top canonical pathways significantly up- or down-regulated in the NMP group of kidneys in comparison to CS kidneys. Pathways are ordered by magnitude of –log (P value). Left panel—Indication of pathway activation or repression based on the z score. A positive z score suggests pathway induction, and a negative z score denotes pathway suppression. Right panel—Percentage (indicated by colored bars) of total number of genes (indicated by numbers to right of bars) in a specific pathway that are differentially expressed in NMP compared with CS kidneys. DBD, donation after brain death; DCD, donation after circulatory death; IL, interleukin.
After simulated transplantation, paired kidneys subjected to either NMP or CS alone displayed highly disparate gene signatures characterized by the differential expression of 495 genes (435 up- and 60 down-regulated, respectively) (Figure 6A). These are indicated in the scatter plot displayed in Figure 6A. A full list characterizing gene expression and pathway changes is provided in Table S6 (SDC, http://links.lww.com/TXD/A226). The top 20 (plausible) pathways that were significantly impacted by NMP as determined by IPA are summarized in Figure 6B, ordered based on the –log (P value). Diseases/functions activated or repressed by NMP in comparison to CS alone, as predicted by IPA based on differential gene expression, are outlined in Fig. S4 and Table S7 (SDC, http://links.lww.com/TXD/A226). Overall, gene signatures were strongly consistent with a decrease in cell death and apoptosis in NMP kidneys, with a corresponding increase in cell survival, viability, and proliferative functions.
Renal Hemodynamics and Function, and Ischemia-Reperfusion Injury-Related Parameters in NMP Kidneys Comparison to Kidneys Undergoing CS Alone
Over the period of ex vivo whole blood reperfusion, RBF was greater, and IRR was lower in every NMP kidney compared with the corresponding CS pair (Figure 7A). RBF and IRR at the 1 hour time point after ex vivo reperfusion in the NMP and CS pairs respectively was 250.3 ± 79.7 mL/min/250 g versus 152.1 ± 138.8 mL/min/250 g (P = 0.175), and 0.4 ± 0.1 mm Hg/mL/min/250 g versus 0.9 ± 0.6 mm Hg/mL/min/250 g (P = 0.137). Aggregated (AUC) RBF was significantly higher (P = 0.023), and IRR was significantly lower in the NMP-“treated” kidneys (P = 0.009).
FIGURE 7.
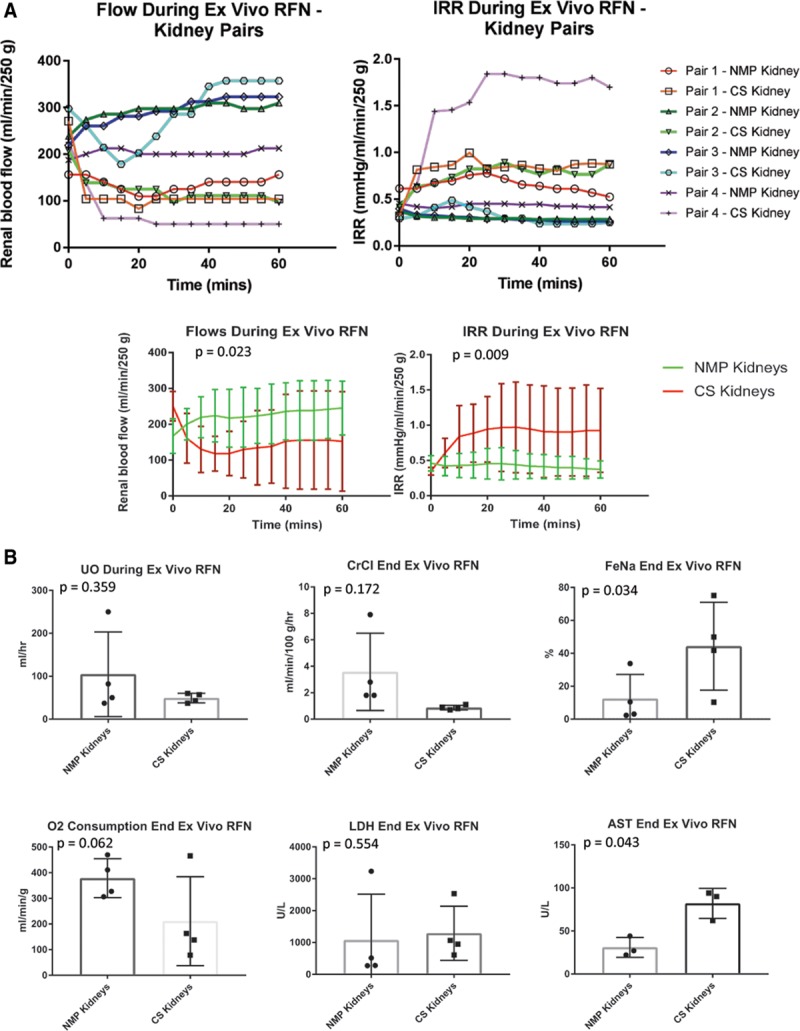
Perfusion and functional parameters following cold static storage (CS) or normothermic machine perfusion (NMP) and subsequent simulated transplantation. A, Upper panels—Renal blood flow and intra-renal resistance (IRR) graphed for each individual donor kidney. Lower panels—Cumulative flow and IRR for the kidneys stored by CS alone in comparison to contralateral kidneys having CS followed by NMP. B, Comparison of renal functional parameters between the groups after simulated transplantation—urine output (UO), creatinine clearance (CrCl), fractional excretion of sodium (FeNa), oxygen consumption, and perfusion fluid levels of lactate dehydrogenase (LDH) and aspartate aminotransferase (AST). RFN, reperfusion.
Paired comparisons of other functional parameters and injury markers were also performed (Figure 7B) and showed a significantly lower FeNa and perfusate aspartate aminotransferase in the NMP group (P = 0.034 and P = 0.043, respectively). There were trends favoring NMP over CS kidneys with respect to CrCl, oxygen consumption, and UO, although these did not reach statistical significance.
Renal tubular epithelial cell death, as measured by TUNEL staining, was reduced in the NMP-treated kidneys after simulated transplantation (5.9 versus 9.6 TUNEL-positive cells/high power field (HPF); P < 0.001); Figure 8A). Similar significant trends were seen with respect to oxidative stress (quantified using DHE staining; P = 0.022; Figure 7B) and complement C9 activation (P = 0.002; Figure 8C). Interestingly, comparative histologic sections (Figure 8D) were not significantly different with respect to acute tubular injury following ex vivo whole blood reperfusion, regardless of the initial treatment (Table S8, SDC, http://links.lww.com/TXD/A226).
FIGURE 8.
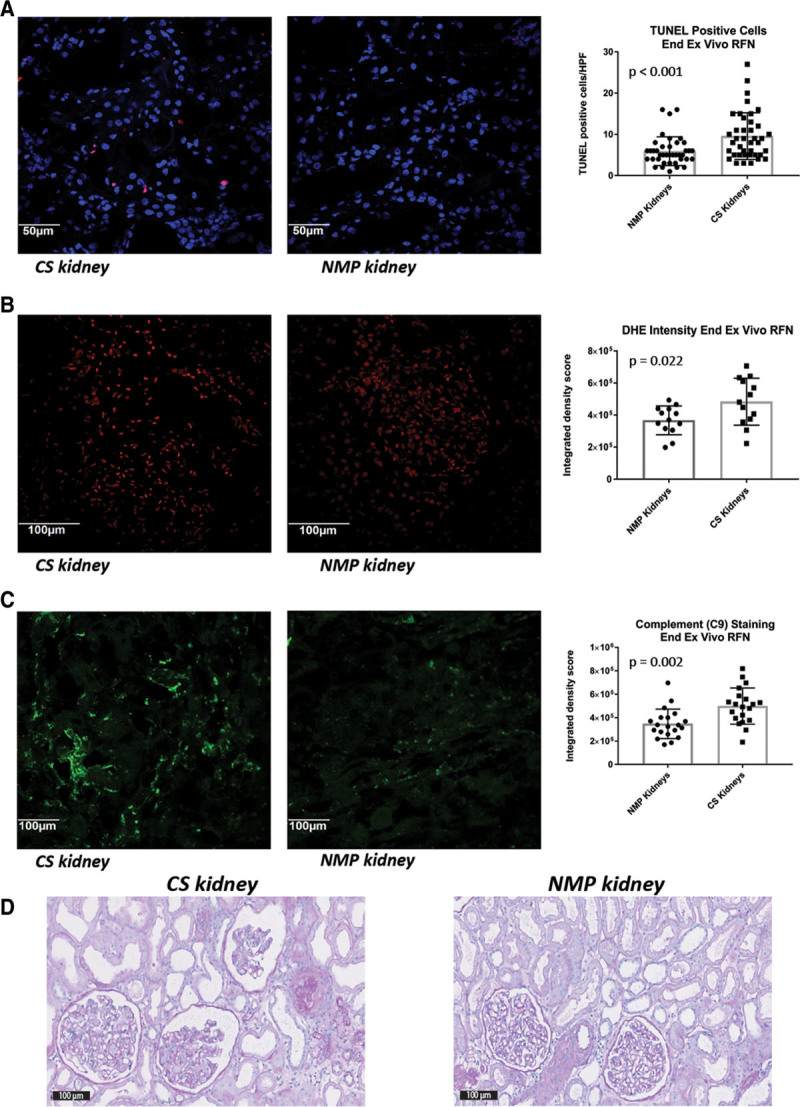
Histopathology and ischemia-reperfusion injury in kidney pairs having normothermic machine perfusion (NMP) or cold static storage (CS) followed by simulated transplantation. A, Representative photomicrograph (pair 2; DBD-D3) and cumulative comparison of renal cell death/apoptosis in both study groups as determined by TUNEL staining (×40). Similar immunofluorescence-based comparisons of (B) oxidative stress (using dihydroethidium [DHE] staining) (pair 3; DBD-D4), and (C) complement C9 staining (pair 2; DBD-D3) after ex vivo whole blood reperfusion (×20). D, Representative photomicrograph of a kidney pair (pair 2; DBD-D3) after simulated transplantation following either CS or NMP; Periodic acid-Schiff stain (×20). DBD, donation after brain death; RFN, reperfusion; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
DISCUSSION
NMP before kidney transplantation represents a paradigm shift in the preservation of deceased donor grafts, with the potential to simultaneously recondition and objectively assess the donor kidney. We present the largest series of discarded human kidney NMP outside of the United Kingdom and demonstrate many novel findings to motivate translation of this technique to clinical practice. Using a paired kidney design and simulated transplantation, we show that brief NMP demonstrates some superiority to CS alone, as evidenced by enhanced early perfusion parameters, glomerular and tubular function, and amelioration of IRI. Transcriptome-wide sequencing demonstrated NMP-based activation of protective stress-related responses, together with promotion of cell survival and proliferation. The capacity of NMP to allow objective assessment of renal allografts and reduce discard rates by gauging perfusion-related parameters was confirmed. We also demonstrated the feasibility of using autologous (donor) blood during NMP compared with the use of third party (banked) blood. Finally, we showed a massive efflux of passenger leukocytes from the donor kidney into the NMP circuit during perfusion, which may be targeted to modulate the acute rejection response in the recipient.
The attractiveness of brief preimplantation NMP lies in its simplicity and the logistical advantages this method affords above longer, continuous methods of NMP, which require perfusion during the whole transportation period. However, this technique largely remains experimental. Transplant centers may be reluctant to adopt this technique due to a lack of understanding of the biological mechanisms through which benefits are conferred. This is notwithstanding the clear advantage offered by brief NMP in reducing graft discard rates, especially in the setting of poorly perfused DCD kidneys.2 Our unique study design comparing paired kidneys has allowed us to confidently explore the impacts of NMP without requiring large patient numbers. In particular, this design removes the confounding influences of different donor and recipient parameters, which contribute to variability in transplantation outcomes.
NMP kidneys displayed better perfusion and functional parameters in comparison to the CS pairs after simulated transplantation. Whole transcriptome sequencing demonstrated a large number of differentially expressed genes in the kidney after NMP in comparison to CS controls. Important gene signatures included the significant upregulation of pro-inflammatory cytokines (including interleukin-6), chemokines, and heat shock proteins (HSPs). Additional differential pathways impacted by NMP included the unfolded protein signaling response, cell death/apoptosis-related cascades, and cell survival. These factors were demonstrated not only in pathway predictions, but confirmed by TUNEL, DHE, and complement staining, which were all significantly improved in NMP kidneys. Overall, the combination of gene expression data, pathway analyses, tissue staining, and finally in vivo renal function, provides a convincing picture of the beneficial impacts attributed to preimplantation NMP.
Passenger leukocytes play a role in the initiation and regulation of the alloimmune response directed against the transplanted organ.39,40 Depletion of these leukocytes typically requires whole body/organ irradiation, which has variable success and is not feasible in the transplant setting.41 Stone et al42,43 demonstrated efflux of passenger leukocytes during NMP of both the porcine kidney and lungs; similar efflux in hypothermic systems is as yet untested. We now demonstrate the efflux of substantial numbers of passenger leukocytes from human donor kidneys into the perfusion circuit, providing obvious therapeutic potential in an attempt to modulate rejection in the recipient. Leukocyte filters have been incorporated into lung perfusion systems to capture circulating leukocytes but have uncertain efficacy, likely due to saturation of the filter.44 Nevertheless, NMP provides the unique opportunity to deliver directed therapies to the kidney, which may include targeting leukocytes. Delivery of other agents that specifically target endothelial cells, ameliorate IRI, and/or attempt to modulate endothelial cell major histocompatibility complex antigen expression using gene therapies have also been demonstrated by several groups.45-47
Existing renal NMP devices have differed in perfusion settings and constituents.1,3,17,18,21 Therefore, parameters such as RBF, IRR, and UO may not be readily compared between different studies in terms of significance and predictive potential. Nevertheless, our use of NMP in discarded human kidneys enhanced RBF and IRR in all but one kidney, possibly suggesting good immediate graft function, as evidenced by early work from Nicholson and Hosgood16 where kidneys with high flows had lower delayed graft function rates upon transplantation. Furthermore, DCD kidneys used here that were discarded due to poor in situ perfusion after retrieval were homogenously and effectively perfused during NMP. Overall, NMP has a remarkable potential to reduce kidney discards and increase utilization rates, and this was also recently reflected in a liver NMP RCT.35
This study was wholly reliant upon the provision of discarded and/or nonutilized deceased donor human kidneys, and therefore all study variables could not be controlled. In particular, depending on resource and staffing availability, not all factors (eg, leukocyte efflux) could be tested for all kidneys. Although kidney numbers are relatively small (n = 15), we included more kidneys than other recently published discarded human NMP series.18,48 More importantly, direct comparisons of CS and NMP using paired kidneys from the same donor have added greater reliability to our results, although admittedly only 4 kidney pairs are included in this comparison. Although final result validation requires kidney transplantation, ex vivo perfusion as a simulation of transplantation is an acceptable alternative when transplantation is not possible.15,23,24,49
In summary, this study has utilized brief NMP of discarded human kidneys to important insights with respect to the mechanistic basis and possible benefits of NMP. Strength has been added to the notion that NMP may reduce kidney discard rates, especially in poorly perfused kidneys.
ACKNOWLEDGMENTS
The authors would like to thank—the NSW Organ and Tissue Donation Service for provision of tissue for research purposes, and Donation Specialist Coordinators from the Organ and Tissue Donation Service for facilitating this process; the Australian Red Cross Blood Service and Dr. John-Paul Tung for the provision of blood products and advice regarding blood isolation/utilization; Westmead Institute for Medical Research Core Facilities staff, in particular Virginia James (histology staining), Hong Yu (assistance with immunofluorescence), and Suat Dervish (design and printing of 3D-printed perfusion chamber); Hsiufen Chua for assistance with transcriptome work and interpretation; Dr. Hien Nguyen for assistance with kidney back-table preparation; and all kidney donors and their families, without which this study would not have been possible.
Supplementary Material
Footnotes
Published online 8 October, 2019.
W.J.H., N.M.R., and H.C.P. are co-senior authors.
This work was supported by Royal Australasian College of Surgeons—Sir John Loewenthal Project Grant.
B.X. is an employee of Thermo Fisher Scientific. The other authors declare no conflicts of interest.
A.M.H. designed and performed the experiments, collected and interpreted data, and wrote the manuscript; D.B.L., B.X., M.H., Y.V.C., K.K., and R.M. performed and/or advised on certain aspects of experiments and were involved in manuscript revision; E.P. and C.H.P. provided statistical and pathological analyses, respectively; R.G., C.Z., P.R., J.L., R.D., and L.Y. assisted in the performance and/or coordination of experiments or organ retrieval, and also assisted in manuscript writing; S.A., G.T., G.W., and G.O. advised on experimental design, data interpretation, and manuscript writing; and W.J.H., N.M.R., and H.C.P. designed experiments, provided advice and assistance in the performance of experiments and data analysis/interpretation, and wrote the manuscript.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013131246–1252 [DOI] [PubMed] [Google Scholar]
- 2.Hosgood SA, Thompson E, Moore T, et al. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br J Surg 2018105388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaths JM, Echeverri J, Linares I, et al. Normothermic ex vivo kidney perfusion following static cold storage-brief, intermediate, or prolonged perfusion for optimal renal graft reconditioning? Am J Transplant 2017172580–2590 [DOI] [PubMed] [Google Scholar]
- 4.Jochmans I, Nicholson ML, Hosgood SA. Kidney perfusion: some like it hot others prefer to keep it cool. Curr Opin Organ Transplant 201722260–266 [DOI] [PubMed] [Google Scholar]
- 5.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 20093607–19 [DOI] [PubMed] [Google Scholar]
- 6.Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant 2010101991–1999 [DOI] [PubMed] [Google Scholar]
- 7.Moers C, Varnav OC, van Heurn E, et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 201090966–973 [DOI] [PubMed] [Google Scholar]
- 8.Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg 2010252756–764 [DOI] [PubMed] [Google Scholar]
- 9.Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 201588241–249 [DOI] [PubMed] [Google Scholar]
- 10.Rege A, Irish B, Castleberry A, et al. Trends in usage and outcomes for expanded criteria donor kidney transplantation in the United States characterized by kidney donor profile index. Cureus. 2016;8:e887. doi: 10.7759/cureus.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia GG, Harden P, Chapman J; World Kidney Day Steering Committee 2012 The global role of kidney transplantation. Lancet 2012379e36–e38 [DOI] [PubMed] [Google Scholar]
- 12.Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int 201528657–664 [DOI] [PubMed] [Google Scholar]
- 13.Hosgood SA, Saeb-Parsy K, Wilson C, et al. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7:e012237. doi: 10.1136/bmjopen-2016-012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res 2013182153–160 [DOI] [PubMed] [Google Scholar]
- 15.Bagul A, Hosgood SA, Kaushik M, et al. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg 200895111–118 [DOI] [PubMed] [Google Scholar]
- 16.Hosgood SA, Barlow AD, Hunter JP, et al. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg 20151021433–1440 [DOI] [PubMed] [Google Scholar]
- 17.Kaths JM, Cen JY, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant 201717957–969 [DOI] [PubMed] [Google Scholar]
- 18.Weissenbacher A, Lo Faro L, Boubriak O, et al. Twenty-four-hour normothermic perfusion of discarded human kidneys with urine recirculation. Am J Transplant 201919178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Transplantation Society of Australia and New Zealand. A Guide to the Australian Kidney Donor Profile Index (KDPI). 2016. Available at https://www.tsanz.com.au/standalonepages/documents/AustralianKDPIINFOv1.0.pdf. Accessed March 2, 2017.
- 20.Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI). 2012. Available at https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf. Accessed May 15, 2019.
- 21.Hameed AM, Miraziz R, Lu DB, et al. Extra-corporeal normothermic machine perfusion of the porcine kidney: working towards future utilization in australasia. ANZ J Surg 201888E429–E434 [DOI] [PubMed] [Google Scholar]
- 22.Hameed A, Dervish S, Rogers N, et al. A novel, customized 3D-printed perfusion chamber for normothermic machine perfusion of the kidney. Transpl Int 201932107–109 [DOI] [PubMed] [Google Scholar]
- 23.Adams TD, Patel M, Hosgood SA, et al. Lowering perfusate temperature from 37°C to 32°C diminishes function in a porcine model of ex vivo kidney perfusion. Transplant Direct. 2017;3:e140. doi: 10.1097/TXD.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schopp I, Reissberg E, Lüer B, et al. Controlled rewarming after hypothermia: adding a new principle to renal preservation. Clin Transl Sci 20158475–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remuzzi G, Cravedi P, Perna A, et al. ; Dual Kidney Transplant Group Long-term outcome of renal transplantation from older donors. N Engl J Med 2006354343–352 [DOI] [PubMed] [Google Scholar]
- 26.Hu M, Wang C, Zhang GY, et al. Infiltrating Foxp3(+) regulatory T cells from spontaneously tolerant kidney allografts demonstrate donor-specific tolerance. Am J Transplant 2013132819–2830 [DOI] [PubMed] [Google Scholar]
- 27.Perico L, Morigi M, Rota C, et al. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat Commun. 2017;8:983. doi: 10.1038/s41467-017-00937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017548471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law CW, Chen Y, Shi W, et al. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 20002525–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Gene Ontology Consortium Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res 201745D331–D338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabregat A, Jupe S, Matthews L, et al. The reactome pathway knowledgebase. Nucleic Acids Res 201846D649–D655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krämer A, Green J, Pollard J, Jr, et al. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 201430523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia-important lessons from the first 12 cases. Transplantation 20171011084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasralla D, Coussios CC, Mergental H, et al. ; Consortium for Organ Preservation in Europe A randomized trial of normothermic preservation in liver transplantation. Nature 201855750–56 [DOI] [PubMed] [Google Scholar]
- 36.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 20153852585–2591 [DOI] [PubMed] [Google Scholar]
- 37.Obrador GT, Macdougall IC. Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol 20138852–860 [DOI] [PubMed] [Google Scholar]
- 38.Leffell MS, Kim D, Vega RM, et al. Red blood cell transfusions and the risk of allosensitization in patients awaiting primary kidney transplantation. Transplantation 201497525–533 [DOI] [PubMed] [Google Scholar]
- 39.Harper IG, Ali JM, Harper SJ, et al. Augmentation of recipient adaptive alloimmunity by donor passenger lymphocytes within the transplant. Cell Rep 2016151214–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberhuber R, Heinbokel T, Cetina Biefer HR, et al. CD11C+ dendritic cells accelerate the rejection of older cardiac transplants via interleukin-17A. Circulation 2015132122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai HC, Zhu X, Lin YJ, et al. Attempted depletion of passenger leukocytes by irradiation in pigs. J Transplant. 2011;2011:928759. doi: 10.1155/2011/928759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone JP, Critchley WR, Major T, et al. Altered immunogenicity of donor lungs via removal of passenger leukocytes using ex vivo lung perfusion. Am J Transplant 20161633–43 [DOI] [PubMed] [Google Scholar]
- 43.Stone JP, Ball AL, Critchley WR, et al. Ex vivo normothermic perfusion induces donor-derived leukocyte mobilization and removal prior to renal transplantation. Kidney Int Rep 20161230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luc JGY, Aboelnazar NS, Himmat S, et al. A leukocyte filter does not provide further benefit during ex vivo lung perfusion. ASAIO J 201763672–678 [DOI] [PubMed] [Google Scholar]
- 45.Tietjen GT, Hosgood SA, DiRito J, et al. Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hameed A, Rogers N, Pleass H, et al. Intra-renal delivery of drugs targeting ischemia-reperfusion injury of the kidney using normothermic machine perfusion. Transplantation. 2018;102:S700. [Google Scholar]
- 47.Figueiredo C, Carvalho Oliveira M, Chen-Wacker C, et al. Immunoengineering of the vascular endothelium to silence MHC expression during normothermic ex vivo lung perfusion. Hum Gene Ther 201930485–496 [DOI] [PubMed] [Google Scholar]
- 48.Kabagambe SK, Palma IP, Smolin Y, et al. Combined ex vivo hypothermic and normothermic perfusion for assessment of high-risk deceased donor human kidneys for transplantation. Transplantation 2019103392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Horn C, Minor T. Improved approach for normothermic machine perfusion of cold stored kidney grafts. Am J Transl Res 2018101921–1929 [PMC free article] [PubMed] [Google Scholar]


