Background.
Active malignancy diagnosed within 5 years is an absolute contraindication for lung transplantation. In this study, we evaluated the rate of incidental malignancies detected in explanted lungs at our institution and assessed the posttransplant survival in patients with nonsmall cell lung cancer (NSCLC).
Methods.
A retrospective chart review of lung transplant recipients at our institution from February 1999 to June 2017 was conducted. A literature review was performed to evaluate the prevalence and survival outcomes in patients with unexpected malignancies.
Results.
From 407 patients who underwent lung transplantation, 9 (2.2%) were discovered to have malignant neoplasms. There were 3 cases of adenocarcinoma, 3 cases of adenocarcinoma in situ, 2 cases of squamous cell carcinoma, and 1 case of metastatic renal cell carcinoma. An extensive literature review found 12 case reports or case series reporting malignancy discovered at the time of lung transplantation. The overall prevalence of incidental neoplasms among 6746 recipients is around 1.5% (n = 103). The most common neoplasms discovered included adenocarcinoma (n = 56, 54%) and squamous cell carcinoma (n = 29, 28%). The overall 3-year survival was 54.4% for patients with localized NSCLC compared to 5.7% for those with nonlocalized disease.
Conclusions.
Unidentified malignancies occur despite aggressive radiographic surveillance with poor posttransplant outcomes in patients with advanced malignancy. Malignancy-related radiographic findings may be missed pretransplant secondary to architectural distortion of lung parenchyma related to end-stage lung disease or because of the critical timing of surgery when donor lungs are available.
INTRODUCTION
Since the first successful lung transplant in 1983, lung transplantation remains an established and viable therapeutic option for the treatment of a variety of end-stage lung disease, extending survival and quality of life beyond the expected natural course of disease at the time of transplant referral.1,2 Smoking-related lung disease, mainly in the form of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), comprise >50% of all lung transplants worldwide.1 This population is at high risk for developing malignancy with the causal relationship between malignancy, COPD, and IPF being firmly established in several studies.3-7
Active malignancy remains an absolute contraindication for lung transplantation. In most cases of hematologic and solid organ cancers, a minimum 5-year cancer free period is often required before lung transplantation can be entertained.8 Despite stringent preoperative screening protocols, incidental findings of malignancy are not uncommon with several centers reporting malignant tumors in explanted lungs at the time of transplantation.9-21
In this study, we reviewed the prevalence of incidentally detected malignancies in explanted lungs at the time of lung transplantation at our institution. We also evaluated pretransplant radiologic features and performed a literature review to evaluate cumulative posttransplant survival of aggregate data.
MATERIALS AND METHODS
Patient Selection
This study was approved by the Institutional Review Board at Emory University (IRB00097690). We performed a retrospective chart review of the electronic medical record of patients who underwent lung transplantation at our institution from February 1999 to June 2017. Patients who underwent retransplantation were excluded from this study. Basic demographic information including age, gender, referral diagnosis, and transplantation type (single versus double) was collected for 407 patients.
Pathologic Assessment and Staging
The surgical pathology reports of explanted lungs were reviewed for all patients included in this study. For patients who were found to have unexpected neoplasms, descriptive information involving histologic type, histologic grade, tumor size, regional lymph node involvement, surgical margins, and presence of pleural, vascular, or lymphatic invasion were reviewed. To evaluate for metastatic disease, both pre and posttransplant imaging including CT and PET/CT scans were reviewed by our transplant team following diagnosis. Final histologic and clinical staging was assigned in accordance to the American Joint Committee on Cancer (AJCC) lung cancer staging criteria.22
Radiographic Assessment
To explore whether these unexpected neoplasms may have been present and unidentified on transplant evaluation or longitudinal follow-up, the authors of this study assessed radiology reports and digitally archived images of pretransplant CT and PET/CT scans in an unblinded manner. An emphasis was placed on the most recent imaging study before a patient’s transplant. As part of our center’s pretransplant screening protocol, all patients undergo a CT scan at their initial transplant evaluation. For nodules >1 cm and those that are suspicious for malignancy, a follow-up PET/CT is performed. Nodules <1 cm are followed with serial CTs every 3 months. For those without concerning features, an annual CT is obtained while subjects are continuing to be evaluated for transplant candidacy and while they remain on the waitlist.
Literature Review
We searched PubMed and article reference lists for case series, case studies, and abstracts describing incidentally detected malignant neoplasms at the time of lung transplantation. Search terms included the following: malignancy in explanted lungs, lung cancer in lung transplant explants, and lung cancer in lung transplantation. Articles that exclusively reported development of malignancy following lung transplantation and survey type studies were excluded. Patient cases and data were reviewed from a total of 12 selected articles that clearly described incidental malignancies in lung transplant explants. Data involving referral diagnosis, tumor type, tumor stage, and survival or follow-up time were collected, whereas individual patient cases and data pertaining to posttransplant malignancy were excluded.
To determine whether there was an effect on posttransplant survival, the combined aggregate patient pool from our center and our literature review was narrowed to only include patients who had a clearly defined follow-up or survival period along with enough clinical information to determine clinical staging in accordance with AJCC staging criteria. As a result, the majority of the data that was pertinent involved patients with nonsmall cell lung cancer (NSCLC). This allowed us to limit the confounding effect of other malignancies on the survival analysis.
Statistical Analysis
Data for subject characteristics including descriptive statistics were analyzed using SPSS (version 24, IBM). Data are expressed as either means ± SD or number (percentage). Further, subject age was analyzed for normality with Shapiro-Wilk test for normality. Subject characteristics with continuous variables were analyzed by one-way ANOVA and categorical variables by Chi-squared analysis. Kaplan-Meier curves were generated for survival analysis. Overall survival data for all lung transplant recipients were imported from the International Society for Heart and Lung Transplantation (ISHLT) registry. Statistical significance was set at a P < 0.05.
RESULTS
Demographics
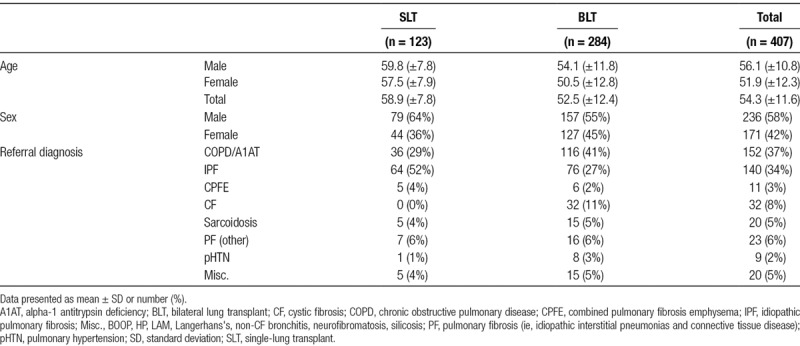
The electronic medical records for 407 patients were reviewed from February 1999 to June 2017. During this time period, 284 (70%) patients underwent bilateral lung transplantation while the remaining 123 (30%) underwent single-lung transplantation. The most common indications for transplantation included COPD (n = 152, 37%), IPF (n = 140, 34%), and cystic fibrosis (CF; n = 32, 8%). Cohort characteristics are available in Table 1. The mean age of recipients at the time of transplant was 54.3 ± 11.6 years. As one would expect with lung transplantation, there was a significantly skewed distribution of ages toward the 6th and 7th decades of life, with a sharp drop off after the age of 70. This fits with national distribution of lung transplant age range.1 Importantly, there was no difference in age of those who were found to have incidental malignancy (60.8 ± 4.3) and those who did not (54.3 ± 11.7).
TABLE 1.
Characteristics of study population
Malignancy in Explanted Lungs
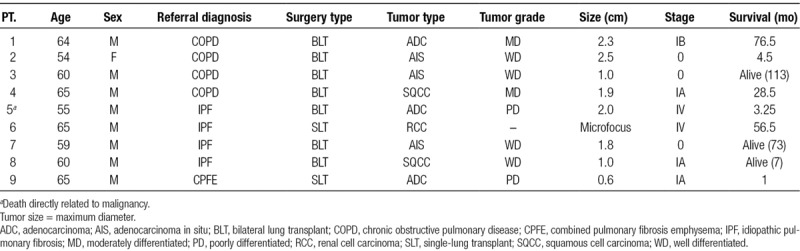
Of the 407 patients who underwent lung transplantation, 9 (2.2%) were found to have malignant neoplasms at the time of transplantation. There were 4 cases (44%) that occurred in patients with COPD, another 4 in IPF, and the remaining case occurred in a patient with combined pulmonary fibrosis and emphysema (CPFE). Patients who underwent bilateral lung transplantation were found to have a higher proportion of malignant neoplasms (78% versus 22%). The most common malignancies identified were adenocarcinoma (ADC; n = 3, 33%) and adenocarcinoma in situ (AIS, formerly known as bronchoalveolar carcinoma; n = 3, 33%) followed by squamous cell carcinoma (SQCC; n = 2, 22%) and a single case of metastatic clear cell renal cell cancer (RCC; n = 1, 11%). With the exception of 2 patients who had metastatic disease, the majority of cases (n = 7, 78%) were found to have localized involvement. All patients with malignant neoplasms were transplanted after the introduction of the Lung Allocation Score (LAS) and had a significant smoking history (Table 2).
TABLE 2.
Patient and tumor characteristics
Patients With Metastatic Disease
Two patients transplanted for IPF were found to have Stage IV disease at the time of transplantation.
Patient 5 was a 55-year-old Caucasian male, who underwent bilateral lung transplantation and was found to have ADC involving the left lower lobe. Additional tumor characteristics at the time of pathologic examination revealed a poorly differentiated 2.0 × 1.0 cm neoplasm with peritumoral angiolymphatic invasion, infiltration of the visceral pleura, and involvement of one ipsilateral hilar lymph node. Preoperative CT scan 15 weeks before transplantation was remarkable for extensive pulmonary fibrosis without any nodules or masses. Postoperative full body PET and MRI brain were unremarkable for metastatic disease; however, he developed cytology confirmed malignant effusion shortly following transplantation. He died 13 weeks following transplantation from septic shock 3 days after talc pleurodesis.
Patient 6 was a 65-year-old Caucasian male, who underwent single-lung transplantation. He had a remote history of renal cell carcinoma treated 5 years before transplantation with unilateral nephrectomy. Pathologic examination of the explanted lung revealed a 1.5 mm microfocus in the left upper lobe and involvement of 2 hilar lymph nodes, the largest of which was 1.9 × 2.3 × 1.4 cm. Preoperative imaging included a full-body PET and CT scan 2 weeks before transplantation revealed an enlarged mediastinal lymph node of similar size with a maximal standardized uptake value of 3.8, which was unremarkable for malignancy on explant pathology. Fortunately, he had a favorable postoperative course with tyrosine kinase inhibitor therapy surviving 56 months and ultimately dying from a spontaneous pneumothorax.
Patients With Localized Disease
Of the 7 patients with localized disease, 3 were found to have AIS, closely followed by 2 cases of adenocarcinoma and SQCC each. Mean tumor size was determined to be 1.58 cm (range 0.6–2.5 cm). All patients underwent surveillance CT scans in accordance with standard protocol set at our institution. The mean interval time from the last CT to transplant date was approximately 6.7 months (range 1–12 mo). On review of selected pretransplant radiology reports and archived digital images, there was no obvious evidence of malignancy. Two patients (2 and 8) were found to have stable <0.6 cm nodules on at least 2 scans, 2 patients (3 and 4) had nonspecific findings (ie, small clustered or scattered nodularity), and 1 patient (1) had a fibrous scar that all correlated with the location of the neoplasm noted on explant pathology. Patients 7 and 9, both with fibrotic disease, had no nodules or masses noted (Table 2). There were no deaths directly related to malignancy.
Total Cases Reported in Literature
From 13 separate cases series, case studies, or abstracts that met our inclusion criteria (including our study), there were a total of 103 cases of incidental neoplasms reported at the time of transplantation among 6746 recipients (1.5%). Of these, adenocarcinoma was the most common neoplasm discovered (n = 56, 54%), followed by SQCC (n = 29, 28%), AIS (n = 4, 4%) small cell lung cancer (n = 3, 3%), and carcinoid (n = 2, 3%). The remaining 7 (7%) cases comprised various neoplasms such as lymphoma or other metastatic solid organ malignancies. Staging data were provided or extrapolated for 94 patients of which 49 (52%) had localized disease (Stage 0 or I) compared to 45 (48%) cases of regional or distant metastatic disease (Stages II–IV) disease. Most neoplasms were discovered in patients who were transplanted for COPD (n = 42, 41%), IPF/usual interstitial pneumonia (n = 38, 37%), or other forms of parenchymal lung disease (n = 21, 21%). Interestingly, there was a single case of lepidic predominant ADC reported in a patient with CF.10
Aggregate Survival for Primary NSCLC
The overall 3-year survival was 54.4% for patients with localized disease (Stage I, n = 32) compared to 5.7% for those with nonlocalized disease (Stages II–IV, n = 22) (Figure 1).
FIGURE 1.
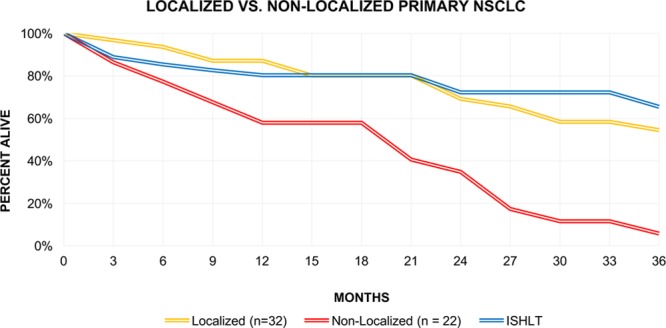
Three-y survival for patients with localized NSCLC (stage I), nonlocalized NSCLC (stages II–IV), and from ISHLT registry data for adult transplants. ISHLT, International Society for Heart and Lung Transplantation; NSCLC, nonsmall cell lung cancer.
DISCUSSION
Incidentally detected malignancies are not uncommonly found in explanted lungs at the time of lung transplantation with many centers reporting rates between 0.4% and 2.8% (overall 1.5%, 6746 patients) (Table 3).12-18,20,21 In this study, we report a similar rate of 2.2% (n = 9) among 407 patients who underwent initial lung transplantation at our center from 1999 to 2017. ADC, along with AIS, was the most commonly discovered neoplasm, which reflects its prevalence among reported cases in the literature (n = 56, 55%; Tables 2 and 3). Fortunately, with the exception of 2 cases of metastatic disease, most cases (7) were found to have localized disease on pathologic evaluation of explanted lungs and posttransplant imaging. Only 1 death (Patient 5) was directly attributed to malignancy.
TABLE 3.
Literature review
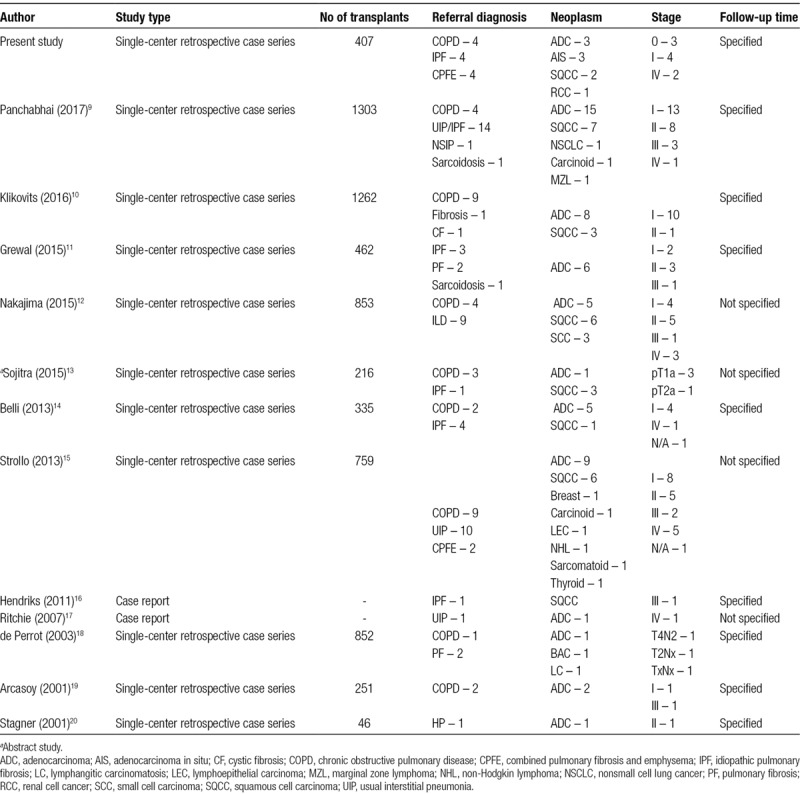
To evaluate the clinical significance of these neoplasms on posttransplant survival, we sought to search the literature for reported cases of incidentally discovered malignancies. These data, represented in Table 3, are similarly reported by Panchabhai with the exception of utilizing reported survival times and exclusion of a survey study conducted by de Perrot that reported 69 cases of bronchogenic carcinoma and multifocal bronchoalveolar carcinoma among 150 surveyed institutions before 2003.9,21 This study was excluded to avoid including overlapping data from institutions we have included as part of our literature review that would have otherwise responded to that survey.
For patients with localized primary NSCLC, the 3-year survival was found to be 54.4%, which is slightly lower to that of all adult transplant recipients registered by the ISHLT (65%) (Figure 1).1 Although this may suggest that transplantation is a reasonable therapeutic option as it would offer a surgical alternative for patients with poor pulmonary reserve or bypassing the need for adjuvant therapy, it remains inferior to lobectomy, segmental resection, and wedge resection, all of which offer optimal estimated 3-year survival rates between 70% and 85% for Stage IA NSCLC.23,24 Many of these patients continue to be at risk for postoperative recurrence, which in turn could affect posttransplant survival, management, and quality of life. For example, in a series of 1703 patients, recurrent NSCLC occurred in 445 of whom 76 had Stage IA and 84 had Stage IB disease.25 This is also not an unusually encountered phenomenon among recipients who have already undergone transplantation in whom malignant neoplasms were discovered in explanted lungs.9,12,18,19
Conversely, patients who had regional or distant metastatic primary NSCLC (stages II–IV) discovered at the time of transplantation had significantly worse 3-year survival of 5.7% which is similar to that of stage IV disease (Figure 1).26 Although available data on the proportion of those patients who underwent adjuvant chemotherapy or radiation therapy are sparse, it does raise the question of whether the added effect of immunosuppression and even arguably, comparatively poorer posttransplant performance status may have played in survival outcomes. Rarely, those with more indolent or therapy responsive forms of cancer, such as Patient 6 who survived for 56.5 months with metastatic renal cell carcinoma, have more favorable outcomes.
Interestingly, in our series, despite a mean tumor size of 1.58 cm (range 0.6–2.5 cm, excluding RCC microfocus) that should have been expectedly detected on pretransplant chest CT or PET/CT (mean 6.7 mo, range 1–12 mo), there were minimal features to suggest malignancy. Possible correlative lesions in 6 of 9 patients were fairly nonspecific with either <0.6 cm nodules that were stable on 2 subsequent scans, small scattered nonspecific nodularity, or the presence of fibroelastic scars. In 3 of 9 patients, all of whom had fibrotic lung disease, there were no correlative lesions on pretransplant evaluation. This is in contrast to a series published in 2013 where 10 of 22 patients with incidental neoplasms had CT positive correlative lesions whereas in the remaining 12 cases, malignancy was not confidently detected. The mean interval time from chest CT to lung transplantation in this study was 4 months (range 0–17 mo).15
Overall, it appears that despite acceptable radiographic evaluation and appropriateness of timing before transplantation, malignancies are often unidentified. Specifically, in patients with extensive fibrotic or emphysematous disease, evaluation of even relatively larger lesions by CT may be difficult due to visuospatial limitations related to architectural distortion of the lung parenchyma or radiographic heterogeneity of these lesions. While retrospective correlation of radiologic and pathologic features may make identification of malignant neoplasms more apparent, prospective radiographic evaluation at the time of imaging is much more challenging. Even when suspicious features are readily identified, many lesions may be benign mimickers of malignancy when pathologically correlated.15 Although several of these lesions may warrant further exploration, many patients are often precluded from transbronchial, mediastinal exploration, or surgical lung biopsy due to poor pulmonary reserve. Lastly, due to the critical timing between the availability of a transplant, many patients are often transplanted before follow-up imaging that may reveal more apparent malignant features.
18F-fluorodeoxyglucose (FDG)-PET is a valuable tool in the evaluation of solitary pulmonary nodules and clinical staging for many solid malignancies; however, there are many limitations that may make its use in routine evaluation of all transplant candidates challenging. First, the diagnostic sensitivity, specificity, and accuracy is decreased for subcentimeter nodules <0.8 cm, lesions with a predominant subsolid component (pure ground glass, part-solid), and certain histological subtypes (carcinoid, AIS, well differentiated and minimally invasive ADC).27-30 Next, additional visuospatial limitations may make radiographic determination of the invasiveness of the tumor difficult along with its inability to specifically distinguish malignancies from other FDG avid lesions and accurately determine lesion size, which is a crucial component of follow-up surveillance imaging. This is challenging in situations where the correlative sequential CT component is of limited use. For example, malignancies may be nested in areas of dense fibrosis and honeycombing, which have shown to have higher than normal SUV uptake and target-to-background ratios in patients with IPF and other diffuse parenchymal lung diseases.31-33
Limitations to our study are primarily those associated with retrospective studies. Notably, given that our results include data over an extended period of time, transplant and oncologic survival may be influenced by an evolving spectrum of standardized practice. Next, the data, which are currently available in the literature, lacks clearly defined survival times and staging data. As a result, the available data pool to perform a survival analysis is relatively small. This precludes further exploration by individual cancer stage or type and is not powered to perform a robust multivariate regression analysis while correcting for known confounders of malignancy. We aim to further evaluate this aspect in greater detail by expanding our analysis to a larger and more comprehensive dataset available from the ISHLT registry. Conversely, the data from our center are strengthened by a well-defined patient population with accurate pathologic and radiographic historical data with near complete follow-up.
In conclusion, unidentified malignancies occur despite aggressive radiographic surveillance with expectedly poor posttransplant outcomes in patients with advanced malignancy. The challenges primarily center around extensive parenchymal changes seen in end-stage lung disease that make it difficult to identify or distinguish malignant features with current radiographic tools, contraindications to follow-up pathologic evaluation due to poor pulmonary reserve, and the critical timing between organ availability and transplant listing. As a result, the necessity for developing and implementing alternative diagnostic methods involving serum or sputum biomarkers, surveillance bronchoscopy, or novel radiographic modalities as adjuvants in screening high risk populations is critical in capturing disease early and appropriately in patients with parenchymal lung disease.
Footnotes
Published online 8 October, 2019.
The authors declare no funding or conflicts of interest.
D.A. participated in research design, performance of research, data collection, data analysis, and writing of the article. W.H. participated in research design, data analysis, and writing of the article. D.N. participated by reviewing the article. S.V. participated in research design and reviewing the article.
REFERENCES
- 1.Yusen RD, Edwards LB, Dipchand AI, et al. ; International Society for Heart and Lung Transplantation The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016351170–1184 [DOI] [PubMed] [Google Scholar]
- 2.Smeritschnig B, Jaksch P, Kocher A, et al. Quality of life after lung transplantation: a cross-sectional study. J Heart Lung Transplant 200524474–480 [DOI] [PubMed] [Google Scholar]
- 3.Powell HA, Iyen-Omofoman B, Baldwin DR, et al. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol 201386–11 [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ, Adcock IM. Chronic obstructive pulmonary disease and lung cancer: a lethal association. Am J Respir Crit Care Med 2011184866–867 [DOI] [PubMed] [Google Scholar]
- 5.Jung HI, Park JS, Lee MY, et al. Prevalence of lung cancer in patients with interstitial lung disease is higher than in those with chronic obstructive pulmonary disease. Medicine (Baltimore) 2018;97:e0071. doi: 10.1097/MD.0000000000010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki T, Katsura H, Sawabe M, et al. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Intern Med 200342483–489 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Yang M, Li P, et al. Idiopathic pulmonary fibrosis will increase the risk of lung cancer. Chin Med J (Engl) 20141273142–3149 [PubMed] [Google Scholar]
- 8.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant 2015341–15 [DOI] [PubMed] [Google Scholar]
- 9.Panchabhai TS, Arrossi AV, Patil PD, et al. Unexpected neoplasms in lungs explanted from lung transplant recipients: a single-center experience and review of literature. Transplant Proc 201850234–240 [DOI] [PubMed] [Google Scholar]
- 10.Klikovits T, Lambers C, Ghanim B, et al. Lung transplantation in patients with incidental early stage lung cancer-institutional experience of a high volume center. Clin Transplant 201630912–917 [DOI] [PubMed] [Google Scholar]
- 11.Grewal AS, Padera RF, Boukedes S, et al. Prevalence and outcome of lung cancer in lung transplant recipients. Respir Med 2015109427–433 [DOI] [PubMed] [Google Scholar]
- 12.Nakajima T, Cypel M, de Perrot M, et al. Retrospective analysis of lung transplant recipients found to have unexpected lung cancer in explanted lungs. Semin Thorac Cardiovasc Surg 2015279–14 [DOI] [PubMed] [Google Scholar]
- 13.Sojitra P, Muralidhar A, Quddus S, et al. Incidental primary neoplasms in explanted lungs at transplantation. J Heart Lung Transplant 201534SupplementS317–S318 [Google Scholar]
- 14.Belli EV, Landolfo K, Keller C, et al. Lung cancer following lung transplant: single institution 10 year experience. Lung Cancer 201381451–454 [DOI] [PubMed] [Google Scholar]
- 15.Strollo DC, Dacic S, Ocak I, et al. Malignancies incidentally detected at lung transplantation: radiologic and pathologic features. AJR Am J Roentgenol 2013201108–116 [DOI] [PubMed] [Google Scholar]
- 16.Hendriks LE, Drent M, van Haren EH, et al. Lung cancer in idiopathic pulmonary fibrosis patients diagnosed during or after lung transplantation. Respir Med Case Rep 2012537–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie AJ, Mussa S, Sivasothy P, et al. Single-lung transplant complicated by unexpected explant carcinoma: a management dilemma. J Heart Lung Transplant 2007261206–1208 [DOI] [PubMed] [Google Scholar]
- 18.de Perrot M, Fischer S, Waddell TK, et al. Management of lung transplant recipients with bronchogenic carcinoma in the native lung. J Heart Lung Transplant 20032287–89 [DOI] [PubMed] [Google Scholar]
- 19.Arcasoy SM, Hersh C, Christie JD, et al. Bronchogenic carcinoma complicating lung transplantation. J Heart Lung Transplant 2001201044–1053 [DOI] [PubMed] [Google Scholar]
- 20.Stagner LD, Allenspach LL, Hogan KK, et al. Bronchogenic carcinoma in lung transplant recipients. J Heart Lung Transplant 200120908–911 [DOI] [PubMed] [Google Scholar]
- 21.de Perrot M, Chernenko S, Waddell TK, et al. Role of lung transplantation in the treatment of bronchogenic carcinomas for patients with end-stage pulmonary disease. J Clin Oncol 2004224351–4356 [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag New York; 2010. [Google Scholar]
- 23.Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the national cancer data base. J Thorac Oncol 2015101625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai C, Shen J, Ren Y, et al. Choice of surgical procedure for patients with non-small-cell lung cancer ≤ 1 cm or > 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol 2016343175–3182 [DOI] [PubMed] [Google Scholar]
- 25.Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 200783409–17. Discussion 417 [DOI] [PubMed] [Google Scholar]
- 26.Ries LAG, Young JL, Keel GE, et al. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215. Bethesda, MD: 2007. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. [Google Scholar]
- 27.Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013143825–839 [DOI] [PubMed] [Google Scholar]
- 28.Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013143840–846 [DOI] [PubMed] [Google Scholar]
- 29.Suzawa N, Ito M, Qiao S, et al. Assessment of factors influencing FDG uptake in non-small cell lung cancer on PET/CT by investigating histological differences in expression of glucose transporters 1 and 3 and tumour size. Lung Cancer 201172191–198 [DOI] [PubMed] [Google Scholar]
- 30.Lococo F, Cesario A, Paci M, et al. PET/CT assessment of neuroendocrine tumors of the lung with special emphasis on bronchial carcinoids. Tumour Biol 2014358369–8377 [DOI] [PubMed] [Google Scholar]
- 31.Groves AM, Win T, Screaton NJ, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med 200950538–545 [DOI] [PubMed] [Google Scholar]
- 32.Justet A, Laurent-Bellue A, Thabut G, et al. [18F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir Res. 2017;18:74. doi: 10.1186/s12931-017-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Win T, Screaton NJ, Porter JC, et al. Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging 201845806–815 [DOI] [PMC free article] [PubMed] [Google Scholar]


