Abstract
Alpha-fetoprotein (AFP), as the most widely used biomarker of hepatocellular carcinoma (HCC), was correlated with ongoing liver damage. The aim of this study was to evaluate the ability of inflammatory correction-based AFP to identify HCC from other liver diseases.
From March 2012 to March 2017, among 926 participants, a total of 501 patients whose transaminases were higher than the upper limit of normal range, including 166 treatment-naïve HCC patients were enrolled in our retrospective study. The liver function, white blood cell (WBC) count and serum AFP level of all patients were collected at the initial stage of admission. The area under the receiver-operating curve (AUROC) of AFP, AFP/(Aspartate aminotransferase∗Alanine aminotransferase) [AFP/(AST∗ALT)] and AFP/WBC were compared between the HCC group and the control groups for the quantifying diagnostic efficacy.
AUROCs of our novel index AFP/(AST∗ALT) were up to 0.853 (95% confidence interval, CI 0.818–0.887, P < .001) and 0.825 (95% CI 0.782–0.868, P < .001), respectively, when differentiating HCC from non-HCC patients and from cirrhosis patients, which was superior to AFP and AFP/WBC. Diagnostic performance of AFP/(AST∗ALT) could be verified in hepatitis B virus (HBV)- or hepatitis C virus (HCV)-associated HCC patients as well. What's more, AFP/(AST∗ALT) had a significant positive and moderate correlation with tumor diameter and presence of cancerous emboli or not (Spearman correlation coefficients were 0.323 and 0.305, respectively; both P < .001). For predicting HCC, the optimal cut-off value of AFP/(AST∗ALT) is 1.603, and the sensitivity and specificity were 82.8% and 72.7%, respectively, which were significantly higher than the AFP and AFP/WBC.
The serum AFP levels based on correction of liver inflammation can effectively improve the diagnostic performance of HCC, providing a new indicator that is simple, economical and pervasive for clinic.
Keywords: alpha-fetoprotein, diagnosis, hepatocellular carcinoma, inflammatory correction
1. Introduction
Hepatocellular carcinoma (HCC), one of the most common malignancies in the world, highly occurs in Eastern Asia, South-Eastern Asia, and Sub-Saharan Africa. The most frequently underlying aetiologies of HCC are chronic viral hepatitis, alcohol intake, and aflatoxin exposure.[1] Worldwide, approximately 80% of cases can be attributed to HBV and HCV infection, however, the data do not reflect co-morbidities, such as non-alcoholic steatohepatitis and metabolic syndrome.[2] Furthermore, the prevalence of non-alcoholic fatty liver disease (NAFLD) has risen conspicuously. The morbidity of HCC varies from 0% to 3% in NAFLD patients and from 2.4% to 12.8% in NAFLD associated cirrhosis patients.[3]
China has the heaviest burden of HCC where about 50% of all new cases and deaths related liver cancer worldwide occurred,[4] and the major risk factors are still HBV (63.9%) or HCV (27.7%) infection. The latest data indicated that the morbidity and mortality of HCC ranked the fourth and third, respectively, among all malignant tumors reported in China,[5] where much more attention should be payed to the prediction of HCC.
Diagnosis of HCC is based on a characteristic combination of serological, imaging, and pathological features. AFP is the most widely used biomarker, however, the elevated serum AFP levels are also frequently observed in some patients with benign liver diseases,[6,7] especially in cirrhosis. It was demonstrated that AFP levels increased as pathological levels of inflammation and fibrosis increased in chronic HBV or HCV infections,[6,8,9] and there were studies indicating that AFP levels had a significant positive correlation with AST and ALT.[6,8,9] What's more, about 30% of HCC patients have normal AFP levels. Studies have demonstrated a serum-based tool called GALAD score for the surveillance of HCC based on logistic regression for age, sex, and the 3 serologic biomarkers of AFP, AFP-L3, and prothrombin induced by vitamin K absence-II (PIVKA-II).[10,11] Nevertheless, the measurement tools and units used in international GALAD score do not meet the requirements in China and too many testing indexes will increase the financial burden on patients.
AST and ALT represent disruption of hepatocytes, and hepatic inflammation itself could lead to an increase in AFP levels, rather than a tumor state. Moreover, AFP levels are positively correlated with transaminase levels to some extent, which has the interference with HCC diagnosis. In order to correct the effect of inflammation on the increase of AFP levels, AST, and ALT were used as denominators, so we developed an optimal indicator: AFP/(AST∗ALT) to make a diagnosis of HCC by applying the most accessible clinical indexes. Besides, our study also explored the influences of the peripheral inflammatory response on AFP.
2. Patients and methods
2.1. Study design and participants
In our retrospective study, the participants, including HCC group (newly diagnosed), and control patients with chronic hepatitis B infections (CHB), chronic hepatitis C infections (CHC), non-viral liver diseases, cirrhosis, cholangiocarcinoma were enrolled from the First Hospital of Jilin University (Changchun, China) between March 2012 and March 2017. Non-viral liver diseases mainly consisted of autoimmune liver disease, alcoholic liver disease, non-alcoholic fatty liver disease and drug-induced liver injury. In order to determine whether AFP was associated with the liver inflammation, only patients with abnormal liver function (defined as AST and ALT exceeding the upper limit of normal value at the same time) were included in the study. The exclusion criteria were:
-
1.
unavailable AFP value;
-
2.
undergoing extrahepatic acute diseases;
-
3.
any types of malignancy for patients with the exception of hepatobiliary system.
The HCC diagnosis was confirmed with histological findings or typical imaging characteristics according to the guidelines of the European Association for the Study of the Liver (EASL) [1] and the diagnosis of cirrhosis or other liver diseases were based on clinical indicators and imageological examination in accordance with the international guidelines.[12–16] The study was conducted in accordance with Declaration of Helsinki and approved by the Ethics Committee of the First Hospital of Jilin University.
2.2. Treatment of serum and data analysis
All serum samples were collected and measured in the morning with all patients being told over-night fasting at the initial stage of admission. Serum AFP was measured quantitatively by electrochemiluminescence (Cobas e601, Roche) in the clinical laboratory of the First Hospital of Jilin University, with the normal reference value of 0–7 ng/ml and an upper limit of detection of 1210 ng/ml. There were several inspectors executed and read the index tests and the reference standard with rich experience and professional accomplishment from clinical laboratory of the First Hospital of Jilin University.
Statistical analyses were performed with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were shown as median (25th/75th percentile) due to skewness distribution of data and categorical variables were displayed as numbers or percentages. The nonparametric Mann-–Whitney U test and χ2 test were used for statistical comparisons as appropriate. For evaluating the diagnostic performance of AFP, AFP/(AST∗ALT), and AFP/WBC, the sensitivity (true-positive rate) and specificity (true-negative rate) of optimal cut off values for the diagnosis of HCC were calculated as described. The ROC curve is a plot of sensitivity vs 1-specifcity for all possible cut off values, so the direct comparison of diagnostic values of these indexes for predicting HCC were assessed by calculating the areas under the ROC. Optimal cut-off value is determined by the best Youden's index, which is calculated by the following formula. All of the differences were considered statistically significant at P < .05.
Formulae:
3. Results
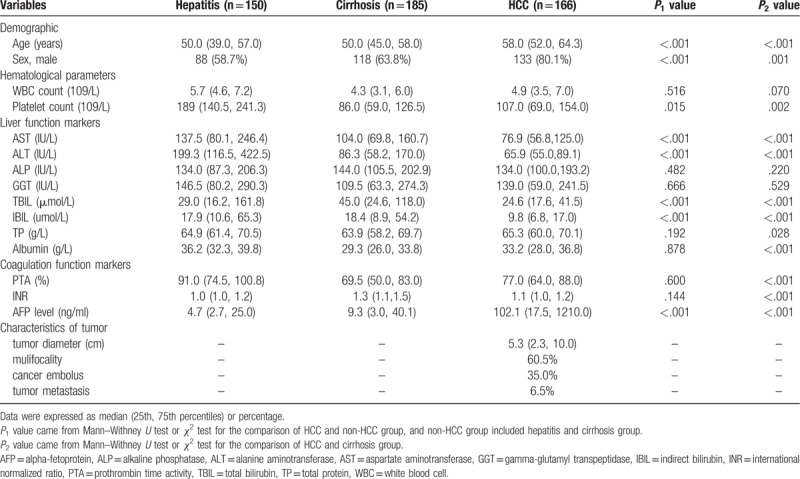
3.1. Demographic characteristics
Overall, 926 participants were enrolled in this study, of which 501 participants were eligible for the condition of increased transaminase levels and became the study population, including 166 treatment-naïve HCC patients, 185 patients with cirrhosis and 150 patients with other liver diseases (Hepatitis group). Among these 501 patients, 254 patients (50.70%) were infected with HBV and 86 patients (17.17%) were infected with hepatitis C virus (HCV). The baseline characteristics were described between different study groups in Table 1. Overall, there were 339 (67.7%) males and 162 females, with a median age of 52.0 years (interquartile range, IQR 46.0–61.0). Hematological parameters, liver function markers, coagulation function markers, AFP level, and characteristics of tumors were also placed into the Table 1. Different P values were calculated in terms of demographic and clinical characteristics. P values demonstrated statistically significant differences for comparisons of HCC with non-HCC and HCC with cirrhosis group in platelet, AST, ALT, total bilirubin (TBIL), indirect bilirubin (IBIL), AFP. For the HCC group, the median of tumor diameter was 5.3 cm (IQR 2.3–10.0), multifocal tumors were present in 60.5% HCC patients, and 35%, 6.5% had cancer emboli in blood vessels and metastasis, respectively.
Table 1.
Baseline characteristics of study population.
3.2. Comparison of diagnostic values of biomarkers for diagnosing HCC
According to the analysis of AUROC, the novel index AFP/(AST∗ALT) (AUROC = 0.853; 95% confidence interval, CI 0.818–0.887, P < .001) was superior to AFP (AUROC = 0.787; 95% CI 0.744–0.829, P < .001) and AFP/WBC (AUROC = 0.786; 95% CI 0.744–0.828, P < .001) in distinguishing the tumors between HCC patients and non-HCC patients (Fig. 1A). For predicting HCC, the optimal cut-off value of AFP/(AST∗ALT) is 1.603, in this case, the sensitivity and specificity were 82.8% and 72.7%, respectively, which were significantly higher than the AFP itself.
Figure 1.

AUROC for AFP, AFP/WBC and AFP/(AST∗ALT) for the diagnosis of HCC in different groups: (A) HCC vs Non-HCC; (B) HCC vs Cirrhosis; (C) HBV-HCC vs HBV and (D) HCV-HCC vs HCV. Non-HCC represented the patients without HCC, HBV-HCC represented HBV related HCC, HCV-HCC represented HCV related HCC. AFP = alpha-fetoprotein, ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUROC = area under the receiver-operating curve, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, WBC = white blood cell.
Cirrhosis, as one of the most important risk factors for HCC, needed to be separately compared with the HCC group to highlight the practicability and validity of our diagnostic indicators and Fig. 1B has been showed between these 2 groups. AFP/(AST∗ALT) still showed a high predictive value (AUROC = 0.825; 95% CI 0.782–0.868, P < .001), accompanied by a superior sensitivity and a cut-off value of 2.907. In addition, CHB or CHC, as the main components of causes in HCC patients, were identified as nontumor characteristics that influenced serum AFP levels, the data was further analyzed by subgroups of etiology (Fig. 1C and D). In HBV group, we compared different predictors between patients with HBV infection or HBV-associated cirrhosis and patients with HBV-associated HCC (HBV-HCC), and similarly in HCV group. Diagnostic performance of HCC markers in the above four groups were summarized in Table 2. Above all, AFP/(AST∗ALT) performed the best diagnostic efficacy, while AFP/WBC showed the worst.
Table 2.
Diagnostic performance of HCC markers in different groups.

Correlation analysis has been made in order to discuss the relationship between AFP/(AST∗ALT) and tumor characteristics. We found it had a significant positive and moderate correlation with tumor diameter and presence of cancerous emboli or not (Spearman's correlation coefficients were 0.323 and 0.305, respectively; both P < .001), however, it was not associated with mulifocality or metastases, and the above results were similar to AFP.
4. Discussions
According to the guidelines from European Association for the Study of the Liver 2018, Liver cancer is the fifth most common cancer and the second most frequent cause of cancer-related death globally. HCC, which accounts for about 90% of primary liver cancer, is a major health problem.[6] In epidemiology, HCC occurs more frequently in male, with a male to female ratio estimated to be 2–2.5:1 in different regions.[6] Similarly, HCC had a strong male (80.1%) preponderance in our study.
Although the combination of AFP, AFP-L3 and PIVKA-II achieved a superior diagnostic performance, in most areas, limited by economic or medical conditions, examination of AFP-L3 and PIVKA-II are not widely available. And subsequent studies found AFP was still the most useful single biomarker and adding AFP-L3 did not enhance the ability to distinguish HCC from non-HCC patients.[17] Up to now, AFP is the only tumor marker widely used in screening and diagnosing HCC. In China, AFP > 400 ng/ml has been set as a diagnostic threshold for clinical diagnosis and staging criteria, excluding chronic or active hepatitis, cirrhosis, testicular or ovarian embryogenic tumor or pregnancy. However, hepatitis or cirrhosis, the main causes of HCC, could not be ruled out, and in our study, sensitivity of AFP using the cut-off at 400 ng/ml was 39.3% (HCC vs Non-HCC) and 39.3% (HCC vs Cirrhosis), both of which were low and the missed diagnosis rate was high.
In addition to hepatic malignancy, the elevation of serum AFP level could be seen in non-cancerous liver diseases, caused by regeneration after liver cell necrosis. Numerous studies have found that AFP decreased or increased in parallel with the levels of AST and ALT synthesized by the liver, so AFP could indirectly reflect the degree of liver inflammation and liver injury, thereby affecting its diagnosis of HCC. Asahina et al even indicated ALT levels were significantly associated with hepatocarcinogenesis.[18] It has been clinically emphasized that AFP levels were susceptible to liver inflammation, but so far, there are no indicators that can effectively avoid the influences of inflammatory factors. Initially, our study aimed to design a prediction model through logistic regression, and yet, interestingly, the ratio of AFP to transaminases was better than the model of logistic regression. We finally found the optimal index to predict HCC over repeated trials. AUROCs of this novel index AFP/(AST∗ALT) were up to 0.853 and 0.825, respectively, when differentiating HCC from non-HCC patients and from cirrhosis patients, with its sensitivity and specificity significantly increasing.
Hong et al suggested that the selection of candidate blood-based mRNA biomarkers for distinguishing cancer from inflammation-associated diseases may yield misleading results,[19] meaning that many serologic tumor markers were affected by inflammation in peripheral blood. Our study further explored the diagnostic capacity of AFP to WBC ratio, which was the worst for the prediction of HCC. Analyzing the reason of this phenomenon, it was considered to be connected with hypersplenism caused by cirrhosis, which led to the destruction and reduction of platelets and WBC.
Differently from the etiology of HCC in Europe and America, most of HCC in China is related to the infection of HBV or HCV. Soresi et al mentioned that the positive predictive value of AFP in viral-associated HCC patients was significantly lower than that of non-viral HCC[20] and serum AFP levels in HBV-HCC patients were significantly higher than those in non HBV-HCC patients.[21,22] Therefore, it was necessary to focus on HBV- or HCV-associated HCC separately with new indicators. From Figure 1 and Table 2, we found that diagnostic efficacy of AFP/(AST∗ALT) could be verified in different underlying diseases, with AUROC more than 0.8. What's more, AFP/(AST∗ALT) was obviously superior to AFP in HBV-HCC patients.
Although viral infection is the leading cause of liver disease, the new epidemic is related to the burden of NAFLD, paralleling the worldwide increase of obesity.[23] NAFLD, with the global prevalence estimated to be 24%, might represent the missing link between cryptogenic cirrhosis and HCC.[24] We expect to explore the diagnostic efficacy of our new indicator for NAFLD-associated HCC in future studies.
There were several limitations in our study. Firstly, the patients in our study came from a single-center, our model has not been validated in large-scale studies. Secondly, although all HCC patients enrolled in the study were newly diagnosed, we could not guarantee that all patients did not have their own oral medication to reduce transaminase levels before admission. Thirdly, in our study, AFP/(AST∗ALT) and AFP had a moderate correlation with tumor diameter and presence of cancerous emboli or not, however, the results of related studies about AFP were inconsistent, and further studies are necessary.
In conclusion, our study findings indicated that the novel index AFP/(AST∗ALT) could provide useful information for the prediction of HCC, which could aid in minimizing the interference of liver inflammation to AFP. Meanwhile, AFP/(AST∗ALT) showed the highest AUROC than AFP and AFP/WBC with reasonable sensitivity and specificity in different groups. The optimal cut-off values of AFP/(AST∗ALT) could be used for the clinical diagnosis of HCC, also the prediction of therapeutic effect and evaluation of prognosis.
Author contributions
Data curation: Xu Liu, Jing Meng.
Investigation: Xu Liu, Junqi Niu.
Methodology: Xu Liu, Hongqin Xu.
Resources: Jing Meng.
Software: Hongqin Xu.
Writing – original draft: Xu Liu.
Writing – review & editing: Xu Liu, Junqi Niu.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUROC = area under the receiver-operating curve, CHB = chronic hepatitis B infections, CHC = chronic hepatitis C infections, CI = confidence interval, EASL = European Association for the Study of the Liver, GGT = gamma-glutamyl transpeptidase, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, IBIL = indirect bilirubin, INR = international normalized ratio, IQR = interquartile range, PIVKA-II = prothrombin induced by vitamin K absence-II, PTA = prothrombin time activity, TBIL = total bilirubin, TP = total protein, VS = versus, WBC = white blood cell.
Data came from the First Hospital of Jilin University of China between March 2012 and March 2017, which are available from the corresponding author upon request.
There are no conflicts of interest to disclose, in addition, we have not received any financial grants and other funding.
References
- [1].Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- [2].Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:1342–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [5].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [6].Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005;43:434–41. [DOI] [PubMed] [Google Scholar]
- [7].Wang T, Zhang KH, Hu PP, et al. Combination of dual serum fluorescence, AFP and hepatic function tests is valuable to identify HCC in AFP-elevated liver diseases. Oncotarget 2017;8:97758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu YR, Lin BB, Zeng DW, et al. Alpha-fetoprotein level as a biomarker of liver fibrosis status: a cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC Gastroenterol 2014;14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim CY, Bo RK, Sang SL, et al. Clinical features of hepatitis B and C virus infections, with high a-fetoprotein levels but not hepatocellular carcinoma. Medicine 2017;96:e5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Toyoda H, Kumada T, Kagebayashi C, et al. Diagnosis of hepatocellular carcinoma using a GALAD model by objectiveclinical and serological factors. Hepatology 2013;58:1230A. [Google Scholar]
- [11].Berhane S, Toyoda H, Tada T, et al. Role of the galad and balad-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol 2016;14:875–86. e6. [DOI] [PubMed] [Google Scholar]
- [12].Chalasami N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- [13].European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- [14].European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017;66:153–94. [DOI] [PubMed] [Google Scholar]
- [15].Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193–213. [DOI] [PubMed] [Google Scholar]
- [16].Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. [DOI] [PubMed] [Google Scholar]
- [17].Park SJ, Jang JY, Jeong SW, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine 2017;96:e5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Asahina Y, Tsuchiya K, Nishimura T, et al. Alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology 2013;58:1253–62. [DOI] [PubMed] [Google Scholar]
- [19].Hong G, Chen B, Li H, et al. Similar source of differential blood mRNAs in lung cancer and pulmonary inflammatory diseases: calls for improved strategy for identifying cancer-specific biomarkers. PLoS One 2014;9:e108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Soresi M, Magliarisi CP, Leto G, et al. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res 2003;23(2C):1747–53. [PubMed] [Google Scholar]
- [21].Liu C, Xiao GQ, Yan LN, et al. Value of a-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol 2013;19:1811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Giannini EG, Sammito G, Farinati F, et al. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: implications for its clinical use. Cancer 2014;120:2150. [DOI] [PubMed] [Google Scholar]
- [23].Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- [24].Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 2016;36:317–24. [DOI] [PubMed] [Google Scholar]


