Supplemental Digital Content is available in the text
Keywords: chronic kidney disease, costs, dialysis, multidisciplinary care, nephrology referral
Abstract
Evidence-based studies have revealed outcomes in patients with chronic kidney disease that differed depending on the design of care delivery. This study compared the effects of 3 types of nephrology care: multidisciplinary care (MDC), nephrology care, and non-nephrology care. We studied their effects on the risks of requiring dialysis and the differences between these methods had on long-term medical resource utilization and costs.
We conducted a retrospective cohort study involving patients with an estimated glomerular filtration rate of (eGFR) ≤45 mL/min/1.73 m2 from 2005 to 2007. Patients were divided into MDC, non-MDC, and non-nephrology referral groups. Between-group differences with regard to the risk of requiring dialysis and annual medical utilization and costs were evaluated using a 5-year follow-up period.
In total, 661 patients were included. After other covariates and the competing risk of death were taken into account, we observed a significant (56%) reduction in the incidence of dialysis in both the MDC and non-MDC groups relative to the non-nephrology referral group. Costs were markedly lower in the MDC group relative to the other groups (average savings: US$ 830 per year; 95% confidence interval: 367–1295; P < .001).
For patients without nephrology referrals, MDC can substantially reduce their risk of developing end-stage renal disease and lower their medical costs. We therefore strongly advocate that all patients with an eGFR of ≤45 mL/min/1.73 m2 should be referred to a nephrologist and receive MDC.
1. Introduction
Chronic kidney disease (CKD) is a global public health problem associated with increased morbidity, mortality, and substantial health care costs.[1] CKD care is complex, usually requiring the knowledge, skills, and experience of various clinical professionals. Studies have demonstrated that CKD outcomes can be improved by the delivery of appropriate health care services, with timely nephrology department referral and multidisciplinary care (MDC) having the most remarkable effects in patients with advanced CKD. Some[2,3,4] but not all[5] of these studies have demonstrated that patients substantially benefit if they receive early nephrology department referrals. Singhal et al[6] argued that the quantity of predialysis care rather than a nephrology referral alone significantly contributes to survival after dialysis. However, adequate predialysis renal care is multidimensional and thus difficult to be defined and performed by a single specialist.[7]
MDC is a common form of care that brings together health care professionals from various disciplines. It has been widely applied in CKD treatment to manage complex therapies and deliver comprehensive health care services. Studies have demonstrated that compared with other forms of care, MDC results in more effective medication prescriptions, a lower renal progression rate, a decreased risk of temporal catheterization for dialysis, and a decreased use of medical services.[8,9,10,11,12] In addition, relative to those receiving care from a nephrologist only, better survival outcomes and slower progress to end-stage renal disease (ESRD) have been observed in patients with CKD receiving MDC.[13] Although these studies have similar results, none have compared the effects of MDC relative to those of nephrology and other types of care in a hospital-based population. It is common for patients with CKD to receive care from various specialists. A lack of information on patients who receive care from specialists other than nephrologists—such as cardiologists, endocrinologists, general practitioners, and practitioners of traditional Chinese medicine—can lead to an incomplete understanding of CKD care in the real world and lost opportunities to improve current practices.[14] There is an urgent need to clarify healthcare gaps and formulate an optimal predialysis care model through clinical practice. This study will also be helpful in the design of a more effective care delivery system for patients with CKD.
This study aimed to investigate the proportion of patients with advanced CKD who were receiving MDC, care from nephrologists, and care from specialists other than nephrologists. In addition, the differences between groups with regard to their results from laboratory monitoring, risk of requiring dialysis, and medical service utilization were also evaluated.
2. Methods
2.1. Design and setting
We conducted a retrospective cohort study using claims and laboratory databases from 1 regional hospital. The regional hospital had 489 beds and provided acute and chronic medical services for the 150,000 residents of a region in Southern Taiwan. There were no nearby hospitals of comparable size. We first identified the patients with CKD who had received care at the hospital between 2005 and 2007. We used an approach adapted from a previous report that classified patients using codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). CKD was indicated by a patient having 1 or more inpatient visits or 2 or more outpatient visits within 1 year.[15] To ensure that we precisely identified the patients with CKD, we calculated their estimated glomerular filtration rate (eGFR) using the CKD–EPI equation and traced their first eGFR measurement after their diagnosis of CKD.[16] Patients with a first eGFR of ≤45 mL/min/1.73 m2 were selected for our study cohort. The date of first eGFR measurement after a diagnosis of CKD was considered the index date.
The non-MDC group comprised patients who had 1 or more nephrology depart visits after the index date but received no MDC. The MDC group comprised patients who had received both nephrology referrals and MDC. The non-nephrology referral group comprised all the other patients. We followed-up these patients from the index date to December 31, 2012.
This study was approved by the ethical review board of Kaohsiung Medical University (KMUH-IRB-20130072). All researchers in this study followed the directives of the Declaration of Helsinki.
2.2. Multidisciplinary care program
In our professional practice, we have conducted an MDC program since 2004. It has brought together various healthcare professionals—such as nephrologists, nurses, dieticians, pharmacists, and social workers—to deliver comprehensive CKD services. Patients with an eGFR of ≤45 mL/min/1.73 m2 and who were diagnosed as having CKD by nephrologists during outpatient or admissions visits were referred to nurses who informed them of the program. If patients agreed to join the MDC program, the nursing staff conducted a series of surveys with patients. The survey comprised questions on a patient's evaluation of medical and nursing care, their physical functions, knowledge of CKD, psychosocial status, and economic status. The patients were requested to return to the clinic every 1 to 3 months, depending on their health conditions. Routine laboratory tests and the educational programs provided by the nursing staff were conducted at least once every 3 months. Dietitian consultations and drug safety education sessions were carried out by pharmacists every 6 months. Social workers in the MDC program provided active social support when necessary.
3. Measurement of study outcomes
Differences in the risk of ESRD and annual healthcare utilization and costs between groups were the primary focuses of this study. A patient's commencement of dialysis during the period of observation was considered a study event. Patients with ESRD who require dialysis can apply to have their requirement to make copayments waived via a catastrophic illness certificate. The event date was determined using the Registry for Catastrophic Illness Patients. Information on mortality and date of death were obtained from the Registry of Death at the hospital. Death before dialysis was a competing event; those who had not received dialysis and survived to the end of the follow-up period were considered to be censored. The extent of a patient's annual utilization of medical services before dialysis was indicated by their number of outpatient visits, number of hospital admissions, and length of stay. Their annual health care expenditure was indicated by their outpatient expenses, inpatient expenses, and overall expenses. These data were assessed from claim databases. All expenses were discounted by 3% annually and reported in US dollars (where US$ 1 = NT$ 30). To compare the frequency of biochemistry examinations between these groups, laboratory data on CKD control (serum albumin, blood urea nitrogen, serum creatinine, urine creatinine, and urine total protein), electrolyte control (calcium, phosphate, sodium, and potassium), and metabolic disease control (hemoglobin A1c, cholesterol, triglyceride, and fasting glucose) were obtained during the observation period.
4. Covariates of interest
We also collected data on patient characteristics—namely their age, sex, baseline eGFR, comorbidities (diabetes, hypertension, hyperlipidemia, cerebrovascular disease, and chronic obstructive pulmonary disease), Charlson comorbidity index score, and use of confounding drugs (antihypertensive, diabetic, antilipid, and nonsteroidal anti-inflammatory drugs, and analgesic drugs other than nonsteroidal anti-inflammatory drugs) as adjusted covariates. The present comorbidities were defined using ICD-9-CM codes (eTable 1 in Supplement 1) when they appeared 2 or more times in outpatient claims or 1 or more times in admission claims within 1 year before the index date. The Charlson comorbidity index score was calculated according to the weights of various diseases, as listed in a previous study.[17] Patients who had ever used a prescribed drug during the follow-up period were defined to have been treated with these drugs.
5. Statistical analysis
The distribution of patient characteristics was expressed as mean ± standard deviation, or median (interquartile range) for continuous variables and count (percentage) for categorical variables. The differences in the characteristics and frequency of biochemistry examinations among groups were compared using the Chi-squared test for categorical variables and one-way analysis of variance or the Kruskal–Wallis test for continuous variables. We used a competing risk analysis to both estimate the 5-year cumulative ESRD incident rates and compare the statistical difference in incident rates between groups using a modified log-rank test. Fine and Gray's[18] subdistribution hazard model with adjustments for various covariates was conducted to obtain the subdistribution hazard ratio (SDHR) and 95% confidence interval (CI). Generalized linear mixed models were used to analyze the differences in annual levels of expenditure on and utilization of medical services between the groups during the follow-up period. Because fitting a complicated model to our massive data set can be difficult, we simplified the model by assuming that the random effects had a normal distribution. This assumption is justified by studies that have demonstrated that even if the distribution of random effects is misspecified, little bias exists in the estimation of the covariates of effects.[19,20] Coefficients were estimated using robust standard errors to correct misspecifications of the correlation structures. Results were represented as adjusted mean differences and their 95% CIs between the 3 groups. The cumulative incidence in competing risk analyses was calculated using the cmprsk package in R (version 3.3.4, R Foundation for Statistical Computing, Vienna, Austria).[21] Statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc, Cary, NC), and figures were created using GraphPad Prism 5.0 (GraphPad Software Inc, San Diego, CA). A 2-sided P < .05 indicated statistical significance.
6. Results
6.1. Demographic characteristics
During 2007 to 2009, 1508 patients had an eGFR of ≤45 mL/min/1.73 m2 after CKD diagnosis. Patients who were excluded were aged <20 years (n = 1), had cancer (n = 18), or had no outpatient visits within the 5-year follow-up period (n = 828). Thus, 661 patients were eligible for follow-up. The non-nephrology referral, non-MDC, and MDC groups comprised 26.8%, 33.2%, and 40.0% of the study cohort, respectively. In general, relative to the other groups, the MDC group had a significantly lower average eGFR, a higher average Charlson score, and higher proportions of female patients, patients with diabetes, patients with hypertension, patients with hyperlipidemia, as well as patients who used more antihypertensive and diabetes drugs (Table 1).
Table 1.
Characteristics of the study cohort.
6.2. Quality of predialysis care
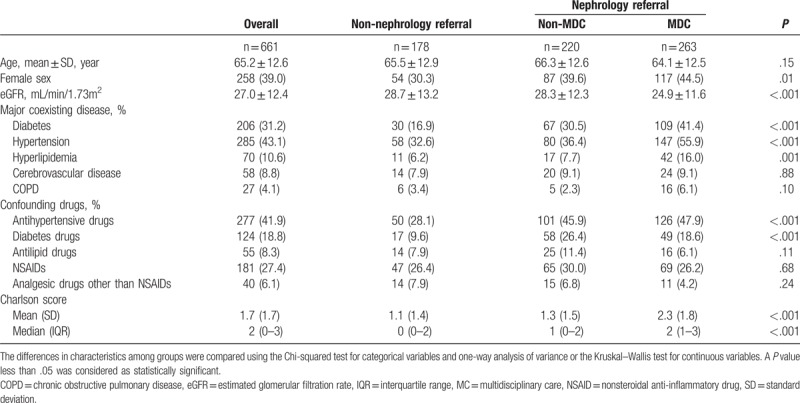
Figure 1 displays the proportions of biochemistry examinations in the observed period among the 3 groups. More than 70% of patients with an eGFR of ≤45 mL/min/1.73 m2 had ever had measurements of their blood urea nitrogen, serum creatinine, triglyceride, and fasting glucose taken. However, the proportions for serum albumin, urine creatinine, and protein, as well as the results obtained from examinations pertaining to electrolyte control, were diverse among the groups. The proportions for albumin, calcium, phosphate, as well as the urine creatinine–protein ratio were substantially higher in the non-MDC and MDC groups relative to the non-nephrology referral group. By contrast, the proportions for blood urea nitrogen and serum creatinine exhibited only slight differences between the 3 groups (Fig. 1A and B). More than 80% of the patients in the MDC group had undergone laboratory tests for electrolyte control (calcium, phosphate, sodium, and potassium). These proportions were significantly higher than those of the other 2 groups (Fig. 1B). Thorough laboratory tests for metabolic disease monitoring and control were also performed for patients in the MDC group and for patients in this group who had diabetes (Fig. 1C).
Figure 1.
Proportion of laboratory monitoring in patients with an estimated glomerular filtration rate of ≤45 mL/min/1.73 m2. (A) Laboratory data for chronic kidney disease control; (B) laboratory data for electrolyte control; and (C) laboratory data for metabolic disease control. BUN = blood urea nitrogen, Ca = calcium, Chol = total cholesterol, Cr = creatinine, Gluco = fasting blood glucose, HbA1c = hemoglobin A1c, K = potassium, Na = sodium, P = phosphate, TG = triglyceride, U-Cr = urine creatinine, U-TP = urine total protein. #, Only the data of patients with diabetes were included in the denominator.
6.3. Five-year cumulative incidence rate and hazard ratios of dialysis
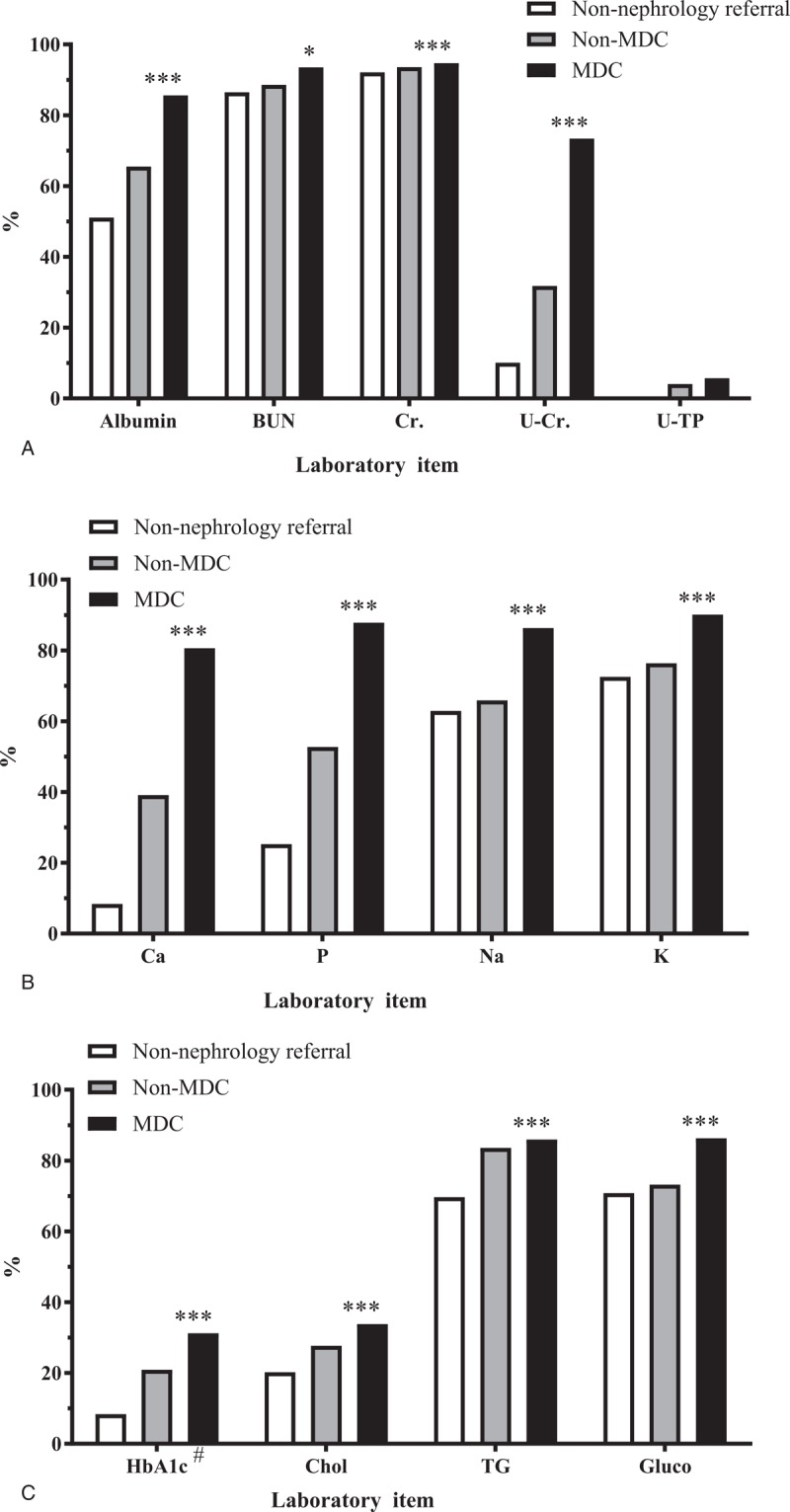
Figure 2 details the differences in the cumulative incident rate of dialysis of the 3 groups. During the first 3 years, the incidence of dialysis was higher in the non-nephrology referral group than the MDC and non-MDC groups (cumulative incident rate = 21.9%, 18.6%, and 13.1%, respectively). However, differences in the overall cumulative incidence of ESRD were not significant (Fig. 2). Similar results were observed in the subdistribution hazard model (both without adjustments, and with adjustments for age and sex).
Figure 2.
Risk of end-stage renal disease. The cumulative incidence of end-stage renal disease was estimated with consideration for the competing risk of mortality. Differences between the MDC, non-MDC, and non-nephrology referral groups were analyzed using modified Kaplan–Meier and Grey methods. MDC = multidisciplinary care.
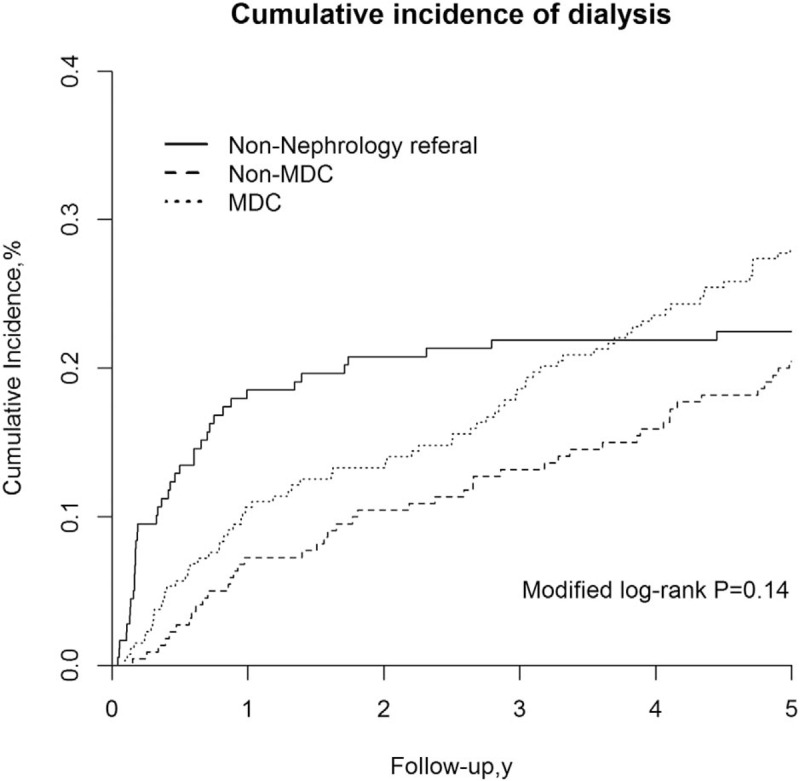
After adjustments were made for age, sex, and eGFR, our multiple regression results indicated that the non-MDC group was associated with a 54% reduction in the risk of ESRD (adjusted SDHR [aSDHR]: 0.46, 95% CI: 0.29–0.72, P < .001), and the MDC group was associated with a 47% reduction in the risk of ESRD (aSDHR: 0.53, 95% CI: 0.36–0.76, P < .001) compared with the non-nephrology referral group. After potential covariates were accounted for, a similar protective effect on ESRD risk was observed among the patients in the non-MDC and MDC groups (Table 2).
Table 2.
Risk of end-stage renal disease in relation to having received multidisciplinary care.
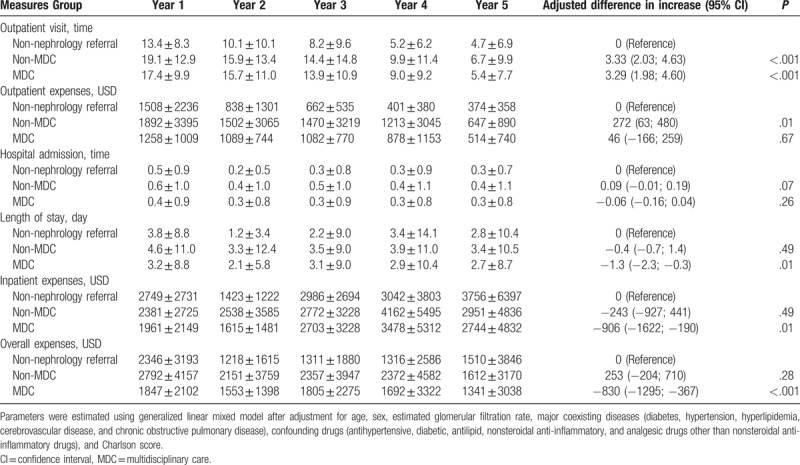
7. Healthcare utilization and expenditure
Table 3 details the differences in expenditure on, and utilization of health care services in the 3 groups. Relative to the non-nephrology referral group, patients in the non-MDC and MDC groups had significantly higher numbers of annual outpatient visits (3.33 and 3.29 times more visits per patient, respectively, P < .001). However, a shorter length of stay (1.3 less days per patient, P = .01) and lower annual inpatient expenses (a decrease of US$ 906 per patient, P = .01) were observed in the MDC group compared with the non-nephrology referral group. The total annual health care expenditure per patient in the MDC group (95% CI = 367–1295, P < .001) was lower, by US$ 830, than for those in the non-nephrology referral group.
Table 3.
Medical utilization in relation to previous multidisciplinary care in patients with chronic kidney disease.
8. Discussion
Many healthcare delivery designs have been suggested over the past decade to provide appropriate care to patients with CKD. However, only a few evidence-based studies have observed differences in the outcomes of various forms of CKD care delivery. This study revealed that only 74% of patients with CKD stage 3b to 5 were cared for by nephrologists, among whom only approximately 55% received MDC. Relative to patients cared for by other specialists, those who had nephrology referrals or who had received MDC similarly exhibited a 56% lower ESRD risk. Relative to the non-nephrology referral group, the non-MDC and MDC groups had significantly higher frequencies of laboratory examinations, particularly examinations related to kidney disease. After tracing the data on the annual expenditure and utilization of health care services, we observed that only the MDC group had significantly lower medical costs.
The National Kidney Foundation Dialysis Outcomes Quality Initiative guidelines suggest that patients should be referred to nephrologists to deal with renal complications. Patients should also be prepared for dialysis once they have an eGFR of <30 mL/min/1.73 m2.[22] Only a few studies have reported on the prevalence of nephrology care among patients with CKD.[23,24,25] Huang et al[23] reported that 77.1% of patients who initiated dialysis from 2006 to 2008 had at least 1 nephrology-related visit during the previous 3 years. However, nephrology visit rates are usually quite low among patients with an eGFR of <60 mL/min/1.73 m2. In Gasparini et al's[24] sample, less than 10% of such patients with CKD were ever seen by a nephrologist. These differences may be attributable to automated eGFR reporting implemented in clinical routines. Such automation has been proven to increase physician awareness and improve their judgment with regard to the timing of nephrology referrals.[26,27]
The avoidance of mortality and slowing of disease progression are the major tasks of pre-ESRD care. Our findings reinforce the importance of nephrology referrals and provide solid evidence for the need for appropriate intensive care during the pre-ESRD phase. The proportions for laboratory monitoring and the results from metabolic disease monitoring tended to be higher in the MDC group than the non-nephrology referral group. Anemia, proteinuria, electrolyte imbalance, and disease controls substantially contribute to mortality and dialysis risk. It is thus reasonable to infer that if adequate laboratory monitoring is provided, prescriptions can be modified as required and complications can be promptly dealt with. This, in turn, could potentially result in a lowered risk of mortality and slower renal progression.[28] In addition, we observed a considerably lower risk of requiring dialysis for patients who had a nephrology referral and patients who had received MDC. This result is strongly corroborated by most findings of previous randomized clinical trials,[29,30,31] but it differs from those of some observational studies.[10,11,32] This difference[9,10,11,32] might be due to these studies’ methods of patient selection for their non-MDC groups. For example, Chen et al[11] selected a non-MDC group using diagnostic codes and observed that MDC reduced the requirement for renal replacement therapy. However, an inverse relationship was observed by another study using propensity score matching.[9] By contrast, this present study included all patients with CKD for comparison, making our results more representative of real clinical practice. Nevertheless, future large randomized trials must be conducted to clarify the effects of MDC on the incidence of dialysis.
Our study revealed similar levels of medical utilization and costs between the non-MDC and MDC groups, which differed from the findings of related studies.[10,11,32] This difference might be due to our different method of measuring the levels of health care costs and utilization. Chen et al[11] observed that without adjustments for covariates, an MDC program resulted in total savings of US$ 1931 per patient annually. Rather than summing the costs over the period of observation, we estimated annual levels of medical utilization and costs and then analyzed the differences between groups while holding other covariates constant. Compared with other methods, our approach was able to more precisely determine the differences in measurements between groups during long-term observations. Differences in our methods of patient selection for the comparison group might also have contributed to these discrepancies. Thus, we did not intend to account for all these discrepancies in detail, and reemphasized that appropriate care in CKD is required and may reduce costs.
This study had several limitations. First, the mindsets of patients who received other specialist, nephrological, or multidisciplinary care could not have been measured and cannot be determined retrospectively. Different attitudes on health behavior, or compliance to care between groups, may have influenced our estimation. Second, we could not obtain electronic medical data from other clinical institutions. Thus, medical service utilization and costs may have been underestimated. However, this would have only slightly influenced the results because our hospital requires relatively low copayments for most medical services. Third, a few patients may have died at home, resulting in a misclassification of death in this study. Finally, the generalizability of our results may be limited to patients with an eGFR of ≤45 mL/min/1.73 m2. These are likely to be patients in health care systems that afford them easy access to specialists.
In conclusion, this study demonstrated that MDC can substantially reduce ESRD risk, increase the frequency of complication monitoring, and lower costs for patients with an eGFR of ≤45 mL/min/1.73 m2. We advocate that all patients with an eGFR of ≤45 mL/min/1.73 m2 should receive MDC and should not be cared for by only 1 type of physician or specialist (nephrologist or otherwise). An appropriate MDC referral mechanism for these patients must be developed.
Acknowledgments
We thank the Department of Medical Informatics, Kaohsiung Municipal Hsiao-Kang Hospital for providing access to their databases.
Author contributions
Conceptualization: Jui-Hsin Chen, Yi-Wen Chiu, Shang-Jyh Hwang, Jer-Chia Tsai, Hon-Yi Shi, Ming-Yen Lin.
Formal analysis: Jui-Hsin Chen, Ming-Yen Lin.
Methodology: Hon-Yi Shi, Ming-Yen Lin.
Supervision: Shang-Jyh Hwang, Jer-Chia Tsai.
Writing – original draft: Jui-Hsin Chen, Hon-Yi Shi, Ming-Yen Lin.
Writing – review & editing: Yi-Wen Chiu, Shang-Jyh Hwang, Jer-Chia Tsai, Ming-Yen Lin.
Supplementary Material
Footnotes
Abbreviations: aSDHR = adjusted subdistribution hazard ratio, BUN = blood urea nitrogen, Ca = calcium, Chol = total cholesterol, CKD = chronic kidney disease, Clinical Modification, COPD = chronic obstructive pulmonary disease, Cr = creatinine, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, Gluco = fasting blood glucose, HbA1c = hemoglobin A1c, I = incidence, ICD-9-CM = International Classification of Disease, IQR = interquartile range, K = potassium, MDC = multidisciplinary care, Na = sodium, Ninth Revision, NSAID = nonsteroidal anti-inflammatory drug, P = phosphate, PY = patient year, SD = standard deviation, SDHR = subdistribution hazard ratio, TG = triglyceride, U-Cr = urine creatinine, USD = United States dollar, U-TP = urine total protein.
The author(s) received funding from Kaohsiung Municipal Hsiao-Kang Hospital (grant KMHK-102004), Taiwan Ministry of Science and Technology (grant 105-2314-B-037-065-), and Taiwan National Health Research Institutes (grant NHRI-EX105-10505PI) for this work.
All relevant data are within the paper and its supporting information files. Some of the results of this study were presented in abstract form at the Taiwan Society of Nephrology Annual Meeting 2013, and the 55th ERA-EDTA Congress. This manuscript was edited by Wallace Academic Editing.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1]. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- [2]. Smart NA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014;Cd007333. [DOI] [PubMed] [Google Scholar]
- [3]. Baek SH, Ahn Sy, Lee SW, et al. Outcomes of predialysis nephrology care in elderly patients beginning to undergo dialysis. PLoS One 2015;10:e0128715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Lonnemann G, Duttlinger J, Hohmann D, et al. Timely referral to outpatient nephrology care slows progression and reduces treatment costs of chronic kidney diseases. Kidney Int Rep 2017;2:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Winkelmayer WC, Liu J, Chertow GM, et al. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med 2011;171:1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Singhal R, Hux JE, Alibhai SM, et al. Inadequate predialysis care and mortality after initiation of renal replacement therapy. Kidney Int 2014;86:399–406. [DOI] [PubMed] [Google Scholar]
- [7]. Rognant N, Laville M. Early mortality in dialysis and adequacy of predialysis renal care: the picture is more complex than we thought. Kidney Int 2014;86:238–40. [DOI] [PubMed] [Google Scholar]
- [8]. Wu IW, Wang SY, Hsu KH, et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality: a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant 2009;24:3426–33. [DOI] [PubMed] [Google Scholar]
- [9]. Chen YR, Yang Y, Wang SC, et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: a 3-year prospective cohort study. Nephrol Dial Transplant 2013;28:671–82. [DOI] [PubMed] [Google Scholar]
- [10]. Chen YR, Yang Y, Wang SC, et al. Multidisciplinary care improves clinical outcome and reduces medical costs for pre-end-stage renal disease in Taiwan. Nephrology 2014;19:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Chen PM, Lai TS, Chen PY, et al. Multidisciplinary care program for advanced chronic kidney disease: reduces renal replacement and medical costs. Am J Med 2015;128:68–76. [DOI] [PubMed] [Google Scholar]
- [12]. Lin E, Chertow GM, Yan B, et al. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: a modeling study. PLoS medicine 2018;15:e1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Wang SM, Hsiao LC, Ting IW, et al. Multidisciplinary care in patients with chronic kidney disease: a systematic review and meta-analysis. Eur J Intern Med 2015;26:640–5. [DOI] [PubMed] [Google Scholar]
- [14]. Lin MY, Lee CT, Kuo MC, et al. Effects of physician's specialty on regular chronic kidney disease care in predialysis: a population-based cross-sectional study. Medicine (Baltimore) 2018;97:e11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Collins AJ, Chen SC, Gilbertson DT, et al. CKD surveillance using administrative data: impact on the health care system. Am J Kidney Dis 2009;53 3 Suppl 3:S27–36. [DOI] [PubMed] [Google Scholar]
- [16]. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [18]. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- [19]. Neuhaus JM, McCulloch CE, Boylan R. Estimation of covariate effects in generalized linear mixed models with a misspecified distribution of random intercepts and slopes. Stat Med 2013;32:2419–29. [DOI] [PubMed] [Google Scholar]
- [20]. McCulloch CE, Neuhaus JM. Misspecifying the shape of a random effects distribution: why getting it wrong may not matter. Stat Sci 2011;388–402. [Google Scholar]
- [21]. Gray B. cmprsk: Subdistribution Analysis of Competing Risks. Available at: https://cran.r-project.org/web/packages/cmprsk/index.html Accessed February 20, 2017 [Google Scholar]
- [22]. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:139–274. [Google Scholar]
- [23]. Huang CY, Hsu CW, Chuang CR, et al. Pre-dialysis visits to a nephrology department and major cardiovascular events in patients undergoing dialysis. PLoS One 2016;11:e0147508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Gasparini A, Evans M, Coresh J, et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016;31:2086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Perkins RM, Chang AR, Wood KE, et al. Incident chronic kidney disease: trends in management and outcomes. Clin Kidney J 2016;9:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Wang V, Hammill BG, Maciejewski ML, et al. Impact of automated reporting of estimated glomerular filtration rate in the Veterans Health Administration. Med Care 2015;53:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Phillips L, Phillips B, Meran S, et al. The long-term impact of eGFR reporting on referral patterns. Eur J Intern Med 2014;25:97–101. [DOI] [PubMed] [Google Scholar]
- [28]. Mendu ML, Waikar SS, Rao SK. Kidney disease population health management in the era of accountable care: a conceptual framework for optimizing care across the CKD spectrum. Am J Kidney Dis 2017;70:122–31. [DOI] [PubMed] [Google Scholar]
- [29]. Peeters MJ, van Zuilen AD, van den Brand JA, et al. Nurse practitioner care improves renal outcome in patients with CKD. J Am Soc Nephrol 2014;25:390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Shi YX, Fan XY, Han HJ, et al. Effectiveness of a nurse-led intensive educational programme on chronic kidney failure patients with hyperphosphataemia: randomised controlled trial. J Clin Nurs 2013;22:1189–97. [DOI] [PubMed] [Google Scholar]
- [31]. van Zuilen AD, Bots ML, Dulger A, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int 2012;82:710–7. [DOI] [PubMed] [Google Scholar]
- [32]. Hsieh HM, Lin MY, Chiu YW, et al. Economic evaluation of a pre-ESRD pay-for-performance programme in advanced chronic kidney disease patients. Nephrol Dial Transplant 2017;32:1184–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


