Abstract
Noninfectious uveitis (NIU), which pathogenesis is often autoimmune nature, occurs as a symptom of systemic syndromes or only in the eye. The standard treatment of NIU is local, topical, and oral administration of corticosteroids (CS) in combination with immunomodulatory therapy (IMT). However, additional therapeutic strategies involving topical and systemic administration of CS or others to treat relapse or exacerbation of ocular inflammation in NIU which present as various ocular manifestations have not been established. The aim of this study was to investigate therapeutic strategies used for various ocular inflammations in relapse or exacerbation of NIU and to evaluate factors associated with the treatment pattern in Japan. The subjects were 198 eyes of 156 NIU patients with relapse or exacerbation of ocular inflammation at 6 university hospitals in Japan. The most frequent disease was sarcoidosis in 23.7% of the cases, followed by Behçet disease (BD) in 21.2%, Vogt-Koyanagi-Harada (VKH) disease in 13.6%, acute anterior uveitis (AAU) in 5.6%, tubulointerstitial nephritis and uveitis syndrome (TINU) in 4.0%, and juvenile idiopathic arthritis (JIA)-associated uveitis in 3.0%. Common ocular findings were worsened anterior inflammation (AI) in 67.2% of the cases, vitreous opacity (VO) in 46.5%, macular edema (ME) in 26.8%, retinal vasculitis (RV) in 23.7%, serous retinal detachment (SRD) in 9.1%, and optic perineuritis (OPN) in 4.0%. Reinforcement of betamethasone eye drop (ED) monotherapy for only AI in both unilateral and bilateral AI, sub-tenon injection of triamcinolone acetonide (STTA) for unilateral posterior inflammation including VO and ME, and systemic therapy using CS and/or IMT for bilateral anterior and posterior inflammation were significantly more frequent. Frequencies of exacerbated individual ocular findings in sarcoidosis and BD were similar, and severe ocular inflammation associated with panuveitis required both topical and systemic therapies. These results demonstrate that reinforcement of betamethasone EDs, topical administration of triamcinolone acetonide, and long-term administration of systemic corticosteroids are the major therapeutic strategies, and reinforcement of betamethasone EDs was used for exacerbated AI independently from its use for posterior inflammation. In addition, STTA was preferentially used for VO and ME associated with posterior inflammation.
Keywords: noninfectious uveitis, ocular inflammation, treatment, uveitis
1. Introduction
Uveitis is a term referring to diverse intraocular inflammatory disorders which cause visual disturbance, and irreversible damage due to ocular inflammation leads to partial or complete loss of vision.[1] The incidence of uveitis in developed countries is approximately 17 to 52 cases per 100,000 in a year, and prevalence estimates range from 38 to 714 cases per 100,000 in a population.[2–7] Uveitis is responsible for approximately 5% to 20% of legal blindness in both the United States and Europe, and perhaps as much as 25% of blindness in the developing world.[5,8–10] Early diagnosis and treatment are required to prevent vision-threatening irreversible tissue damage caused by uveitis and complications including cataract, glaucoma, retinopathy, and macular edema (ME).[11] The goal of uveitis treatment is to suppress inflammation before exacerbation and to maintain visual function.[12,13]
The pathogenesis of noninfectious uveitis (NIU) is often autoimmune nature and occurs as a symptom of systemic syndromes, such as sarcoidosis, Behçet disease (BD), and Vogt-Koyanagi-Harada (VKH) syndrome, or only in the eye. The standard treatment of NIU is local, topical, and oral administration of CS in combination with immunomodulatory therapy (IMT). The therapeutic strategies are determined based on impaired visual function, the severity of ocular inflammation, the clinical course of uveitis, risk of serious complications, the cause of uveitis, and the patients’ background. On the other hand, since long-term use of moderate-to-high doses of corticosteroids can cause various adverse events in the eye (e.g., glaucoma and cataract) and systemically (e.g., diabetes, hypertension, and osteoporosis),[14–16] concomitant IMT agents are required for effectively managing severe NIU.[17] Although guidelines for NIU treatment were reported in 2000,[14] additional therapeutic strategies for relapse of ocular inflammation or exacerbation of NIU which present as various ocular manifestations have not been established. Requirement of rescue therapy using systemic administration of CS with IMT would differ between relapse and exacerbation of ocular inflammation in NIU with or without systemic diseases. It is important to understand current treatment patterns used by uveitis specialists. In the present study, we investigated therapeutic strategies used for various ocular inflammatory conditions in relapse or exacerbation of NIU and evaluated factors associated with the treatment pattern.
2. Materials and methods
A retrospective uncontrolled cross-sectional multicenter study was conducted by reviewing clinical charts using a study-specific questionnaire. The study subjects comprised 198 eyes 156 NIU patients with recurrent or exacerbated ocular inflammation treated at six university hospitals in Japan between January and December 2017. The institutional review board of each center approved the study protocol according to the tenets of the Declaration of Helsinki. Patient demographics, diagnosis of uveitis, concomitant systemic and ocular conditions, and therapy regimens were extracted. Ocular findings of relapse or exacerbation of uveitis were classified into anterior inflammation (AI), vitreous opacity (VO), ME, serous retinal detachment (SRD), retinal vasculitis (RV) and/or exudates, and optic perineuritis (OPN) including overlaps. The anatomical classification of ocular inflammation was based on the most severe ocular inflammation observed in each eye. If there were several ocular inflammations in the same eye during this study period, rescue therapy for the most severe grade of inflammation was indicated. A questionnaire was filled out by the ophthalmologist who treated the patients at each participating institution, and the data were collected and analyzed in National Defense Medical College. Statistical analyses were performed using JMP software package version 12 (Business Unit of SAS, Cary, NC). The chi-square test was used for analysis of patient background and the basic data, and Fisher exact test was used to compare data of individual treatments with ocular findings or the causative diseases. Logistic regression analysis was applied to detect correlation between ocular findings and therapeutic strategies. A P value less than .05 was considered significant.
3. Results
3.1. Backgrounds and characteristics of enrolled patients
Demographic and disease characteristics of patients enrolled in the present study are summarized in Table 1. The age (mean ± standard deviation) at treatment was 50.3 ± 19.7 years (range 13–72 years). Of 156 NIU patients enrolled, 70 (44.9%) were men and 86 (55.1%) were women. Ninety-six eyes were granulomatous uveitis, 87 eyes were nongranulomatous uveitis, and 15 eyes were unidentified conditions. The most frequent disease was sarcoidosis in 47 eyes (23.7%), followed by BD in 42 eyes (21.2%), VKH disease in 27 eyes (13.6%), acute anterior uveitis (AAU) in 11 eyes (5.6%), tubulointerstitial nephritis and uveitis syndrome (TINU) in 8 eyes (4.0%), and juvenile idiopathic arthritis (JIA)-associated uveitis in 6 eyes (3.0%). Of 198 eyes with relapse or exacerbation of ocular inflammation, 91 (46.0%) had anterior and posterior inflammation, 61 (30.8%) had posterior inflammation, and 46 (23.2%) had AI. Ocular findings based on relapse or exacerbation of uveitis including overlaps were AI in 133 eyes (67.2%), VO in 92 eyes (46.5%), ME in 53 eyes (26.8%), RV in 47 eyes (23.7%), SRD in 18 eyes (9.1%), and OPN in 8 eyes (4.0%). Therapeutic strategies using corticosteroids comprised reinforcement of betamethasone eye drops (ED) in 107 eyes (54%), subconjuctival injection (SCI) of betamethasone in 17 eyes (8.6%), posterior sub-tenon triamcinolone acetonide injection (STTA) in 69 eyes (34.8%), intravitreous injection of triamcinolone acetonide (IVTA) in 2 eyes (1.0%), short-term (<3 months) systemic administration of prednisolone (S-cor) in 6 eyes (3.0%), long-term (>3 months) systemic administration of prednisolone (L-cor) in 37 eyes (18.7%), and methylprednisolone pulse therapy in 2 eyes (1.0%). Immunomodulatory agents were also used, with cyclosporine (Cyc) in 9 eyes (4.5%) and infliximab (Inf) in 10 eyes (5.1%).
Table 1.
Demographic and clinical characteristics of patients enrolled in this study.

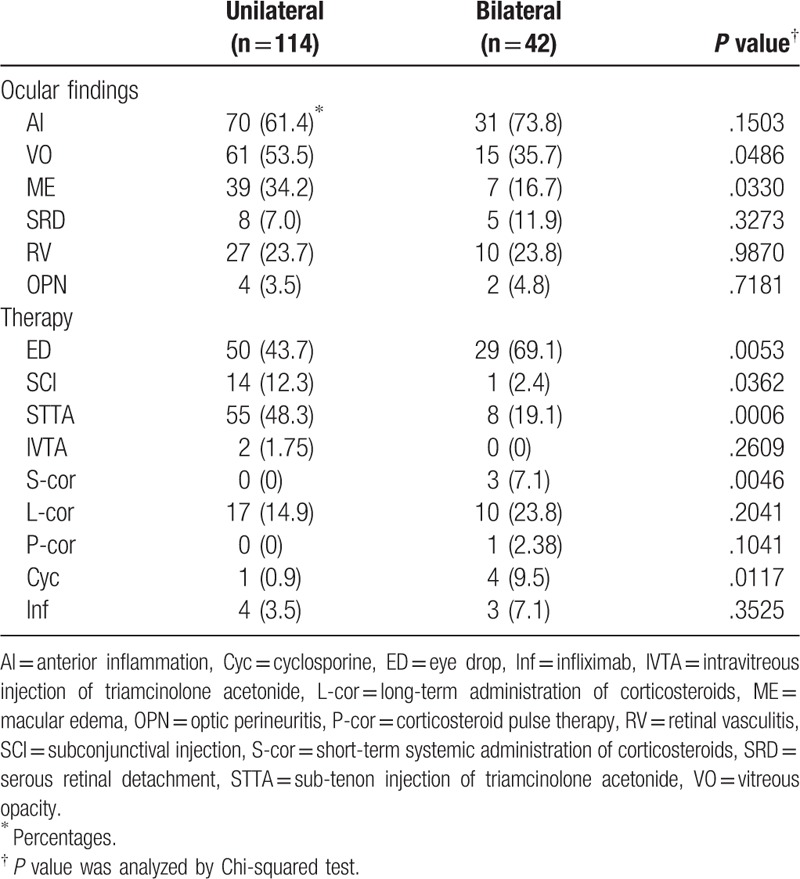
3.2. Exacerbated ocular findings in unilateral and bilateral inflammation and the therapy used
Visual disturbance by bilateral exacerbated ocular inflammation is functionally and spiritually and mentally much more severe condition for NIU patients than unilateral ocular inflammation. Therefore, it is important to evaluate differences in ocular features and therapeutic strategies between unilateral and bilateral exacerbated ocular inflammation. Frequency of ocular findings in unilateral or bilateral ocular inflammation and the therapy used are shown in Table 2. VO and ME were significantly more frequent in unilateral ocular inflammation than in bilateral ocular inflammation. SCI, STTA, and IVTA as topical treatments were used more often to treat unilateral ocular inflammation than to treat bilateral ocular inflammation with statistically significant differences between the use of SCI and STTA, although reinforcement of ED was used significantly more often to treat bilateral inflammation than to treat unilateral inflammation. On the other hand, systemic treatments such as S-cor, L-cor, corticosteroid pulse therapy (P-cor), Cyc, and Inf were used more often to treat bilateral ocular inflammation than to treat unilateral ocular inflammation, with statistically significant differences between the use of S-cor and Cyc.
Table 2.
Ocular findings and therapeutic strategies in unilateral or bilateral exacerbated ocular inflammation.
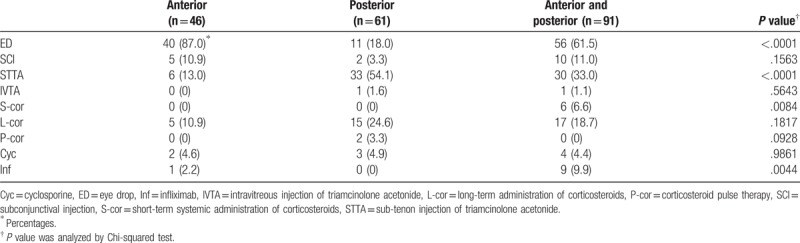
3.3. Therapeutic strategies classified based on anatomical location of exacerbated ocular inflammation
Frequency of individual therapeutic strategies classified based on anatomical location of exacerbated ocular inflammation is shown in Table 3. Statistically significant differences were noted in the reinforcement of ED, STTA, S-cor, and Inf among the 3 groups, wherein ED to treat AI with or without posterior inflammation, STTA to treat posterior inflammation with or without AI, and S-cor and Inf to treat both anterior and posterior inflammation were used significantly and more frequently
Table 3.
Therapeutic strategies for anatomical location of exacerbated ocular inflammation.
3.4. Frequent ocular findings occurred by exacerbated ocular inflammation in individual uveitis cases
Frequency of ocular findings occurred by exacerbated ocular inflammation in individual uveitis cases including overlaps are shown in Table 4. AI was approximately 75% in sarcoidosis and BD, 50% in VKH, and 100% in AAU, TINU, and JIA-associated uveitis. VO was approximately 70% in sarcoidosis and BD, 25% in TINU, and less than 20% in VKH, AAU, and JIA-associated uveitis. ME was 30% to 40% in sarcoidosis, BD, and JIA-associated uveitis, and 20 to 25% in VKH. SRD was mostly in VKH. RV was 30% to 40% in sarcoidosis and BD, and 50% in TINU. The frequency of ON was less than 10% and was observed in sarcoidosis, BD, and VKH.
Table 4.
Frequency of ocular findings occurred by exacerbated ocular inflammation in individual uveitis.

3.5. Therapeutic strategies based on ocular findings
Therapeutic strategies used based on individual ocular findings are shown in Table 5. Reinforcement of ED monotherapy was performed in 58 eyes, which was significant more frequent applied for eyes with only AI (27 eyes, P <.005). On the other hand, STTA monotherapy was significantly less frequent in treatment for eyes with only AI (3 out of 37 eyes, P <.05) or AI and VO (3 out of 32 eyes, P <.05), however was significantly more frequent in therapy for eyes with only VO (8 out of 11 eyes, P <.005), VO + ME (4 out of 6 eyes, P <.05), and only ME (9 out of 14 eyes, P <.005). In addition, ED + STTA was applied for 16 eyes, in which eyes with AI + ME were the most significantly (5 eyes, P <.005). ED + SCD + STTA + L-cor was only used for eye with AI + VO + ME + SRD. ED + S-cor was administrated for 6 eyes, which was preferentially performed for eyes with AI + VO + ME (2 eyes, P <.05) or AI + OPN (2 eyes, P <.05). ED + L-cor + Cyc was applied for 3 eyes, in which 2 eyes are AI + SRD (P <.05). L-cor monotherapy was significantly more in eyes with AI + RV (4 out of 4 eyes, P <.005), or VO + SRD + OPN (2 out of 3 eyes, P <.05). L-cor + Cyc or P-cor was significantly more used for treatment of eyes with only RV (P <.05, P <.005). In treatment with Inf, Inf monotherapy for eyes with AI + VO + SRD (2 out of 16 eyes, P <.05) or AI + ME (2 out of 10 eyes, P <.05), and ED + Inf for eyes with AI + VO (2 out of 2 eyes, P <.05) were significant.
Table 5.
Therapeutic strategies for individual exacerbated ocular findings.

3.6. Therapeutic strategies based on the causative diseases
Therapeutic strategies classified based on the causative diseases are shown in Table 6. ED + STTA for sarcoidosis in 9 out of 16 eyes (37.5%, P < 0.005), ED + STTA + Inf for BD in all 3 eyes (100%, P <.005), L-cor for VKH in 8 out of 20 eyes (40%, P <.005), L-cor + Cyc for VKH in 2 out of 4 eyes (50%, P <.05), and SCI for AAU in 2 out of 6 eyes (33.3%, P <.05) were significantly more frequent. TINU was treated by ED monotherapy (6 out of 8 eyes, 75%, P <.005) or ED + L-cor (2 out of 8 eyes, 25%, P <.05) with statistical differences. On the other hand, ED monotherapy was significantly less in treatment of VKH (1 out of 27 eyes, 3.7%, P <.005).
Table 6.
Therapeutic strategies for exacerbated ocular findings classified by the causative diseases.

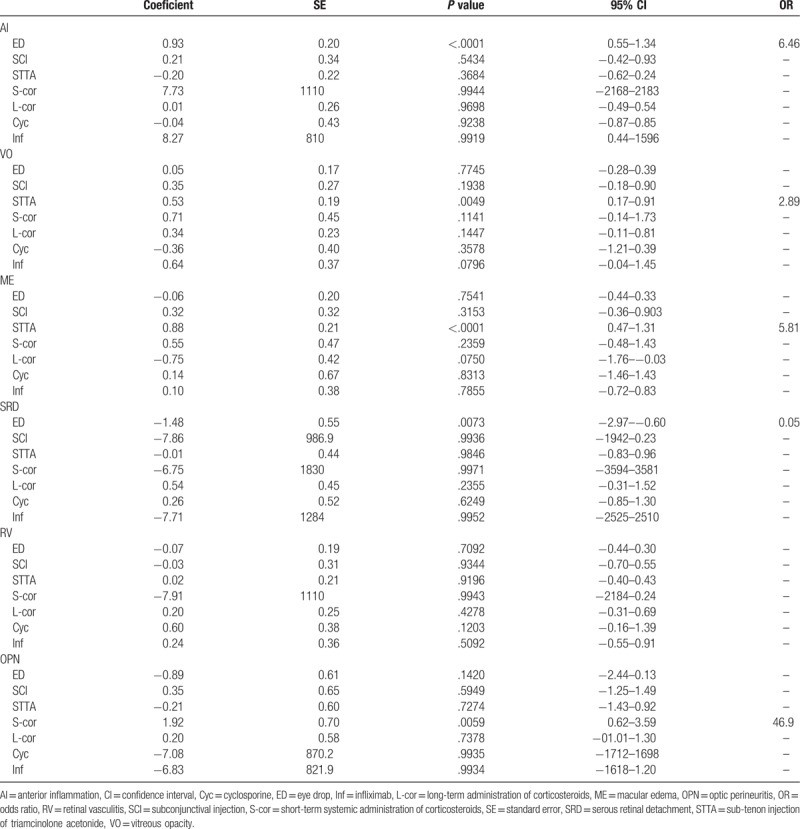
3.7. Correlations between therapeutic strategies and ocular findings
Correlations between ocular findings (AI, VO, ME, SRD, and ON) and therapeutic strategies (ED, SCD, STTA, S-cor, L-cor, Cyc, and Inf) analyzed using multivariable logistic regression analysis are shown in Table 7. ED for AI (OR: 6.46, 95% CI: 0.55–1.34, P <.0001), STTA for VO (OR: 2.89, 95% CI: 0.17–0.91, P <.005), STTA for ME (OR: 5.81, 95% CI: 0.47–1.31, P <.0001), and S-cor for OPN (OR: 46.9, 95% CI: 0.62–3.59, P <.05) were positively correlated, while there was negative correlation between ED and SRD (OR: 0.05, 95% CI: −2.97 to −0.60, P <.05).
Table 7.
Correlations between ocular findings and individual therapeutic strategies.
4. Discussion
Therapeutic strategies for relapse or exacerbation of ocular inflammation in NIU need to be chosen considering a number of factors. First, the extent of uveitis and associated inflammation including
-
1)
unilaterality or bilaterality,
-
2)
anatomical location within the eye,
-
3)
severity of ocular inflammation, and
-
4)
presence of active systemic disease.
Second, the etiology, which helps define the type of treatment required. Third, potential complications of the disease, irreversible tissue damage due to ocular inflammation, and/or adverse effects of the treatments. In general, local and topical treatments for exacerbated ocular inflammation are more common in unilateral and/or idiopathic uveitis, and systemic regimens are likely to be used to treat more severe cases or bilateral ocular inflammation due to exacerbation of posterior uveitis or panuveitis, especially those associated with systemic diseases. In the present study, reinforcement of ED was found to be used significantly more often to treat exacerbated AI, and less often for limited posterior inflammation such as SRD (Table 3, Table 5, and Table 7). SCI and STTA as local treatments were used significantly more frequent to treat unilateral inflammation, and systemic administration was used more often to treat bilateral uveitis (Table 2). On the other hand, reinforcement of ED was used significantly less frequent for unilateral uveitis than for bilateral uveitis (Table 2). One possibility is that AI was more frequently involved as an ocular finding in exacerbated bilateral ocular inflammation (73.8%) than in unilateral inflammation (61.3%), although there was no statistically significant difference. Second, it may result from the fact that VO and ME as posterior inflammation, which does not usually require reinforcement of ED, were significantly more observed in unilateral inflammation than in bilateral inflammation. Because of the heterogeneity in uveitis and wide geographical variation in both clinical features and disease etiology, it is challenging to compare estimated prevalence between different regions.[18,19] Among the enrolled patients, 44.9% were male and 55.1% were female, with a mean age of 50 ± 19.7 years. The gender ratio was similar to that reported in uveitis prevalence studies from various countries, and the mean age was equal or higher.[5,20,21] The most common causative disease of uveitis in the present study was sarcoidosis, followed by BD, VKH, AAU, and TINU (Table 1). In agreement with our finding, the most frequent cause of uveitis identified in Japan in 2002 was sarcoidosis (13.3%), followed by VKH (6.7%) and BD (6.2%).[22] In uveitis prevalence studies conducted in 2009, the most common causative disease was found to be sarcoidosis (10.6%) followed by VKH (7.0%), but the third was AAU (6.5%) instead of BD; scleritis (6.1%) and herpetic iridocyclitis (4.2%) were next, and BD (3.9%) was the sixth.[23] In addition to this decrease in BD patient number in the recent decades, ocular inflammation associated with BD uveitis has been found to be less severity compared to that in the past.[24] However, repeated ocular attacks are characteristics of BD uveitis, with higher frequency and numbers than other types of uveitis. Therefore, although total numbers of BD patients are decreasing, it is not contradicted that BD is the second frequent disease in this study.
ME occurs in approximately 40% of patients with intermediate uveitis, posterior uveitis, or panuveitis,[8,15] and is the most common complication of uveitis leading to visual disturbance. It is also responsible for a third of the blindness caused by uveitis.[8] In the present study, ME was observed in 53 out of 198 eyes (26.8%). However, because 25 eyes were posterior uveitis and 28 eyes were panuveitis among the 53 eyes with ME, the incidence of ME in posterior and pan uveitis was found to be 34.9%, which is comparable with previous reports.
STTA and IVTA are widely used in the treatment of ME and posterior inflammation associated with uveitis. [25,26,27–31] STTA for ME, VO, and ME + VO, and ED + STTA for AI + ME were significantly more performed, although IVTA was used only twice for ME and AI + VO + SRD (Table 5). Although IVTA has been reported to be more effective to treat ME related to uveitis than STTA,[31] the incidence of intraocular pressure elevation is higher in the use of IVTA than STTA.[32] Therefore, STTA would be more likely to be used to treat ME and other cases of posterior inflammation than IVTA.
The ocular features and clinical course of uveitis in sarcoidosis are apparently different from those in BD; however, the frequency of individual ocular findings due to exacerbated ocular inflammation was similar between them, as shown in Table 4. Further, although therapeutic strategies based on ocular findings were consistent between them, there were some differences in therapeutic patterns of systemic regimens (Table 6). In Japan, treatment of uveitis using Inf is approved only for BD-associated uveitis. Treatment of Inf for other kinds of uveitis including sarcoidosis is considered off-label use. Therefore, Inf was found to be used significantly more often to treat exacerbated uveitis in BD. Adalimumab was newly approved for treatment of refractory uveitis including BD at 2016. It is possible that use of adalimumab would increase for treatment of exacerbated ocular inflammation in NIU.
This study was limited to therapeutic strategies for recurrence or exacerbation of ocular inflammation in NIU, however, the use of IMT agents was fewer kinds and lower frequency compared with other reports. As well as biologics, Cyc is the only immunosuppressive drug authorized by the National Health Insurance of Japan for treatment of refractory uveitis and is used to treat various forms of ocular inflammation. Masuda et al conducted a randomized controlled trial of Cyc versus colchicine in BD patients and demonstrated that both frequencies of ocular attacks and severity of ocular inflammation were reduced more in the Cyc group than in the colchicine group.[33] Other studies have also indicated valuable anti-inflammatory effects of Cyc in patients with severe refractory posterior uveitis that included sarcoidosis, idiopathic RV, and BD.[34,35] On the other hand, Cyc acts fast but takes 7 to 15 days from initiation to reach peak efficacy.[36] Therefore, Cyc would be likely to be used as rescue therapy to treat relapse or exacerbation of ocular inflammation in NIU, as found in the present study.
There were independent positive correlations between ED and AI, STTA and VO or ME, and S-cor and OPN. However, ED was negatively correlated with SRD. Sixteen out of 18 eyes with SRD were VKH (88.9%), of which only 4 eyes were AI (25%).
Because AI was observed in 13 eyes out of all eyes with VKH (48.2%), SRD was likely to have preferentially occurred due to relapse of ocular inflammation confined to the posterior segment.
Several limitations of this study, given its cross-sectional design, need to be mentioned. The results were analyzed with the only domestic data and the number of subjects was small. The subjects from 6 centers participating in this study do not cover all area in Japan. Data regarding therapy details, treatment characteristics based on the severity of uveitis, clinical course, residual visual functions, side effects, and systemic conditions were not collected as part of the study. Therapeutic patterns found represented only the practices of uveitis specialists who had agreed to participate in the study. Therefore, certain findings may have been different from the practices of general ophthalmologists.
5. Conclusions
The present study indicates the following with regard to the therapeutic strategies used for relapse or exacerbation of NIU in Japan.
-
1)
Reinforcement of betamethasone EDs, topical administration of triamcinolone acetonide, and long-term administration of systemic corticosteroids were the major therapeutic strategies;
-
2)
reinforcement of betamethasone EDs was used for exacerbated AI independently from its use for posterior inflammation;
-
3)
sub-tenon injection of triamcinolone acetonide (STTA) was preferentially used for VO and ME associated with posterior inflammation;
-
4)
STTA was provided equally for ocular findings in sarcoidosis and BD; and
-
5)
a systemic regimen of corticosteroids was more common than local therapy in VKH.
Author contributions
Conceptualization: Masaru Takeuchi.
Data curation: Takayuki Kanda, Toshikatsu Kaburaki, Rie Tanaka, Kenichi Namba, Koju Kamoi, Kazuichi Maruyama, Etsuko Shibuya, Nobuhisa Mizuki.
Formal analysis: Masaru Takeuchi, Takayuki Kanda.
Funding acquisition: Masaru Takeuchi.
Investigation: Toshikatsu Kaburaki, Rie Tanaka, Kenichi Namba, Koju Kamoi, Kazuichi Maruyama, Etsuko Shibuya, Nobuhisa Mizuki.
Validation: Masaru Takeuchi.
Writing – original draft: Masaru Takeuchi.
Writing – review & editing: Masaru Takeuchi, Toshikatsu Kaburaki, Rie Tanaka, Kenichi Namba, Koju Kamoi, Kazuichi Maruyama, Etsuko Shibuya, Nobuhisa Mizuki.
Footnotes
Abbreviations: AAU = acute anterior uveitis, BD = Behçet disease, Cyc = cyclosporine, Inf = infliximab, AI = anterior inflammation, IVTA = intravitreous injection of triamcinolone acetonide, L-cor = long-term administration of corticosteroids, ME = macular edema, NIU = noninfectious uveitis, OPN = optic perineuritis, P-cor = corticosteroid pulse therapy, RV = retinal vasculitis, SCI = subconjunctival injection, S-cor = short-term systemic administration of corticosteroids, SRD = serous retinal detachment, STTA = sub-tenon injection of triamcinolone acetonide, TINU = tubulointerstitial nephritis and uveitis syndrome, JIA = juvenile idiopathic arthritis. ED = eye drop, VKH = Vogt-Koyanagi-Harada disease, VO = vitreous opacity.
This work was supported by Grant-in-Aid 16K11337 for Scientific Research from the Japan Society for the Promotion of Science.
The authors have declared that no conflict of interest exists.
References
- [1].Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol 2004;88:1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis: incidence and prevalence in a small urban community. Arch Ophthalmol 1962;68:502–14. [DOI] [PubMed] [Google Scholar]
- [3].Tran VT, Auer C, Guex-Crosier Y, et al. Epidemiology of uveitis in Switzerland. Ocul Immunol Inflamm 1994;2:169–76. [DOI] [PubMed] [Google Scholar]
- [4].Päivönsalo-Hietanen T, Tuominen J, Vaahtoranta-Lehtonen H, et al. Incidence and prevalence of different uveitis entities in Finland. Acta Ophthalmol Scand 1997;75:76–81. [DOI] [PubMed] [Google Scholar]
- [5].Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California epidemiology of uveitis study. Ophthalmology 2004;111:491–500. [DOI] [PubMed] [Google Scholar]
- [6].Suhler EB, Lloyd MJ, Choi D, et al. Incidence and prevalence of uveitis in veterans affairs medical centers of the Pacific Northwest. Am J Ophthalmol 2008;146:890–6. [DOI] [PubMed] [Google Scholar]
- [7].Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the pacific ocular inflammation study. JAMA Ophthalmol 2013;131:1405–12. [DOI] [PubMed] [Google Scholar]
- [8].Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine 2001;80:263–70. [DOI] [PubMed] [Google Scholar]
- [10].Pavesio CE, DeCory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol 2008;92:455–9. [DOI] [PubMed] [Google Scholar]
- [11].Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica 2004;218:223–36. [DOI] [PubMed] [Google Scholar]
- [12].Nguyen QD, Callanan D, Dugel P, et al. Treating chronic noninfectious posterior segment uveitis: the impact of cumulative damage. Proceedings of an expert panel roundtable discussion. Retina 2006;Suppl:1–6. [DOI] [PubMed] [Google Scholar]
- [13].Gupta R, Murray PI. Chronic non-infectious uveitis in the elderly: epidemiology, pathophysiology and management. Drugs Aging 2006;23:535–58. [DOI] [PubMed] [Google Scholar]
- [14].Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000;130:492–513. [DOI] [PubMed] [Google Scholar]
- [15].Kempen JH, Altaweel MM, et al. Multicenter Uveitis Steroid Treatment Trial Research Group. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 2011;118:1916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Suhler EB, Thorne JE, Mittal M, et al. Corticosteroid-related adverse events systematically increase with corticosteroid dose in noninfectious intermediate, posterior, or panuveitis: post hoc analyses from the VISUAL-1 and VISUAL-2 trials. Ophthalmology 2017;124:1799–807. [DOI] [PubMed] [Google Scholar]
- [17].You C, Sahawneh HF, Ma L, et al. A review and update on orphan drugs for the treatment of non infectious uveitis. Clin Ophthalmol 2017;11:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miserocchi E, Fogliato G, Modorati G, et al. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol 2013;23:705–17. [DOI] [PubMed] [Google Scholar]
- [19].Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996;383:787–93. [DOI] [PubMed] [Google Scholar]
- [20].Kazokoglu H, Onal S, Tugal-Tutkun I, et al. Demographic and clinical features of uveitis in tertiary centers in Turkey. Ophthalmic Epidemiol 2008;15:285–93. [DOI] [PubMed] [Google Scholar]
- [21].Yang P, Zhang Z, Zhou H, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res 2005;30:943–8. [DOI] [PubMed] [Google Scholar]
- [22].Goto H, Mochizuki M, Yamaki K, et al. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol 2007;51:41–4. [DOI] [PubMed] [Google Scholar]
- [23].Ohguro N, Sonoda KH, Takeuchi M, et al. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol 2012;56:432–5. [DOI] [PubMed] [Google Scholar]
- [24].Yoshida A, Kawashima H, Motoyama Y, et al. Comparison of patients with Behçet's disease in the 1980s and 1990s. Ophthalmology 2004;111:810–5. [DOI] [PubMed] [Google Scholar]
- [25].Lafranco Dafflon M, Tran VT, Guex-Crosier Y, et al. Posterior sub-Tenon's steroid injections for the treatment of posterior ocular inflammation: indications, efficacy and side effects. Graefes Arch Clin Exp Ophthalmol 1999;237:289–95. [DOI] [PubMed] [Google Scholar]
- [26].Leder HA, Jabs DA, Galor A, et al. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol 2011;152:441–8. [DOI] [PubMed] [Google Scholar]
- [27].Salek SS, Leder HA, Butler NJ, et al. Periocular triamcinolone acetonide injections for control of intraocular inflammation associated with uveitis. Ocul Immunol Inflamm 2013;21:257–63. [DOI] [PubMed] [Google Scholar]
- [28].Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol 1995;120:55–64. [DOI] [PubMed] [Google Scholar]
- [29].Kok H, Lau C, Maycock N, et al. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology 2005;112:1916.e1–7. [DOI] [PubMed] [Google Scholar]
- [30].Androudi S, Letko E, Meniconi M, et al. Safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema. Ocul Immunol Inflamm 2005;13:205–12. [DOI] [PubMed] [Google Scholar]
- [31].Ozkiris A. Intravitreal triamcinolone acetonide injection for the treatment of posterior uveitis. Ocul Immunol Inflamm 2006;14:233–8. [DOI] [PubMed] [Google Scholar]
- [32].Hirano Y, Ito T, Nozaki M, et al. Intraocular pressure elevation following triamcinolone acetonide administration as related to administration routes. Jpn J Ophthalmol 2009;53:519–22. [DOI] [PubMed] [Google Scholar]
- [33].Masuda K, Nakajima A, Urayama A, et al. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet's disease. Lancet 1989;1:1093–6. [DOI] [PubMed] [Google Scholar]
- [34].Nussenblatt RB, Palestine AG, Rook AH, et al. Treatment of intraocular inflammatory disease with cyclosporin A. Lancet 1983;2:235–8. [DOI] [PubMed] [Google Scholar]
- [35].Graham EM, Sanders MD, James DG, et al. Cyclosporin A in the treatment of posterior uveitis. Trans Ophthalmol Soc U K 1985;104:146–51. [PubMed] [Google Scholar]
- [36].Ruhlmann A, Nordheim A. Effects of the immunosuppressive drugs CsA and FK506 on intracellular signalling and gene regulation. Immunobiology 1997;198:192–206. [DOI] [PubMed] [Google Scholar]


