Abstract
EGFR-TKIs have been widely used in the first-line treatment of NSCLC patients harboring EGFR mutations. However, the prognosis indicators are limited. In the present study, the prognostic value of systemic immune-inflammation index (SII), neutrophil to lymphocyte ratio (NLR), and platelet to lymphocyte ratio (PLR) were assessed in EGFR-Mutant lung adenocarcinoma patients treated with first-generation EGFR-TKIs. Two hundred three patients were included in this retrospective analysis. SII was calculated as platelet counts × neutrophil counts / lymphocyte counts. Receiver operating characteristic (ROC) curve was used to evaluate the optimal cut-off value for SII, NLR, and PLR. Univariate and multivariate survival analysis were performed to identify factors correlated with PFS and OS. Applying cut-offs of ≥1066.935 (SII), ≥4.40 (NLR), and ≥182.595 (PLR), higher NLR was associated with worse Eastern Cooperative Oncology Group performance status (ECOG PS) (P = .006), and higher brain metastasis rate (P = .03), higher PLR was associated with smoking history (P = .037), and worse ECOG PS (P = .001), and higher SII groups were associated with worse ECOG PS (P = .002). In univariate analysis, higher NLR (P < .001), higher PLR (P = .002), and higher SII (P < .001) were associated with worse PFS. Higher NLR (P < .001), and higher SII (P < .001) were associated with worse OS. In multivariate analysis, NLR (HR 1.736;95%CI:1.020–2.954; P = .03), PLR (HR 1.823; 95%CI:1.059–3.137; P = .04), and SII (HR2.577; 95%CI:1.677–3.958; P < .001) were independently correlated with PFS. While only SII (HR 2.802; 95%CI:1.659–4.733; P < .001) was independently correlated with OS. The present study demonstrated that SII is an independent prognostic factor for poor survival of advanced EGFR-Mutant lung adenocarcinoma patients treated with first-generation TKIs.
Keywords: EGFR-TKI, non-small cell lung cancer, prognosis, systemic immune-inflammation index
1. Introduction
Lung cancer is the leading cause of cancer-related mortality both in China and worldwide, and non-small cell lung cancer (NSCLC) comprises the majority of all lung cancer cases.[1,2] More than half of the NSCLC patients are diagnosed at advanced stage, and the prognosis is poor. The median survival time was about 12 months before the era of target therapy.[3] During the past decades, the development of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (EGFR-TKIs) has significantly improved the prognosis of the patients harboring EGFR mutation. EGFR-TKIs now act as the standard first-line treatment of the EGFR-mutant advanced NSCLC patients, with a median response rate of about 60%, and median progression free survival (PFS) 10 months.[4] However, most of the patients will develop drug resistance and resulted in treatment failure, which is the major challenge of clinical work. Therefore, exploring prognostic factors that can predict target therapy efficacy is important.
Increasing evidence has demonstrated that host immune-inflammation status plays an important role in the prognosis of the patients. There have been several immune-inflammation based indicators, including neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) which can predict survival in many solid tumors.[5,6,7,8,9] The systemic immune-inflammation index (SII) is also a simple and inexpensive marker which has been reported to be predictive in the prognosis in colorectal cancer,[10] NSCLC,[11] ovarian cancer,[12] pancreatic cancer,[13] and hepatocellular carcinoma.[14] However, the prognostic value of SII in EGFR-Mutant advanced NSCLC patients treated with EGFR-TKI has been rarely reported.
In the present study, a cohort of 203 EGFR-mutant advanced lung adenocarcinoma patients were retrospectively analyzed to evaluate the clinical significance and prognostic value of NLR, PLR, and SII in NSCLC patients receiving EGFR-TKI treatment.
2. Patients and methods
2.1. Patients selection
We retrospectively reviewed the records of newly diagnosed stage IV lung adenocarcinoma patients between Jan 2013 and Nov 2018 at the Second Xiangya Hospital in Changsha, China. The patients who met the following inclusion criteria were included:
-
(1)
pathologically diagnosed as lung adenocarcinoma,
-
(2)
harboring EGFR active mutation,
-
(3)
using first generation EGFR-TKI (gefitinib, erlotinib, or icotinib) as the first-line treatment,
-
(4)
with complete record of blood test results within 1 week prior to the initiation of EGFR-TKI treatment and follow-up record.
Patients who met the following exclusion criteria were excluded:
-
(1)
history of other malignant tumors and chronic inflammatory diseases,
-
(2)
with recent steroid therapy,
-
(3)
with recent clinical evidence of acute infection or inflammation.
Consequently, 203 patients were enrolled in the present study. The study was approved by the ethics committee of the Second Xiangya Hospital, Central South University.
2.2. Data collection
Patient characteristics including gender, age, smoking history, brain metastasis status, ECOG score, EGFR mutation status, and full blood counts were obtained from the electronic medical record system of the Second Xiangya Hospital. The SII, NLR, and PLR were calculated as follows: SII = platelet counts × neutrophil counts / lymphocyte counts, NLR = neutrophil counts / lymphocyte counts, PLR = platelet counts / lymphocyte counts. The last follow-up occurred in December 31, 2018. The overall survival (OS) was calculated from the date of diagnosis to the date of death for any reason or to the last date of follow-up. The progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression based on response evaluation criteria in solid tumors (RECIST) 1.1, or death.
2.3. Statistical methods
The relationship between SII, NLR, and PLR and clinicopathological factors were analyzed using the chi-square test. Receiver operating characteristic (ROC) curves were used to calculate the optimal cut-off value for SII, NLR, and PLR. Survival analysis was performed using Kaplan–Meier method. The differences between the survival curves were compared by log-rank test. The multivariate Cox hazard regression analysis was performed on the factors that were shown to be significant on univariate analysis. All tests were 2-sided and P values less than .05 were considered significant. The SPSS software 20.0 was used.
3. Results
3.1. Patient characteristics
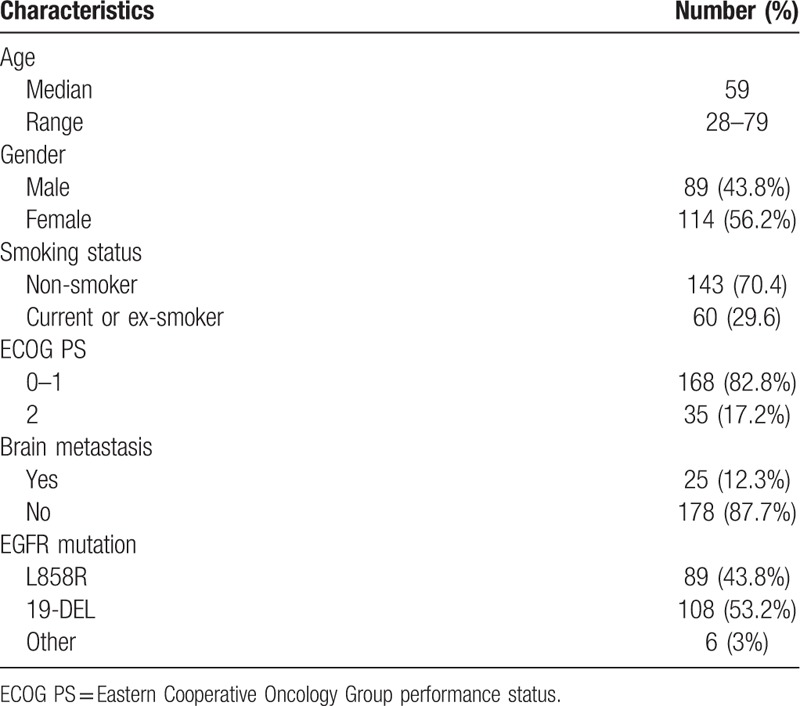
The patient characteristics are shown in Table 1. A total of 203 advanced EGFR-mutant lung adenocarcinoma patients were included, among which 89 (43.8%) were male and 114 (56.2%) were female. The median age was 59 years (range: 28–79 years). Sixty patients (29.6%) had a smoking history. The majority of patients had an ECOG score of 0 to 1 (168, 82.8%). In all the patients, 89 (43.8%) patients had the exon 21 L858R mutation, 108 (53.2%) the exon 19 deletion mutation, and 6 (3%) other rare mutations. There are 25 (12.3%) patients who had brain metastasis, and 5 patients received whole brain radiation therapy (WBRT) concurrent with TKI.
Table 1.
Clinicopathological characteristics of patients.
3.2. Cut-off value of SII, NLR, PLR, and their association with clinicopathological characteristics
The median values for SII, NLR, and PLR of all these patients were 796.85 (range: 167.49–4021.94), 3.26 (range: 0.83–12.74) and 173.55 (range: 55.30–847.37). The optimal cut-off values of SII, NLR, and PLR were determined by ROC analysis. As shown in Figure 1A, the area under the curve (AUC) for PFS were 0.653, 0.625, and 0.644. The optimal values of SII, NLR, and PLR for the prediction of PFS were 1066.935, 4.40, and 182.595. As shown in Figure 1B, the AUC for OS were 0.593, 0.561, and 0.586. The optimal values of SII, NLR, and PLR for the prediction of PFS were 1343.665, 4.60, and 182.595.
Figure 1.
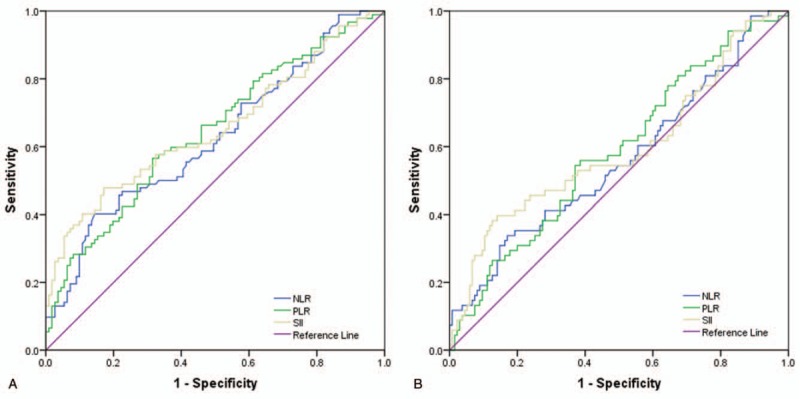
A Receiver operating characteristic curve analysis for optimal cut-off value of NLR, PLR, and SII for PFS. B. Receiver operating characteristic curve analysis for optimal cut-off value of NLR, PLR, and SII for OS. NLR = Neutrophil to Lymphocyte Ratio, OS = Overall Survival, PFS = Progression Free Survival, PLR = Platelet to Lymphocyte Ratio, SII = Systemic Immune-inflammation Index.
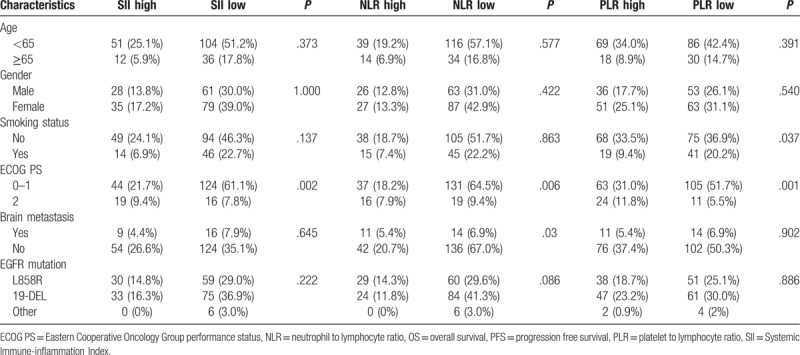
The relationship between SII, NLR, and PLR and patient clinicopathological characteristics is shown in Table 2. Patients with high SII, NLR, and PLR were more likely to have worse Eastern Cooperative Oncology Group performance status (ECOG PS) (P = .002, 0.006, 0.001, respectively). However, SII, NLR, and PLR did not show any significant correlation with age, gender, smoking status, brain metastasis, and EGFR mutation status using the cut-off value mentioned above.
Table 2.
Clinicopathological characteristics according to SII, NLR, and PLR.
3.3. Univariate and multivariate Cox regression analysis for PFS and OS
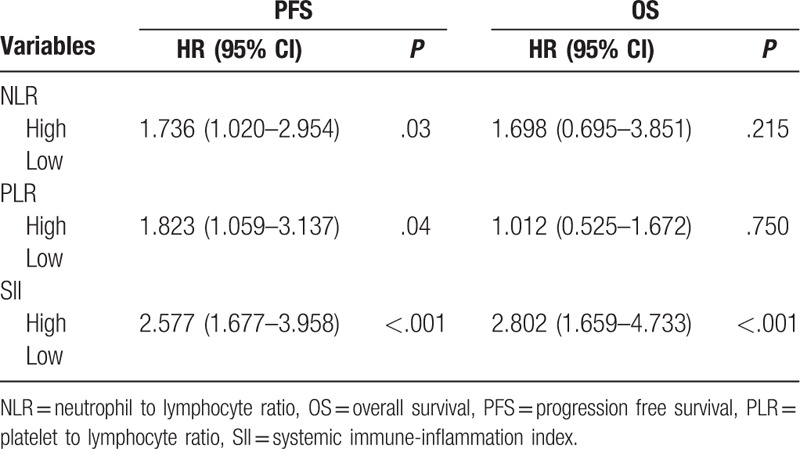
On univariate cox regression analyses, NLR (P < .001), PLR (P = .002), and SII (P < .001) were significantly associated with PFS, and brain metastasis is of boarder significance (P = .052). NLR (P < .001) and SII (P < .001) were significantly associated with OS, while brain metastasis (P = .09) and PLR (P = .08) were of boarder significance (Table 3, Figs. 2 and 3). All other clinicopathological characteristics were not statistically significant. In the multivariate Cox regression analysis, as shown in Table 4, NLR (HR 1.736; 95% CI 1.020–2.954; P = .03), PLR (HR 1.823; 95% CI 1.059–3.137; P = .04) and SII (HR 2.577; 95% CI 1.677–3.958; P < .001) were independent prognostic factors for PFS. Only SII (HR 2.802; 95% CI 1.659–4.733; P < .001) was independent prognostic factors for OS.
Table 3.
Univariate analysis of potential factors associated with PFS and OS.
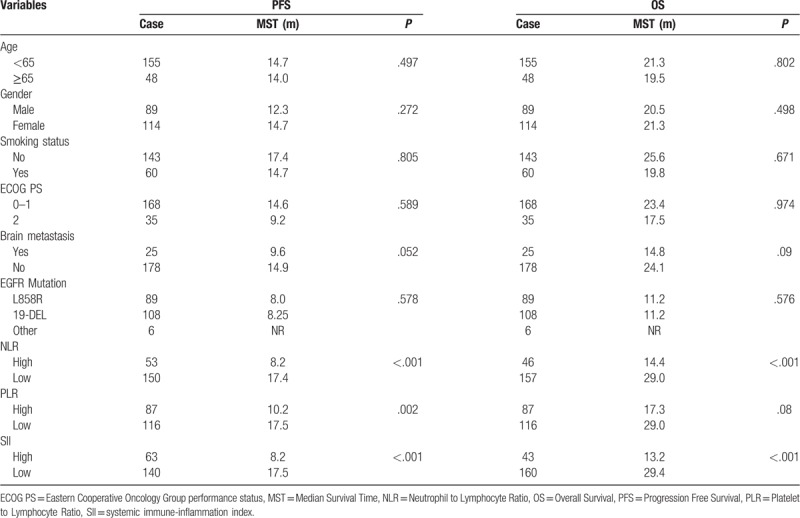
Figure 2.
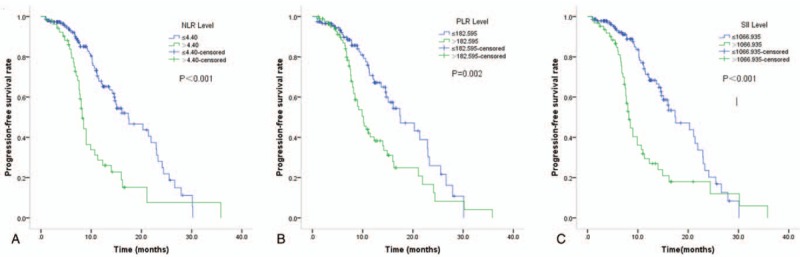
Kaplan–Meier curves of PFS according to NLR (A), PLR (B), and SII (C). NLR = Neutrophil to Lymphocyte Ratio, PLR = Platelet to Lymphocyte Ratio, SII = systemic immune-inflammation index.
Figure 3.
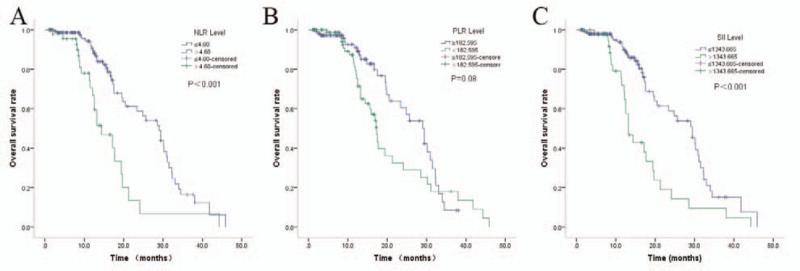
Kaplan–Meier curves of OS according to NLR (A), PLR (B), and SII (C). NLR = Neutrophil to Lymphocyte Ratio, PLR = Platelet to Lymphocyte Ratio, SII = systemic immune-inflammation index.
Table 4.
Multivariable Cox regression analyses for PFS and OS.
4. Discussion
In the present study, we evaluated the prognostic value of blood-based immune-inflammation factors, SII, NLR, and PLR, in EGFR-mutant advanced lung adenocarcinoma patient treating with first-line EGFR-TKIs. We found that patients with SII ≥ 1066.935, NLR ≥ 4.40, and PLR ≥ 182.595 were more likely to have worse ECOG PS scores. Patients with a smoking history were more likely to have higher PLR. Patients with higher NLR were more likely to have brain metastasis. Further, the pretreatment SII, NLR, and PLR were independent prognostic factors for PFS, while only SII was the independent prognostic factor for OS. Compared with NLR and PLR, SII had higher prognostic ability.
Inflammation is a hallmark of cancer, which can promote tumorigenesis and progression of cancer.[15] Tumor cells could secret proinflammatory factors, and systemic inflammation could in return promote tumor cell proliferation, promote angiogenesis, and inhibit host anti-tumor immune response.[16] It has been reported that about 30% to 50% of advanced cancer patients were with evidence of systemic inflammation, who were more likely to have a worse prognosis.[17] Inflammatory cells, including neutrophils, lymphocytes and platelets, and other factors, such as C-reactive protein (CRP) have been reported to be associated with prognosis in various cancers. Neutrophil is an indicator of acute and chronic inflammation which can promote tumor progression and inhibit anti-tumor immunity.[18,19] Lymphocyte, especially T lymphocyte, plays an important role in host anti-tumor immunity,[20] and treatment related lymphopenia is reported to be associated with poor prognosis.[21] Thrombocytosis is a paraneoplastic syndrome which is widely accepted as an adverse prognosis factor of cancer patients.[22] However, one of the major challenges in this area is that there is still lack of consensus of the indicators of systemic inflammation. A series of indicators, based on those factors mentioned above have been established and reported to be associated with prognosis of cancer patients, including NLR,[5] PLR,[7] and Glasgow prognostic score (GPS).[23,24] SII is a comparatively new indicator based on the account of three types of peripheral immune cells, and is suggested to better reflect the host immune and inflammation status compared with NLR or PLR. Previous studies have demonstrated prognostic value of SII and its superiority over NLR and PLR in various cancers.[25,26,27]
The prognostic value of SII in NSCLC has also been reported. Tomita et al[28] conducted a retrospective analysis in NSCLC patients after curative resection and found that SII was an independent predictive indicator for cancer-specific survival, and patients with SII < 471.2 had a significant better 5-year OS (83.61% vs 60.39%, P < .001). Another research in stage III NSCLC patients reported that patients with SII < 660 had a better OS (HR 2.105; 95%CI: 1.481–2.741; P < .001), and SII is superior to NLP and PLR in terms of prognostic ability.[29] For advanced stage patient, Guo et al[11] reported that SII was also a prognosis factor. Patients with SII < 521 had a better PFS (HR 5.009; 95%CI: 1.996–12.570; P = .001) and OS (HR 2.824; 95% CI: 1.285–6.207; P = .01). In another research on lung adenocarcinoma with brain metastasis, Li et al[30] reported that SII ≤ 1218.81 was associated with prolonged OS (HR 2.179; 95% CI: 1.616–2.940; P < .001).
The current principle of cancer treatment is multimodality comprehensive treatment, including surgery, chemotherapy, radiation therapy, target therapy, and immunotherapy. Different modality has different mechanism, resistance mechanism, and different effect on host immune and inflammation status. Previous studies mainly focused on the prognostic role of immune and inflammation indicators in NSCLC patients receiving resection, chemotherapy and radiation therapy. The report on the prognostic role of those indicators in NSCLC patients receiving EGFR-TKI is rare. Aguiar-Bujanda et al[31] reported that NLR is a prognostic factor in European patients with EGFR-Mutant NSCLC Treated with TKIs. In a subgroup analysis of Li's research, they revealed that SII was of boarder significance in predicting the survival of EGFR-mutant lung adenocarcinoma patients with brain metastasis, with a P = .08. In the present study, we found that SII, NLR, and PLR were prognostic factors for PFS, and only SII was the prognostic factors for OS. Our study is partially consistence with previous study. Since there were only 25 patients with brain metastasis were included, and the cut-off value was 1066.935 for PFS, and 1343.665 for OS in our research, the inconsistency may result from different inclusion criteria and different cut-off values.
Besides effecting immune status, systemic inflammation also induces metabolism impairment and results in mal-nutrition, including cancer related cachexia, which also contribute to morbidity and mortality in cancer.[32] Cachexia is a complication of cancer whose major manifestation is loss of muscle and fat mass.[33] It is caused by pro-inflammatory cytokines, including IL-6 and related cytokines, and JAK/STAT and PI3K/Akt/mTOR pathway were demonstrated to be involved in the pathogenesis of cachexia.[32,34] Cachexia is prevalent in advanced stage cancer patients, and is correlated with shorter survival and worse quality-of-life.[35] Therefore, exploring the pathogenesis of systemic inflammation and mal-nutrition provides new insights in interventions to improve the prognosis of cancer patients. Some basic researches have demonstrated that reversal of cancer cachexia could prolong survival in rats.[36] There is early clinical trial showing that agent targeting JAK2 may be effective in advanced nonsquamous NSCLC with systemic inflammation.[37] However, effective interventions and agents are still lacking, which worth further research.
Although our research suggested the prognostic value of SII in EGFR-mutant lung adenocarcinoma patients treated with first-line EGFR-TKI, there are some limitations in our research. First, this was a single center retrospective study with a comparably small sample size. The imbalance between groups may bring some bias to the results. Such as, the number of patients with brain metastasis and ECOG PS score of >2 was small. Second, the cut-off values calculated based-on ROC were different from previous studies, which makes it very hard to compare our results with others. Due to the retrospective nature of most of the studies in this area, multicenter prospective research with larger sample size is still needed to further verify the prognostic value of SII in this group of patients.
In conclusion, the present study showed that the SII, a blood-based immune inflammation index is independently associated with PFS and OS in EGFR-Mutant lung adenocarcinoma patients treated with first-line EGFR-TKIs. It may be an easy-to-derive prognostic factor for these patients which worth further research.
Author contributions
Conceptualization: Tao Hou.
Data curation: Chao Deng, Na Zhang, Yapeng Wang, Shun Jiang, Min Lu, Yan Huang.
Investigation: Na Zhang, Yapeng Wang, Shun Jiang, Min Lu, Yan Huang.
Methodology: Chao Deng, Shun Jiang, Tao Hou.
Resources: Chao Deng.
Supervision: Jin-an Ma, Chunhong Hu.
Writing – original draft: Tao Hou.
Writing – review & editing: Jin-an Ma, Chunhong Hu.
Footnotes
Abbreviations: 95%CI 95% = confidence intervals, AUC = area under the curve, CRP = C-reactive protein, ECOG PS = Eastern Cooperative Oncology Group performance status, EGFR-TKI = Epidermal Growth Factor Receptor-tyrosine Kinase Inhibitor, GPS = Glasgow prognostic score, HR = hazard ratios, IL-6 = interleukin-6, JAK/STAT = the Janus kinase/signal transducer and activator, NLR = neutrophil to lymphocyte ratio, NSCLC = non-small cell lung cancer, OS = overall survival, PFS = progression free survival, PI3K/Akt/mTOR = phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin, PLR = platelet to lymphocyte ratio, RECIST = response evaluation criteria in solid tumors, ROC = receiver operating characteristic, SII = systemic immune-inflammation index, WBRT = whole brain radiation therapy.
The authors have no conflicts of interests to disclose.
References
- [1]. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1–9. [DOI] [PubMed] [Google Scholar]
- [2]. Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol 2015;33:2197–204. [DOI] [PubMed] [Google Scholar]
- [4]. Normando SR, Cruz FM, Del Giglio A. Cumulative meta-analysis of epidermal growth factor receptor-tyrosine kinase inhibitors as first-line therapy in metastatic non-small-cell lung cancer. Anticancer Drugs 2015;26:995–1003. [DOI] [PubMed] [Google Scholar]
- [5]. Yao JJ, Zhu FT, Dong J, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced nasopharyngeal carcinoma: a large institution-based cohort study from an endemic area. BMC Cancer 2019;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Sandfeld-Paulsen B, Meldgaard P, Sorensen BS, et al. The prognostic role of inflammation-scores on overall survival in lung cancer patients. Acta Oncol 2019;1–6. [DOI] [PubMed] [Google Scholar]
- [7]. Jonska-Gmyrek J, Gmyrek L, Zolciak-Siwinska A, et al. Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients. Cancer Manag Res 2018;10:6029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Kim JH, Lee JY, Kim HK, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol 2017;23:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Bojaxhiu B, Templeton AJ, Elicin O, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol 2018;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Xie QK, Chen P, Hu WM, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med 2018;16:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Guo D, Zhang J, Jing W, et al. Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol 2018;14:2643–50. [DOI] [PubMed] [Google Scholar]
- [12]. Nie D, Gong H, Mao X, et al. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol 2019;152:259–64. [DOI] [PubMed] [Google Scholar]
- [13]. Zhang K, Hua YQ, Wang D, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med 2019;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [15]. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [16]. Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [17]. Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134–46. [DOI] [PubMed] [Google Scholar]
- [18]. Vazquez Rodriguez G, Abrahamsson A, Jensen LD, et al. Estradiol promotes breast cancer cell migration via recruitment and activation of neutrophils. Cancer Immunol Res 2017;5:234–47. [DOI] [PubMed] [Google Scholar]
- [19]. Faget J, Groeneveld S, Boivin G, et al. Neutrophils and snail orchestrate the establishment of a pro-tumor microenvironment in lung cancer. Cell Rep 2017;21:3190–204. [DOI] [PubMed] [Google Scholar]
- [20]. Iseki Y, Shibutani M, Maeda K, et al. The impact of the preoperative peripheral lymphocyte count and lymphocyte percentage in patients with colorectal cancer. Surg Today 2017;47:743–54. [DOI] [PubMed] [Google Scholar]
- [21]. Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017;99:128–35. [DOI] [PubMed] [Google Scholar]
- [22]. Gu D, Szallasi A. Thrombocytosis portends adverse prognosis in colorectal cancer: a meta-analysis of 5,619 patients in 16 individual studies. Anticancer Res 2017;37:4717–26. [DOI] [PubMed] [Google Scholar]
- [23]. Lv Y, Pan Y, Dong C, et al. Modified glasgow prognostic score at recurrence predicts poor survival in resected non-small cell lung cancer (NSCLC) patients. Med Sci Monit 2017;23:3780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Kasahara N, Sunaga N, Tsukagoshi Y, et al. Post-treatment glasgow prognostic score predicts efficacy in advanced non-small-cell lung cancer treated with anti-PD1. Anticancer Res 2019;39:1455–61. [DOI] [PubMed] [Google Scholar]
- [25]. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23:6261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol 2016;7:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol 2019;234:1794–802. [DOI] [PubMed] [Google Scholar]
- [28]. Tomita M, Ayabe T, Maeda R, et al. Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo 2018;32:663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Tong YS, Tan J, Zhou XL, et al. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med 2017;15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Li H, Wang G, Zhang H, et al. Prognostic role of the systemic immune-inflammation index in brain metastases from lung adenocarcinoma with different EGFR mutations. Genes Immun 2018. [DOI] [PubMed] [Google Scholar]
- [31]. Aguiar-Bujanda D, Duenas-Comino A, Saura-Grau S, et al. Neutrophil to lymphocyte ratio as a prognostic factor in european patients with epidermal growth factor receptor-mutant non-small cell lung cancer treated with tyrosine kinase inhibitors. Oncol Res Treat 2018;41:755–61. [DOI] [PubMed] [Google Scholar]
- [32]. Madeddu C, Mantovani G, Gramignano G, et al. Muscle wasting as main evidence of energy impairment in cancer cachexia: future therapeutic approaches. Future Oncol 2015;11:2697–710. [DOI] [PubMed] [Google Scholar]
- [33]. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2015;14:58–74. [DOI] [PubMed] [Google Scholar]
- [34]. Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 2016;54:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. van der Meij BS, Schoonbeek CP, Smit EF, et al. Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: an exploratory study comparing two consensus-based frameworks. Br J Nutr 2013;109:2231–9. [DOI] [PubMed] [Google Scholar]
- [36]. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010;142:531–43. [DOI] [PubMed] [Google Scholar]
- [37]. Giaccone G, Sanborn RE, Waqar SN, et al. A placebo-controlled phase ii study of ruxolitinib in combination with pemetrexed and cisplatin for first-line treatment of patients with advanced nonsquamous non-small-cell lung cancer and systemic inflammation. Clin Lung Cancer 2018;19:e567–74. [DOI] [PubMed] [Google Scholar]


