Abstract
Intracerebral hemorrhage (ICH) is a major cause of morbidity and mortality throughout the world. It is reported that the incidence of deep ICH and intracranial artery stenosis (ICAS) are higher in Asian countries. Thus, there are concerns regarding a potential relationship between ICAS and ICH. This study was aimed to investigate this potential relationship between intracranial artery (middle cerebral artery, MCA) stenosis and ICH in the lateral lenticulostriate artery (LLA) territory in Chinese. Totally, 973 in-hospital subjects were retrospectively enrolled including subjects with the diagnosis of ICH, acute ischemic stroke (IS), and prior IS and subjects without cerebral diseases. These subjects were divided into four groups: ICH, acute IS, prior IS, and normal group (without cerebral diseases). Multiple logistic regression analysis showed that severe MCA stenosis was associated with the increased risk of ICH (OR = 5.070) and acute IS (OR = 5.406) in the LLA territory. The moderate MCA stenosis was associated with the increased risk of ICH (OR = 9.899) and was not associated with acute IS in the LLA territory. The increased perfusion pressure to the LLA may be the cause. In conclusion, MCA stenosis, especially moderate MCA stenosis, is associated with ICH in the LLA territory in Chinese.
Keywords: atherosclerotic plaque, cerebral arterial diseases, cerebral hemorrhage, intracranial hemorrhages, middle cerebral artery
1. Introduction
Intracerebral hemorrhage (ICH) is a major cause of morbidity and mortality throughout the world.[1] It is reported that ICH occurs in 10% to 30% of all strokes, and two-thirds of the subjects survive with permanent disabilities.[1,2] The incidence of spontaneous ICH is reported higher in Asian countries,[3] which is two times higher in Asians (51.8 per 100 000) than in the Caucasian population (24.6 per 100 000).[4] In addition, the common location of the hemorrhage differs from Asian and Caucasian populations. There are more deep hemorrhages in Asians, while more lobar hemorrhages in Caucasian.[5,6] This dominant difference may be attributable to different incidence of risk factors of different population. The prevalence of hypertension, alcohol intake, hypercholesterolemia, and smoking is significantly higher in Chinese than in Caucasian,[7] of which hypertension is one of the most important risk factors for deep ICH.[8] It is known that hypertensive angiopathy is responsible for about 35% of ICH cases; amyloid angiopathy, 20%; and undetermined etiology, 21%; cavernomas and arteriovenous malformations, 5%; anticoagulation, 14% and systemic disease, 5%, respectively.[9] Among the cases with undetermined etiology, 67% cases involve deep supratentorial hemorrhages.[9]
In addition to a higher incidence of deep ICH, Asian patients also have a higher incidence of intracranial artery stenosis (ICAS) in comparison with Caucasian patients. ICAS is the underlying cause of up to 30% to 50% of strokes in the Asian population, which is much higher than in Caucasian with a rate of 5% to 10% and in African with a rate of 15% to 29%.[10–15] These previous findings raise concerns regarding the possible role of ICAS in the progression of deep ICH especially the role of in the middle cerebral artery (MCA).
The atherosclerotic plaques in the MCA can impair the normal arterial structure and cause stenosis and hemodynamic changes. We speculated that the MCA stenosis would increase the perfusion pressure to the lateral lenticulostriate arteries (LLA) and consequently cause ICH in the LLA territory. Therefore, our study aimed to investigate the potential association between MCA stenosis and ICH in the LLA territory in Chinese.
2. Methods
Totally, 3959 in-hospital subjects were consecutively enrolled in this study from January 2015 to November 2018 in Xinqiao hospital. Inclusion criteria were as follows: (a) subjects with diagnosis of ICH in the LLA territory and ischemic stroke (IS) in the LLA territory, MCA territory, and both LLA and MCA territories; (b) subjects with suspected cerebral vascular diseases and consequently were proved without cerebral vascular diseases; (c) subjects had underwent computed tomography angiography (CTA); (d) subjects with ICH had underwent cranial computed tomography (CT) to confirm the diagnosis;( e) subjects with IS had underwent diffusion-weighted imaging (DWI) to clarify the diagnosis of the acute IS and prior IS.
Exclusion criteria were: (a) subjects with ICH outside of the LLA territory; (b) subjects with the diagnosis of brain tumors, cerebral vascular malformations, vasculitis, trauma, coagulopathy, and cerebral venous sinus thrombosis; (c) a history of sympathomimetic drug abuse; (d) subjects with missed medical data.
Finally, 973 subjects were included in final analysis, including 115 subjects with ICH in the LLA territory, 350 subjects with acute IS in LLA and/or MCA territory, 161 subjects with prior IS in the LLA and/or MCA territory and 347 subjects with suspected cerebral vascular diseases and consequently proved without cerebral vascular diseases. Based on the CT and DWI results, we divided all study subjects into four groups: ICH, acute IS, prior IS, and normal group. The ICH group included subjects with hemorrhagic lesions on CT in the lateral lenticulostriate arteries (LLA) territory. The acute IS group included subjects with acute infarct lesions on DWI in the LLA territory, MCA territory, and both. The prior IS group included subjects with prior infarct lesions in the LLA and/or MCA territory on T1 weighted image, T2 weighted image, and/or a history of IS. The normal group included subjects with suspected cerebral vascular diseases and consequently proved without cerebral vascular diseases as the control group.
According to the location, cerebral atherosclerotic stenosis was classified into MCA stenosis, ICAS, and extracranial a stenosis (ECAS) group. The MCA stenosis group included subjects with atherosclerotic stenosis occurring in MCA. The ICAS group included subjects with atherosclerotic stenosis occurring in four major intracranial arteries including anterior cerebral artery (ACA), posterior cerebral artery (PCA), intracranial segment of vertebral artery (V4), and basilar artery (BA). The ECAS group included subjects with atherosclerotic stenosis occurring in four major extracranial arteries including the common carotid artery (CCA), cervical segment of internal carotid artery (C1), the first segment of vertebral artery (V1), and subclavian artery (SCA). The MCA stenosis was measured using the WASID (in warfarin-aspirin symptomatic intracranial disease trial) method and further was classified into mild (<50%), moderate (50%–70%), and severe (>70%) stenosis depending on the severity of stenosis.
The study protocol was approved by the ethics committee of Xinqiao Hospital, Army Medical University. Information regarding the risk factors for ischemic and hemorrhagic stroke, including age, sex, history of hypertension, history of diabetes mellitus, creatinine, alanine aminotransferase, triglyceride, total cholesterol, low-density lipoprotein, thyroid-stimulating hormone, free triiodothyronine, free thyroxine, and hemoglobin were collected from subjects’ medical records. Multiple logistic regression models were used to analyze the potential association between MCA stenosis and ICH in the LLA territory. Analyses were performed by using SPSS software (version 22.0, SPSS Inc., Chicago, IL). A two-sided P value <.05 was considered statistically significant.
3. Results
Baseline characteristics of ICH, acute IS, prior IS, and normal groups were in Table 1. Variables included in the multiple logistic regression models were age, sex, hypertension, diabetes mellitus, hyperlipidemia merged by triglyceride, total cholesterol and low-density lipoprotein, MCA stenosis, ICAS, and ECAS. Multiple logistic regression analyses (Table 2) found that the severity of MCA stenosis was associated with the increased risk of ICH (mild MCA stenosis: odds ratio [OR] = 2.929, 95% confidence interval [CI] = 1.278–6.709, P = .011, moderate MCA stenosis OR = 9.899, 95% CI = 2.171–45.143, P = .003, and severe MCA stenosis OR = 5.070, 95% CI = 1.079–23.827, P = .040, respectively). The C1 stenosis was associated with the decreased risk of ICH (OR = 0.450, 95% CI = 0.253–0.798, P = .006). The other cerebrovascular risk factors, such as history of hypertension and male sex, also showed the increased risk of ICH (OR = 15.921, 95% CI = 8.303–30.528, P < .001 and OR = 1.813, 95% CI = 1.072-3.067, P = .026, respectively). In contrast, diabetes mellitus and ECAS showed the decreased risk of ICH (OR = 0.213, 95% CI = 0.106–0.427, P < .001 and OR = 0.459, 95% CI = 0.265–0.794, P = .005, respectively). Hyperlipidemia, age, and ICAS (not including MCA stenosis) were not associated with ICH.
Table 1.
Baseline characteristics of subjects in intracerebral hemorrhage, ischemic stroke, and normal groups.
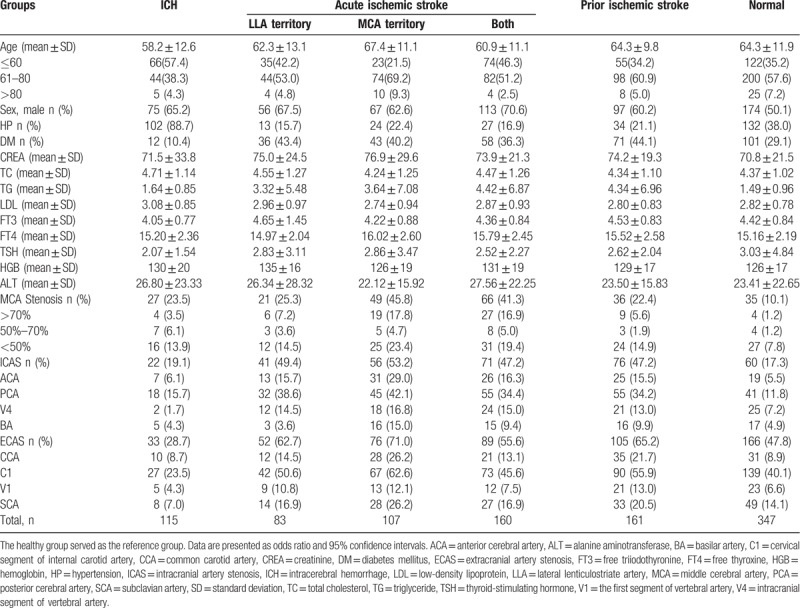
Table 2.
Multiple logistic analysis of middle cerebral artery stenosis and intracerebral hemorrhage and ischemic stroke.
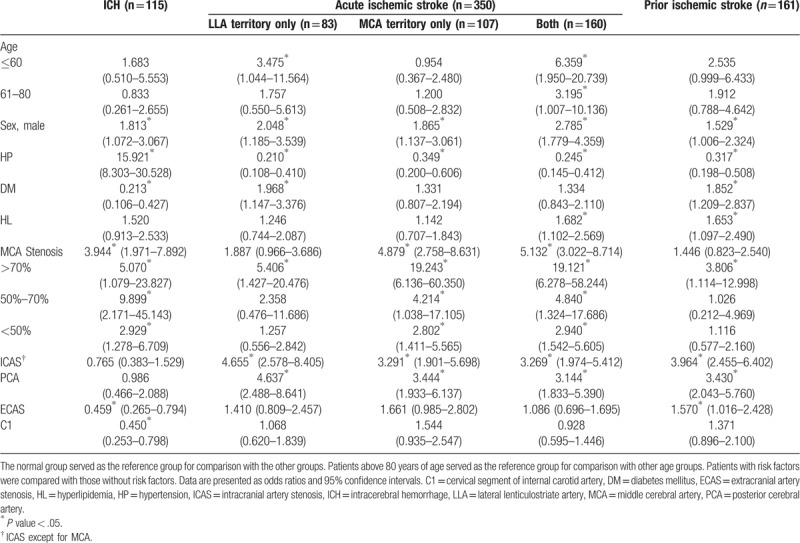
Similarly, MCA stenosis was associated with increased risk of acute IS occurring in the MCA territory only and in both LLA and MCA territories (OR = 4.879, 95% CI = 2.758–8.631, P = .001 and OR = 5.132, 95% CI = 3.022–8.714, P < .001, respectively) including the mild MCA stenosis (OR = 2.802, 95% CI = 1.411–5.565, P = .003 and OR = 2.940, 95% CI = 1.542–5.605, P = .001, respectively). The moderate and severe MCA stenosis were associated with the higher risk of acute IS occurring in the MCA territory only and in both LLA and MCA territories (Table 2). Only severe MCA stenosis was associated with the increased risk of acute IS in the LLA territory only and prior IS. The ICAS (not including MCA stenosis) was also associated with the increased risk of acute and prior IS as well as the PCA stenosis. Hypertension showed the decreased risk of acute and prior IS. Age greater than 60 years was associated with the increased risk of acute IS in both LLA and MCA territories. Diabetes mellitus and hyperlipidemia were also associated with the increased risk of prior IS and acute IS in a specific territory.
4. Discussion
The main findings of this study are: (1) the MCA stenosis was associated with the increased risk of ICH; (2) the moderate MCA stenosis has the higher risk of ICH than the mild and severe MCA stenosis; (3) the ECAS, especially C1 stenosis, was associated with the decreased risk of ICH. Although MCA stenosis associated with the increased risk of IS was well known, the association between MCA stenosis and ICH was seldom studied. Previous studies suggested that ICH and IS may share a common mechanism.[16–18] We speculated that MCA stenosis decreased the arterial diameter and caused a high perfusion pressure to the LLA which lead to ICH and IS in the LLA territory. In this study, we found that MCA stenosis was associated with the increased risk of ICH in the LLA territory (including the mild, moderate, and severe MCA stenosis). The moderate MCA stenosis showed the highest risk of ICH in the LLA territory (OR = 9.899). It was explained that when the MCA stenosis was moderate, the perfusion pressure to the LLA was the highest and thus led to the highest risk of ICH in the LLA territory. When the MCA stenosis increased from moderate to severe stenosis or decreased from moderate to mild, the perfusion pressure decreased and thus led to the lower risk of ICH in the LLA territory (OR = 5.070 and OR = 2.929, respectively). The ECAS, especially C1 stenosis, was associated with the decreased risk of ICH in the LLA territory, which might be contributable to the decreased perfusion pressure to MCA and LLA (Table 2). Similarly, hypertension increased the perfusion pressure to MCA and LLA and thus increased the risk of ICH in the LLA territory (OR = 15.921).
In contrast to ICH, the mild and moderate MCA stenosis did not show the increased risk of acute IS in the LLA territory (Table 2). Only the severe MCA stenosis was associated with the increased risk of acute IS in the LLA territory (OR = 5.406). It suggested that the acute IS and ICH in the LLA territory may share the common mechanism (the increased perfusion pressure to the LLA) and the difference was acute IS in the LLA territory occurred in the advanced stage of atherosclerosis (severe MCA stenosis).
In contrast, acute IS in the MCA territory had different mechanisms from that in the LLA territory. Acute IS in the LLA territory often occurred via artery-to-artery embolism or watershed stroke mechanism. Consequently, the risk of acute IS in the MCA territory exponentially increased as the increased severity of MCA stenosis (Table 2). The severe MCA stenosis showed a fourfold risk of acute IS in the MCA territory than the moderate MCA stenosis.
In conclusion, the moderate MCA stenosis had the highest risk of ICH in the LLA territory than mild and moderate MCA stenosis, which may be caused by the increased perfusion pressure to the LLA. Meanwhile, the severe MCA stenosis rather than the mild and moderate MCA stenosis was associated with the increased risk of acute IS in the LLA territory. The MCA stenosis, especially moderate MCA stenosis, should be considered as a risk factor of ICH in the LLA territory in Asia population.
This study was a retrospective design, which limited the power of the findings. All the data were collected through the medical records and selection bias possibly exists. The lack of clinical data may bias these results including the history of smoking, alcoholism, antihypertension drug, etc. and results of our study should be further proved by prospective observational data. During the whole follow-up, there were nine patients with IS (9/511) and only two patients with MCA stenosis suffered from acute ICH in this study. The reason might be the relatively short follow-up duration and retrospective analysis. Therefore, there was no enough data to describe the whole situation in real world or make any conclusion or further discussion.
Author contributions
Conceptualization: Jie Shuai.
Data curation: Huchuan Zhou.
Formal analysis: Lin Shen, Huchuan Zhou, Fei Wei, Jie Shuai.
Methodology: Huchuan Zhou, Fei Wei, Jie Shuai.
Resources: Huchuan Zhou.
Supervision: Jie Shuai.
Validation: Fei Wei, Jie Shuai.
Writing – original draft: Lin Shen.
Writing – review & editing: Lin Shen, Jie Shuai.
Footnotes
Abbreviations: ACA = anterior cerebral artery, BA = basilar artery, C1 = cervical segment of internal carotid artery, CCA = common carotid artery, CI = confidence interval, CT = cranial computed tomography, CTA = computed tomography angiography, DWI = diffusion-weighted imaging, ICAS = intracranial artery stenosis, ICH = intracerebral hemorrhage, IS = ischemic stroke, LLA = lateral lenticulostriate artery, MCA = middle cerebral artery, OR = odds ratio, PCA = posterior cerebral artery, SCA = subclavian artery, V1 = the first segment of vertebral artery, V4 = intracranial segment of vertebral artery, WASID = warfarin-aspirin symptomatic intracranial disease.
The authors report no conflicts of interest
References
- [1].Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart 2014;9:101–6. [DOI] [PubMed] [Google Scholar]
- [3].Burke TA, Venketasubramanian RN. The epidemiology of stroke in the East Asian region: a literature-based review. Int J Stroke 2006;1:208–15. [DOI] [PubMed] [Google Scholar]
- [4].van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–76. [DOI] [PubMed] [Google Scholar]
- [5].Inagawa T. Recurrent primary intracerebral hemorrhage in Izumo City, Japan. Surg Neurol 2005;64:28–35. discussion 35–6. [DOI] [PubMed] [Google Scholar]
- [6].Hanger HC, Wilkinson TJ, Fayez-Iskander N, et al. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2007;78:836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsai CF, Anderson N, Thomas B, et al. Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in Chinese and White populations: systematic review and meta-analysis. PLoS One 2016;11:e0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Labovitz DL, Halim A, Boden-Albala B, et al. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology 2005;65:518–22. [DOI] [PubMed] [Google Scholar]
- [9].Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 2012;43:2592–7. [DOI] [PubMed] [Google Scholar]
- [10].Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396–9. [DOI] [PubMed] [Google Scholar]
- [11].Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 1995;26:14–20. [DOI] [PubMed] [Google Scholar]
- [12].Qureshi AI, Safdar K, Patel M, et al. Stroke in young black patients. Risk factors, subtypes, and prognosis. Stroke 1995;26:1995–8. [DOI] [PubMed] [Google Scholar]
- [13].Weisberg LA. Clinical characteristics of transient ischemic attacks in black patients. Neurology 1991;41:1410–4. [DOI] [PubMed] [Google Scholar]
- [14].Wityk RJ, Lehman D, Klag M, et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996;27:1974–80. [DOI] [PubMed] [Google Scholar]
- [15].Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158–9. [DOI] [PubMed] [Google Scholar]
- [16].Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol 1971;30:536–50. [DOI] [PubMed] [Google Scholar]
- [17].Azarpazhooh MR, Nicol MB, Donnan GA, et al. Patterns of stroke recurrence according to subtype of first stroke event: the North East Melbourne Stroke Incidence Study (NEMESIS). Int J Stroke 2008;3:158–64. [DOI] [PubMed] [Google Scholar]
- [18].Huhtakangas J, Lopponen P, Tetri S, et al. Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke 2013;44:585–90. [DOI] [PubMed] [Google Scholar]


