Abstract
Background:
This study will systematically assess the efficacy and safety of pirfenidone for the treatment of patients with pulmonary fibrosis (PF).
Methods:
We will search potential records from following literature sources from their inceptions to the present without language, and publication status limitations: Cochrane Library, EMBASE, PUBMED, the Cumulative Index to Nursing and Allied Health Literature, the Allied and Complementary Medicine Database, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure. In addition, we also search grey literature sources, such as dissertations, conference proceedings, as well as the reference lists of included studies. All randomized controlled trials related to the pirfenidone for treating PF will be included. All the process of study selection, data extraction, and methodological evaluation will be carried out by 2 authors independently. The primary outcome comprises of all-cause-mortality, and lung function status, as measured by forced vital capacity. The secondary outcomes consist of 6-minute walk distance, progression-free survival, dyspnea, acute exacerbation, quality of life, and adverse events. Whenever possible, all results data will be pooled and meta-analysis will be performed.
Results:
This study will systematically assess the efficacy and safety of pirfenidone for the treatment of patients with PF.
Conclusion:
The findings of the present study will summarize most recent evidence of pirfenidone for PF.
Ethics and dissemination:
No individual data will be analyzed in this study, thus, no research ethics approval is required in this study. The findings of this study are expected to be disseminated in a peer-reviewed journal or conference presentations.
PROSPERO registration number:
PROSPERO CRD42019126958.
Keywords: efficacy, pirfenidone, pulmonary fibrosis, safety
1. Introduction
Pulmonary fibrosis (PF) is a very tricky lung disorder,[1,2] characterized by severe and progressive fibrosis of the interstitium of lung, which mainly manifests as exertional dyspnoea and cough.[3–6] This condition is very fatal, causing the death of patients within 2 to 5 years from diagnosis.[7–10] It has been reported that the 5-year survival rate of PF varies from 20% to 40% associated with PF,[11] which is similar to non-small cell lung cancer and even worse than other cancers.[12]
Pirfenidone is an antifibrotic drug with anti-inflammatory properties, which is widely used for the treatment of PF.[13–17] Although a most recent systematic review has been published in October 2016,[18,19] it searched the database only up to the February 2016, and most importantly, several high-quality randomized controlled trails (RCTs) have been published after that.[19,20–24] Thus, it is still very necessary to conduct an updated study to systematically summarize the latest evidence for further assessing the efficacy and safety of pirfenidone for the treatment of PF.
2. Methods
2.1. Objectives
This study systematically assesses the efficacy and safety of pirfenidone for patients with PF.
2.2. Study registration
This protocol has registered on PROSPERO (CRD42019126958). It has reported based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Protocol statement.[25]
2.3. Eligibility criteria for study selection
2.3.1. Types of studies
This study will include RCTs of pirfenidone for PF without language and publication status restrictions. Non-RCTs and quasi-RCTs will be excluded in this study.
2.3.2. Types of patients
Patients with PF regardless race, gender, age or educational background will be included in this study.
2.3.3. Types of interventions
In the experimental group, patients can receive any forms of pirfenidone monotherapy. In the control group, patients can undergo any treatments, except the pirfenidone therapy.
2.3.4. Types of Outcomes
2.3.4.1. Primary outcomes
All-cause-mortality;
Lung function status (as measured by forced vital capacity).
2.3.4.2. Secondary outcomes
6-minute walk distance;
Progression-free survival;
Dyspnea (as assessed by University of California San Diego Shortness of Breath Questionnaire, or other instruments);
Acute exacerbation;
Quality of life (as evaluated 36-Item Short Form Health Survey, or other scales);
Adverse events (any expected or unexpected adverse events or reactions).
2.4. Search strategy for identification of studies
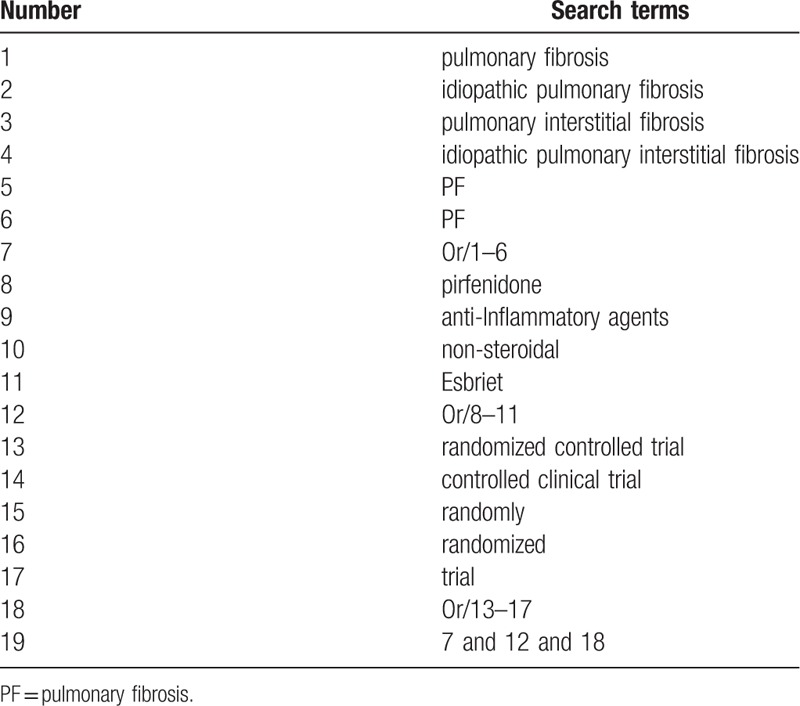
The following literature sources will be searched from their inceptions to the present without language, and publication status limitations: Cochrane Library, EMBASE, PUBMED, the Cumulative Index to Nursing and Allied Health Literature, the Allied and Complementary Medicine Database, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure. Additionally, grey literature sources, including dissertations, conference proceedings, and reference lists of included studies will also be considered for inclusion. We have presented the detailed search strategy for PUBMED in Table 1. We have also used similar search strategy to other electronic databases.
Table 1.
Search strategy used in PUBMED database.
2.5. Study selection
EndNote 7.0 software will be used to manage the searched records, and remove the duplicated literature. Two authors will independently review the title and abstract for each searched record to confirm eligible studies. Full texts will also be read if they can not be identified from titles and abstracts. All disagreements will be arbitrated by a third author through discussion. We will document each excluded study with clearly reason. The results of study selection will be presented in PRISMA flowchart.
2.6. Data extraction
Two authors will perform data extraction using a pre-designed data extraction sheet respectively. Any disagreements will be discussed with a third author. All essential information and data will be collected from each eligible trial for following information: basic information, patients, study methods, details of treatments, outcome measurements, adverse events, and any other relevant information.
2.7. Risk of bias assessment for eligible trials
Risk of bias for each eligible trail will be judged according to the standard criteria of Cochrane Risk of Bias Tool through 7 aspects. Each aspect will be further judged as 3 levels: high risk of bias, unclear risk of bias, and low risk of bias. Two authors will assess the risk of bias for each study respectively. All divisions regarding the risk of bias assessment will be solved by a third author through discussion
2.8. Measurement of treatment effect
Continuous data will be measured by using mean difference or standardized mean difference and 95% confidence intervals (CIs). Dichotomous data will be measured by using risk ratio and 95% CIs.
2.9. Missing data dealing with
We will try our best to contact primary authors of eligible studies to obtain missing or insufficient data. If we can not require those additional data, only available data will be analyzed. However, we will discuss its potential impacts.
2.10. Heterogeneity identification
In this study, I2 test will be used to identify heterogeneity among eligible studies. When I2 ≤50%, low heterogeneity is considered. When I2 >50%, significant heterogeneity is regarded, and subgroup analysis will be conducted.
2.11. Data synthesis
If low heterogeneity is identified, a fixed-effect model will be applied to pool the data and we will also carry out meta-analysis. If significant heterogeneity is detected, a random-effect model will be used to pool the data. Additionally, we will also carry out subgroup analysis to explore any possible causes that may account for significant heterogeneity. We will not pool the data, and will not perform meta-analysis if there is still significant heterogeneity after subgroup analysis. However, we will carry out a systematic narrative synthesis for the findings.
2.12. Subgroup analysis
We will operate subgroup analysis in accordance with different characteristics, interventions, and outcomes.
2.13. Sensitivity analysis
Sensitivity analysis will be performed to test the robustness and stability of the outcome results by removing low quality of eligible studies, and also the possible effects of missing data.
2.14. Reporting bias
We will carry out funnel plot[26] and Egger regression test[27] to identify any possible reporting bias if more than 10 eligible RCTs will be included in this study.
3. Discussion
The protocol of this study will apply rigorous methodology to identify and examine studies reporting the outcomes of pirfenidone for PF. Although most recent similar study has been reported in 2016,[18] there were more than 6 high-quality RCTs after that.[19,20–24] Thus, it is still very necessary to update and summarize latest evidence for assessing the efficacy and safety of pirfenidone for PF.
This study will summarize rigorous evidence of pirfenidone for PF across all published RCTs. The findings of this study will inform our understanding of the value of pirfenidone in treating PF outcomes. This evidence may also provide helpful evidence for clinical practice and health policy-makers for the treatment of PF.
Author contributions
Conceptualization: Shu-Min Li, Yang Lin, Shan-Shan Liang.
Data curation: Shu-Min Li, Yang Lin, Shan-Shan Liang.
Formal analysis: Shu-Min Li, Shan-Shan Liang.
Investigation: Shu-Min Li.
Methodology: Yang Lin, Shan-Shan Liang.
Project administration: Shu-Min Li.
Resources: Shu-Min Li, Yang Lin, Shan-Shan Liang.
Software: Yang Lin, Shan-Shan Liang.
Supervision: Shu-Min Li.
Validation: Shu-Min Li, Shan-Shan Liang.
Visualization: Shu-Min Li.
Writing – original draft: Shu-Min Li, Yang Lin, Shan-Shan Liang.
Writing – review & editing: Shu-Min Li, Yang Lin, Shan-Shan Liang.
Footnotes
Abbreviations: CIs = confidence intervals, PF = pulmonary fibrosis.
YL and S-ML contributed equally to this study.
This work is supported by the Heilongjiang Provincial Health and Family Planning Commission Research Project (NO. 2017-395). Provider does not involve any sections of this study.
The authors have no conflicts of interest to disclose.
References
- [1].van Cleemput J, Sonaglioni A, Wuyts WA, et al. Idiopathic pulmonary fibrosis for cardiologists: differential diagnosis, cardiovascular comorbidities, and patient management. Adv Ther 2019;36:298–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018;378:1811–23. [DOI] [PubMed] [Google Scholar]
- [3].Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017;3:17074. [DOI] [PubMed] [Google Scholar]
- [4].Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 2008;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2015;192:e3–19. [DOI] [PubMed] [Google Scholar]
- [7].Strand MJ, Sprunger D, Cosgrove GP, et al. Pulmonary function and survival in idiopathic vs secondary usual interstitial pneumonia. Chest 2014;146:775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim JH, Lee JH, Ryu YJ, et al. Clinical predictors of survival in idiopathic pulmonary fibrosis. Tuberc Respir Dis (Seoul) 2012;73:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:1390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fernández Pérez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 2010;137:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [13].King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. [DOI] [PubMed] [Google Scholar]
- [14].Azuma A, Taguchi Y, Ogura T, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir Res 2011;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Richeldi L, du Bois RM. Pirfenidone in idiopathic pulmonary fibrosis: the CAPACITY program. Expert Rev Respir Med 2011;5:473–81. [DOI] [PubMed] [Google Scholar]
- [16].Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760–9. [DOI] [PubMed] [Google Scholar]
- [17].Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–7. [DOI] [PubMed] [Google Scholar]
- [18].Rogliani P, Calzetta L, Cavalli F, et al. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Pulm Pharmacol Ther 2016;40:95–103. [DOI] [PubMed] [Google Scholar]
- [19].Aravena C, Labarca G, Venegas C, et al. Pirfenidone for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PLoS One 2015;10:e0136160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Behr J, Bendstrup E, Crestani B, et al. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2016;4:445–53. [DOI] [PubMed] [Google Scholar]
- [21].Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Albera C, Costabel U, Fagan EA, et al. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur Respir J 2016;48:843–51. [DOI] [PubMed] [Google Scholar]
- [23].Fisher M, Nathan SD, Hill C, et al. Predicting life expectancy for pirfenidone in idiopathic pulmonary fibrosis. J Manag Care Spec Pharm 2017;23:S17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Costabel U, Albera C, Lancaster LH, et al. An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (RECAP). Respiration 2017;94:408–15. [DOI] [PubMed] [Google Scholar]
- [25].Shamseer L, Moher D, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- [26].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


