Abstract
Background:
The differences in efficacy between capecitabine and 5-fuorouracil (5-FU) in neoadjuvant chemoradiotherapy (CRT) of locally advanced rectal cancer (LARC) are not well recognized. We performed this meta-analysis to analyze the effect of capecitabine and 5-FU on neoadjuvant CRT to more accurately understand the differences between the 2 drugs.
Methods:
MEDLINE, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and Wanfang Database were performed to identify all published studies investigating the efficacy of capecitabine in neoadjuvant CRT of LARC versus 5-FU before August, 2017. Primary endpoint was the odds ratio (OR) for improving pathological complete response (pCR) rate of patients with LARC. Secondary endpoints were the ORs of efficiency for downstaging tumor and increasing R0 resection in patients with LARC. Safety analyses were also performed. The OR was the principal measurement of effect, which was calculated as capecitabine group versus 5-FU group, and was presented as a point estimate with 95% confidence intervals (CIs). All calculations and statistical tests were performed using RevMan 5.3 software.
Results:
In all, 2916 patients with LARC enrolled in the 10 studies were divided into capecitabine group (n = 1451) and 5-FU group (n = 1465). The meta-analysis showed that capecitabine improved pCR (OR 1.34, 95% CI 1.10–1.63), and R0 resection rate (OR 1.92, 95% CI 1.10–3.36). There were no statistically significant differences either in overall downstaging rate (OR 1.31, 95% CI 0.79–2.16) or in the tumor downstaging rate (OR 1.24, 95% CI 0.79–1.92), but there was a significant difference of the nodal downstaging rate between the 2 groups (OR 1.68, 95% CI 1.11–2.54). There was no statistically significant difference in sphincter preservation rate between the 2 groups (OR 1.36, 95% CI 0.96–1.92). No obvious safety concerns about mortality and complications were raised in these studies. There were no statistically significant differences in 3-year disease-free-survival (OR 1.29, 95% CI 0.75–2.20), and in grade 3 to 4 acute toxicity during CRT (OR 0.63, 95% CI 0.31–1.30).
Conclusions:
Compared with 5-FU-based neoadjuvant CRT, capecitabine-based neoadjuvant CRT can safely improve pCR, nodal down-staging, ad R0 resection of patients with LARC.
Keywords: 5-fluorouracil, capecitabine, meta-analysis, neoadjuvant chemoradiotherapy, rectal neoplasms
1. Introduction
Rectal cancer remains to be an important health issue worldwide, and is among the most commonly diagnosed cancer and leading causes of cancer-related death worldwide.[1] The treatment of the rectal cancer is dependent on the TNM staging of the tumor. Early-stage rectal cancer is curable with surgical treatment alone, with a 5-year overall survival rate of 90%.[2,3] However, the majority of rectal cancer patients are diagnosed with advanced diseases. The advanced rectal cancer without distant metastasis is still a potentially curable disease, but the prognosis is poorer than the early-stage diseases. Treatment of advanced rectal cancer is still a challenge for gastrointestinal surgeons. Localized tumors, limited to the submucosa, can be best treated surgically, with a long-term survival of over 90%,[3] but the prognosis of locally advanced tumors is poor due to a high unresectability rate at presentation, and a much higher relapse and metastasis rate after radical surgery. Locally advanced rectal cancer (LARC) without evidence of distant metastasis are potentially curable, but these tumors usually present with a more advanced stage and are associated with a worse prognosis, so it is necessary and widely accepted that multidisciplinary treatment, including surgery, chemotherapy, and radiotherapy, is often employed. Neoadjuvant chemoradiotherapy (CRT) has been recommended for the treatment of LARC, thus demanding further studies regarding the regimens of neoadjuvant CRT.
The employment of pelvic radiation may be the most significant development of the multidisciplinary treatment of LARC, and preoperative CRT, despite a moderate increase in acute toxicity and no impact on overall survival significantly improves local control, has become the standard of care for patients with LRC.[4–8] The Dutch rectal cancer total mesorectal excision (TME) trial showed that neoadjuvant radiation significantly improved 10-year survival in TNM stage III patients with a negative circumferential margin.[9] Compared with upfront surgery, the possible advantages of neoadjuvant CRT in the treatment of LARC include gained resectability and an increased rate of sphincter preservation.[7,10] According to the results of the German CAO/ARO/AIO-94 randomized phase III trial, preoperative CRT showed improved pelvic control and sphincter preservation and less acute/chronic toxicity than postoperative chemoradiotherapy,[7] and there is a persisting significant improvement of pre versus postoperative CRT on local control.[7,8] There is also evidence that showed that preoperative CRT reduce overall long-term surgical complications compared with postoperative CRT.[11]
The addition of 5-fluorouracil (5-FU) to preoperative radiotherapy has been shown to improve the treatment effect significantly compared with radiotherapy alone.[5,12] Fluorouracil has been limited by poor oral absorption and gastrointestinal toxicity, despite of its vital importance in regimens for the treatment of rectal cancer, whereas capecitabine, which is a prodrug of 5-FU, can be orally administered; the final step of its conversion to the active form of 5-FU is performed by thymidine phosphorylase, which is at higher concentrations in most tumor tissue than in most normal healthy tissue, this theoretically allows low systemic toxicity and should be more effective and much safer than 5-FU.[13,14] Recent large phase III studies have confirmed that capecitabine is noninferior to 5-FU as a component of neoadjuvant CRT for rectal cancer.[15] Therefore, 5-FU and the like, capecitabine-based neoadjuvant CRT is now a standard treatment for LARC.
Achievement of complete pathological response (pCR) after neoadjuvant CRT is associated with greatly improved cancer outcomes in LARC.[16,17] Two meta-analyses[18,19] have shown that pCR is associated with excellent long-term survival, with low rates of local recurrence and distant failure, suggesting that achievement of pCR by neoadjuvant CRT may indicate a favorable biological tumor profile. So, we set the odds ratio (OR) for improving pCR as the primary endpoint of this meta-analysis.
The improvements in magnetic resonance and ultrasound endoscopy allows more accurate preoperative staging can be achieved,[20] and accordingly develop clinical studies with different regimens of neoadjuvant chemoradiotherapy.[15,21–29] Ten studies of 5-FU and capecitabine efficacies of neoadjuvant CRT in LARC were compared in this meta-analysis. All 10 studies have shown that preoperative CRT using capecitabine and 5-fluouracil is feasible and improves the pCR rate. The aim of the current meta-analysis under such circumstances was to evaluate the effectiveness of neoadjuvant CRT in treatment of rectal cancer and explre the optimal strategy for preoperative treating patients with LARC.
2. Materials and methods
2.1. Ethical approval
Because the present study is a meta-analysis of previously published studies, the ethical approval and patient consent are not required.
2.2. Data collection and selection
A comprehensive electronic data retrieval was in MEDLINE, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and Wanfang Database until August, 2017. Electronic searches were carried out to identify all published studies that compared the capecitabine-based neoadjuvant CRT with 5-FU-based one for LARC. The search was done using four sets of terms: “rectal cancer”; “capecitabine”; “5-fluorouracil” and “neoadjuvant chemoradiotherapy/preoperative chemoradiotherapy”, and the terms were set to title/abstract.
2.3. Inclusion and exclusion criteria
The following inclusion criteria were used: studies that compared the capecitabine-based neoadjuvant CRT with 5-FU-based one for LARC; blindness of the trial was not required; patients with pathologically diagnosed rectal adenocarcinoma, without prior treatment before entering the trial, but with a history of potentially curative surgery; and studies which were considered updated.
Exclusion criteria: studies on postoperative chemotherapy; studies that included patients with metastases at enrollment were excluded; and studies with the group not receiving surgery were excluded.
The data of each study were collected by 2 reviewers (J.F.Z. and W.Z.) independently. The results were consistent.
2.4. Data extraction
The following data were extracted from each study and recorded using a predesigned form: authors, year of publication, patient population, country of investigators, sample size (total, eligible, and per arm), CRT regimen, cycles of chemotherapy, follow-up period, curative effect (survival rate, rate of macroscopic radical resection and cancer stage at pathological examination), and adverse events. Two reviewers (J.F.Z. and W.Z.) did the extraction independently.
2.5. Meta-analysis protocol
Data were obtained directly from included articles or calculated by percentage in each article. The meta-analysis was performed using Review Manager 5.3 software (provided by Cochrane Collaboration). Outcomes assessed by this meta-analysis included the overall survival, 3-year progression-free survival rate, tumor down-staging rate, R0 resection rate, and safety analysis. Overall survival was defined as the time between the treatment randomization and the date of the last follow-up or of the patient's death. Patients who were lost to follow-up were considered as dead. Locoregional recurrence was measured either from the date of surgery to the occurrence of the event or to the date of last follow-up. Heterogeneity between the studies was assessed to determine which model would be used in the meta-analysis. A sensitivity analysis was performed by changing the meta-analysis model. An OR was the principal measurement of effect. It was calculated as the capecitabine group versus the 5-FU group.
2.6. Statistical analysis
All statistical analyses were performed spontaneously using Review Manager 5.3 software (Nordic Cochran Centre, Copenhagen, Denmark). Heterogeneity between the studies was assessed using chi-square test. I2 statistics was used for the degree of heterogeneity evaluation, fixed-effects model methodology was applied when I2 < 50%, and random-effects model methodology was applied when I2 > 50%. The OR was calculated for dichotomous data with 95% confidence intervals (CIs) for all analyses. All P values were 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Search results and characteristics of included studies
Figure 1 demonstrates a flow chart of the selection process that yielded a total of 10 studies included in the current meta-analysis. A total of 2916 patients (capecitabine group, n = 1451; fluorouracil group, n = 1465) were included in the analysis. The detailed characteristics of these studies are summarized in Table 1.
Figure 1.
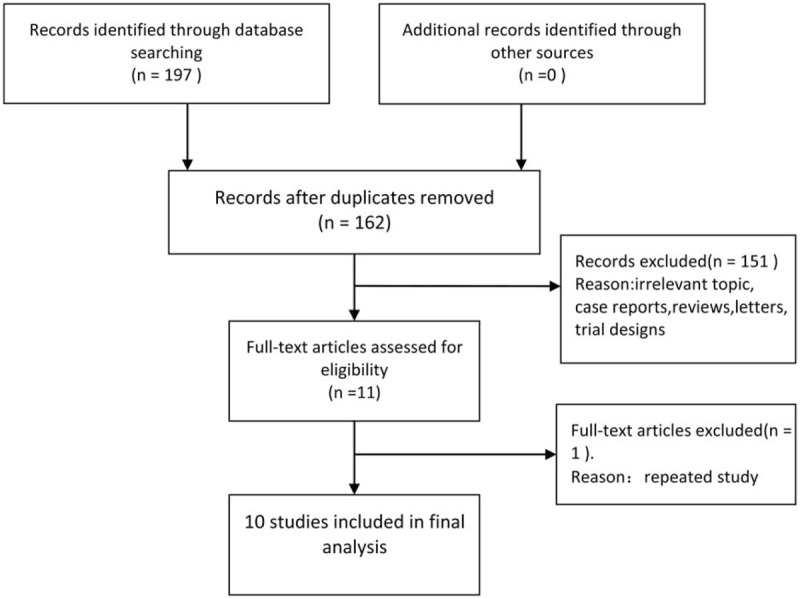
Flow diagram of studies selection process.
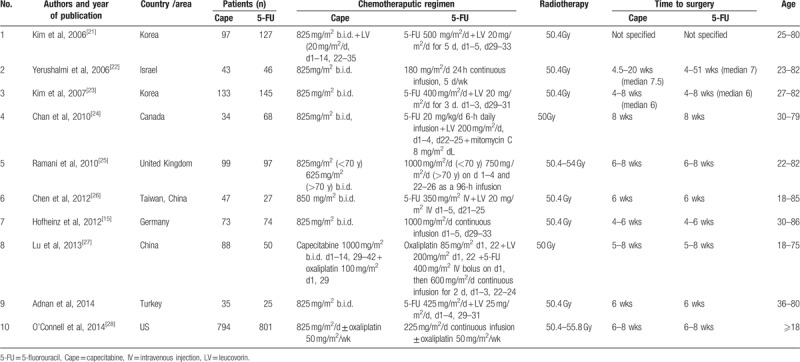
Table 1.
Characteristics of the included studies in the meta-analysis.
3.2. Complete pathological response
There was no significant heterogeneity between the studies (P = .54), and the fixed-effects model was used. The data in all the 10 studies[15,21–29] were available for the analysis of pCR. There were 1408 patients in the capecitabine group and 1382 patients in the 5-FU group in this meta-analysis, and the pCR rates were 19.53% and 15.48%, respectively. The OR, expressed as capecitabine group versus 5-FU group, was 1.34 (95% CI 1.10–1.63, P = .004). The difference of the pCR between capecitabine group and 5-FU group was statistically significant. The pCR was increased by 4.05% in the capecitabine group compared with the 5-FU group (Fig. 2). The number needed to treat (NNT) was 25. Publication bias was not found in the included studies (Fig. 3).
Figure 2.
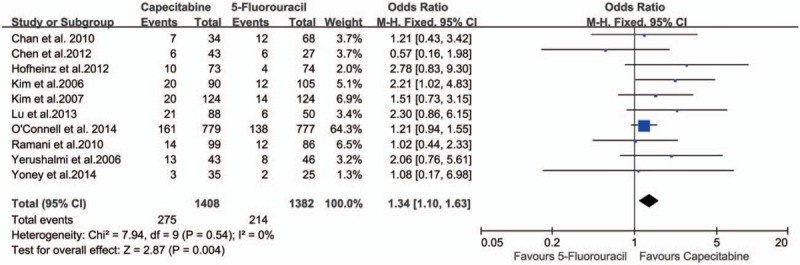
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on pCR rate. CRT = chemoradiotherapy, pCR = pathological complete response.
Figure 3.
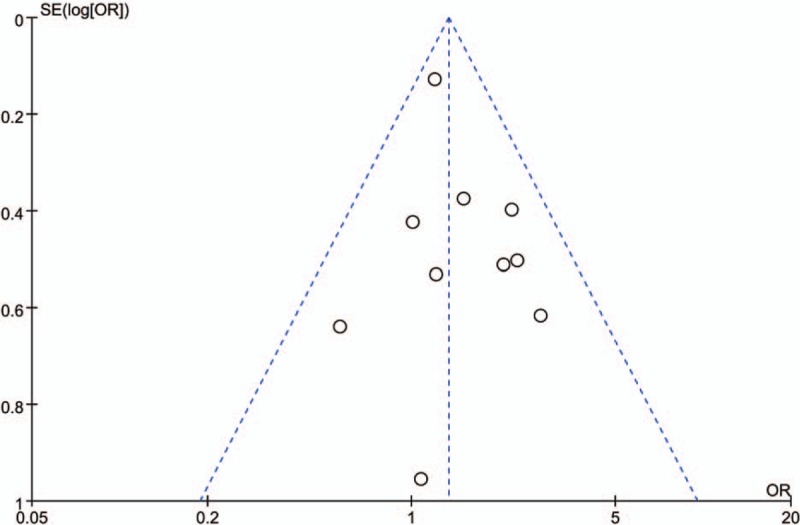
Publication bias in the included studies. Funnel plot analysis of potential publication bias. OR = odds ratio.
3.3. Downstaging rate
Seven studies[21–23,25–27,29] describing the pathological downstaging of rectal cancer after resection (519 in capecitabine group and 466 in 5-FU group) were included in the analysis. Six studies[21,23–26,29] describing the tumor downstaging (425 in capecitabine group and 446 in 5-FU group), 3 studies[21,25,26] describing the nodal downstaging (214 in capecitabine group and 207 in 5-FU group) were included in the analysis respectively. There were no statistically significant differences either in overall downstaging rate (68.02% vs 62.23%; OR 1.31, 95% CI 0.79–2.16, I2 = 67%, P = .29) or in the tumor downstaging rate (51.29% vs 45.52%; OR 1.24, 95% CI 0.79–1.92, I2 = 57%, P = .35)between the 2 groups, but there was a significant difference of the nodal downstaging rate between capecitabine group and 5-FU group (66.36% vs 56.03%; OR 1.68, 95% CI 1.11–2.54, I2 = 42%, P = .01) (Fig. 4A, B, and C).
Figure 4.
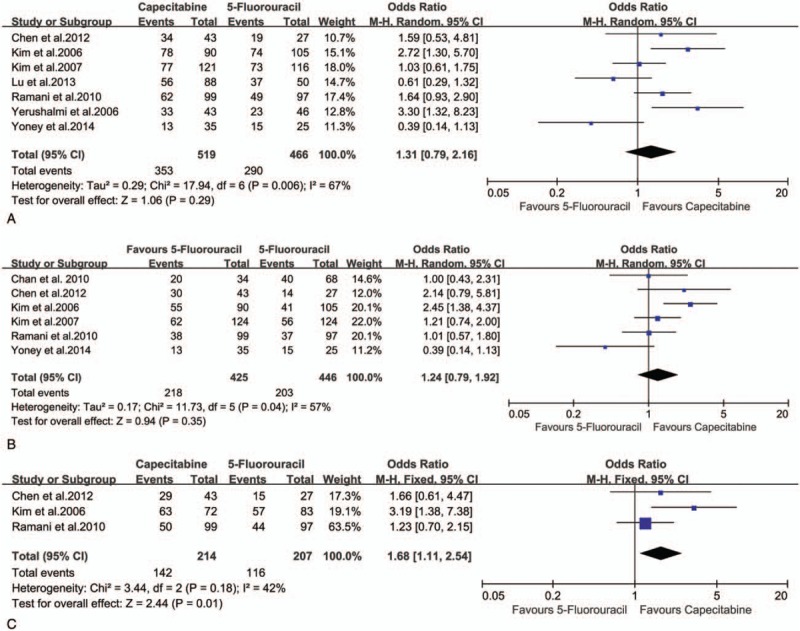
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on downstaging rate. (A) Overall downstaging rate; (B) tumor downstaging rate; (C) nodal downstaging rate.
3.4. R0 resection rate
The R0 resection rate of rectal cancer was reported in 3 studies.[15,25,29] Because no obvious heterogeneity was observed in these studies (P = .76, I2 = 0%), the fixed-effect model was used. The R0 resection rate of the rectal cancer was higher in capecitabine group than in 5-FU group (87.86% vs 80.10%; OR 1.92, 95% CI 1.10–3.36, I2 = 0%, P = .02), and the NNT was 13 (Fig. 5).
Figure 5.
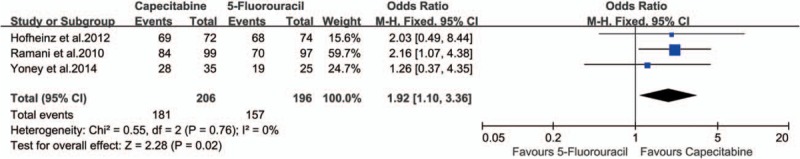
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on R0 resection. CRT = chemoradiotherapy.
3.5. Sphincter preservation
Eight studies[21–26,28,29] comparing the different sphincter preservation rate between the 2 groups (1247 in capecitabine group and 1272 in 5-FU group) were included in the analysis. There was no statistically significant difference in sphincter preservation rate between the 2 groups (63.67% vs 59.75%; OR 1.36, 95% CI 0.96–1.92, I2 = 53%, P = .09) (Fig. 6).
Figure 6.
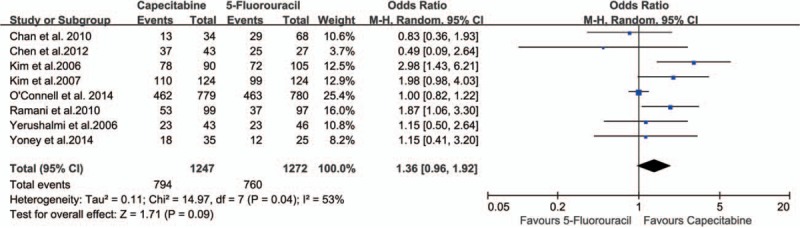
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on sphincter preservation. CRT = chemoradiotherapy.
3.6. Three-year disease-free survival
Three studies[15,24,29] compared the 3-year disease-free survival (DFS) rates between the 2 groups. There were no statistically significant differences in 3-year DFS (76.67% vs 75.72%; OR 1.29, 95% CI 0.75–2.20, I2 = 0%, P = .35) (Fig. 7).
Figure 7.
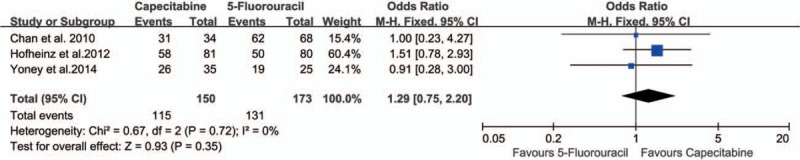
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on three-year disease-free survival. CRT = chemoradiotherapy.
3.7. Safety analysis
Safety analysis included both chemotherapy-induced acute adverse effects (grade 3/4, defined according to the Common Toxicity Criteria of the National Cancer Institute, version 2.0) and postoperative complication and mortality. Eight studies[21–26,28,29] reported grade 3/4 acute adverse effects of neoadjuvant CRT, and there were no statistically significant differences in grade 3 to 4 acute toxicity during CRT (26.82% vs 28.21%; OR 0.63, 95% CI 0.31–1.30, I2 = 82%, P = .21) (Fig. 8). Three studies[21,26,29] reported perioperative mortality without death in either group. Four studies[21,26,28,29] reported anastomotic leakage with no statistically significant difference (P = .68) between the 2 groups (2.18% vs 2.40%; OR 0.88, 95% CI 0.48–1.62, I2 = 0%) (Fig. 9).
Figure 8.
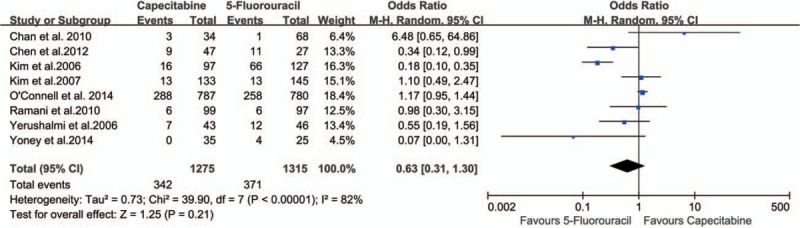
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on acute chemoradiotherapeutic toxicity. CRT = chemoradiotherapy.
Figure 9.
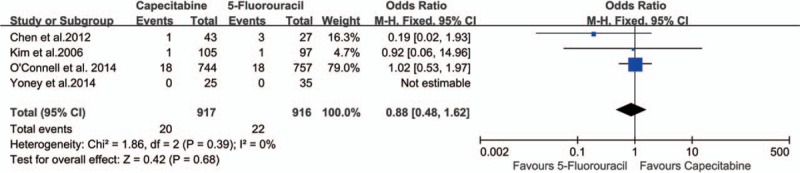
Effect of capecitabine-based and 5-fluorouracil-based neoadjuvant CRT on anastomotic leakage. CRT = chemoradiotherapy.
4. Discussion
Our meta-analysis demonstrated the feasibility of capecitabine-based neoadjuvant CRT for LARC patients. Compared with 5-FU-based neoadjuvant CRT, capecitabine-based regimen could improve pCR (NNT = 25) and increase the R0 resection rate (NNT = 13) of rectal cancer. To examine the role of capecitabine-based neoadjuvant CRT in improving the 3-year DFS rate of LARC patients compared with the patients who received 5-FU-based regimen, data from 3 studies[15,24,29] were further analyzed, showing that capecitabine had no effect on the 3-year DFS rate of LARC patients (OR 1.29, 95% CI 0.75–2.20). Although local failure reduced significantly, DFS has remained stable over these years.[4,8] Many studies showed that some of the complete responders with careful selection and strict surveillance may avoid surgery, and can get a similar oncological outcome compared with surgery, so the “watch and wait” policy can be considered instead of surgery.[30–33] Some studies showed that patients with pathological complete response have significant longer DFS and overall survival after neoadjuvant CRT and can be an early response indicater.[17,18] What's more, in recent years, there has been growing interest in total neoadjuvant therapy (TNT), and a retrospective cohort analysis shows that TNT cohort has a higher CR rate (both pCR and cCR), suggest TNT may be adopted to avoid surgery and preserve organ.[34] Our study showed that capecitabine-based neoadjuvant CRT benefit the pCR of rectal cancer patients, which means more selected patients who get a cCR after neoadjuvant CRT or TNT may avoid surgery and can get a similar oncological outcome.
The regimen of the neoadjuvant chemotherapy affects the outcome of the treatment. Several regimens have been used in the neoadjuvant CRT of LARC. Our present meta-analysis showed that capecitabine-based combination regimen had a high efficiency for LARC patients. The effective response rate will help downstage tumors to the greatest extent and increase the probability of R0 resection. Outcome parameter as R0 resection depends highly on the quality of surgery; however, only 1 of the 3 included studies provided limited information about surgery.[15] Although there is a nonsignificant increase in sphincter preservation rate in this study, some oncologists hold the point that patients with a complete response managed by “watch and wait” strategy may avert permanent colostomy, thus improving the quality of life of the patients.[31,35]
Another major concern in our meta-analysis is the efficiency and safety of neoadjuvant CRT in the studies included. Our meta-analysis showed that LARC patients could well tolerate neoadjuvant CRT. Grade 3/4 gastrointestinal and leukopenia adverse events of neoadjuvant CRT occurred in 27.53% (713/2590) of LARC patients. The difference of complication rate between those 2 groups was not obvious, indicating that capecitabine-based neoadjuvant CRT is a safe modality for LARC (OR 0.63, 95% CI 0.31–1.30). The R0 resection rate of the rectal cancer was higher in capecitabine group than in 5-FU group (OR 1.92, 95% CI 1.10–3.36, NNT = 13). These results suggest that capecitabine can be considered as an alternative to 5-FU in the setting of CRT. Capecitabine was not inferior to 5-FU in safety concern.
There are several limitations to this study. The included studies recruited patients with different amount of capecitabine and 5-FU. The variable grades and clinical stages of disease could have potentially influenced overall survival and the incidence of local or distal recurrence. Only 2 of the included studies were randomized and 8 of them were retrospective.
Based on the evidence from 2 randomized and 8 retrospective studies, capecitabine-based neoadjuvant CRT appears to have clinically measurable advantages in patients with LARC. The reckonable impact on oncological endpoints awaits the findings from large high-quality randomized trials.
5. Conclusions
In conclusion, our study provides information on the efficacy of neoadjuvant CRT with capecitabine-based and 5-FU-based regimen in LARC patients. These regimens were regarded as the most effective ones in neoadjuvant CRT for treating LARC patients. The meta-analysis showed that compared with 5-FU-based neoadjuvant CRT, capecitabine-based neoadjuvant CRT can safely improve pCR, nodal downstaging and R0 resection of patients with LARC. With all these clinical and scientific efforts, these treatment strategies will definitely continue to further improve the outcome of LARC patients.
Author contributions
Conceptualization: Jinfeng Zhu, Wei Zeng.
Data curation: Jinfeng Zhu, Wei Zeng, Lei Ge, Xinhui Yang.
Formal analysis: Jinfeng Zhu.
Investigation: Jinfeng Zhu, Wei Zeng, Lei Ge.
Supervision: Qisan Wang, Haijiang Wang.
Validation: Xinhui Yang.
Writing – original draft: Jinfeng Zhu.
Writing – review & editing: Qisan Wang, Haijiang Wang.
Jinfeng Zhu orcid: 0000-0002-8726-5030.
Footnotes
Abbreviations: 5-FU = 5-fluorouracil, cCR = clinical complete response, CI = confidence interval, CR = complete response, CRT = chemoradiotherapy, DFS = disease-free survival, IV = intravenous injection, LARC = locally advanced rectal cancer, LV = Leucovorin, NNT = number needed to treat, OR = odds ratio, pCR = pathological complete response.
The authors report no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Miller K, Fedewa S, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- [3].Miller K, Siegel R, Lin C, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [4].Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 2014;32:1554–62. [DOI] [PubMed] [Google Scholar]
- [5].Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23. [DOI] [PubMed] [Google Scholar]
- [6].Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol 2016;34:3300–7. [DOI] [PubMed] [Google Scholar]
- [7].Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- [8].Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–33. [DOI] [PubMed] [Google Scholar]
- [9].van Gijn W, Marijnen C, Nagtegaal I, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–82. [DOI] [PubMed] [Google Scholar]
- [10].Ke T, Liao Y, Chiang H, et al. Effectiveness of neoadjuvant concurrent chemoradiotherapy versus up-front proctectomy in clinical stage II-III rectal cancer: a population-based study. Asia Pac J Clin Oncol 2016;12:e234–40. [DOI] [PubMed] [Google Scholar]
- [11].Tural D, Selcukbiricik F, Yildiz O, et al. Preoperative versus postoperative chemoradiotherapy in stage T3, N0 rectal cancer. Int J Clin Oncol 2014;19:889–96. [DOI] [PubMed] [Google Scholar]
- [12].Hasegawa S, Goto S, Matsumoto T, et al. A multicenter phase 2 study on the feasibility and efficacy of neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer. Ann Surg Oncol 2017;24:3587–95. [DOI] [PubMed] [Google Scholar]
- [13].Budman DR, Meropol NJ, Reigner B, et al. Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 1998;16:1795–802. [DOI] [PubMed] [Google Scholar]
- [14].Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998;34:1274–81. [DOI] [PubMed] [Google Scholar]
- [15].Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579–88. [DOI] [PubMed] [Google Scholar]
- [16].de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol 2011;18:1590–8. [DOI] [PubMed] [Google Scholar]
- [17].Dinaux A, Amri R, Bordeianou L, et al. The impact of pathologic complete response in patients with neoadjuvantly treated locally advanced rectal cancer: a large single-center experience. J Gastrointest Surg 2017;21:1153–8. [DOI] [PubMed] [Google Scholar]
- [18].Maas M, Nelemans P, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. [DOI] [PubMed] [Google Scholar]
- [19].Martin S, Heneghan H, Winter D. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012;99:918–28. [DOI] [PubMed] [Google Scholar]
- [20].Zhong G, Xiao Y, Zhou W, et al. Value of endorectal ultrasonography in measuring the extent of mesorectal invasion and substaging of T3 stage rectal cancer. Oncol Lett 2017;14:5657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim JS, Kim JS, Cho MJ, et al. Comparison of the efficacy of oral capecitabine versus bolus 5-FU in preoperative radiotherapy of locally advanced rectal cancer. J Korean Med Sci 2006;21:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yerushalmi R, Idelevich E, Dror Y, et al. Preoperative chemoradiation in rectal cancer: retrospective comparison between capecitabine and continuous infusion of 5-fluorouracil. J Surg Oncol 2006;93:529–33. [DOI] [PubMed] [Google Scholar]
- [23].Kim DY, Jung KH, Kim TH, et al. Comparison of 5-fluorouracil/leucovorin and capecitabine in preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007;67:378–84. [DOI] [PubMed] [Google Scholar]
- [24].Chan AK, Wong AO, Jenken DA. Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer: is it equivalent to 5-FU infusion plus leucovorin and radiotherapy? Int J Radiat Oncol Biol Phys 2010;76:1413–9. [DOI] [PubMed] [Google Scholar]
- [25].Ramani VS, Sun Myint A, Montazeri A, et al. Preoperative chemoradiotherapy for rectal cancer: a comparison between intravenous 5-fluorouracil and oral capecitabine. Colorectal Dis 2010;12 suppl 2:37–46. [DOI] [PubMed] [Google Scholar]
- [26].Chen CF, Huang MY, Huang CJ, et al. A observational study of the efficacy and safety of capecitabine versus bolus infusional 5-fluorouracil in pre-operative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 2012;27:727–36. [DOI] [PubMed] [Google Scholar]
- [27].Lu JY, Xiao Y, Qiu HZ, et al. Clinical outcome of neoadjuvant chemoradiation therapy with oxaliplatin and capecitabine or 5-fluorouracil for locally advanced rectal cancer. J Surg Oncol 2013;108:213–9. [DOI] [PubMed] [Google Scholar]
- [28].O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol 2014;32:1927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoney A, Isikli L. Preoperative chemoradiation in locally advanced rectal cancer: a comparison of bolus 5-fluorouracil/leucovorin and capecitabine. Saudi J Gastroenterol 2014;20:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hupkens B, Martens M, Stoot J, et al. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard resection: a matched-controlled study. Dis Colon Rectum 2017;60:1032–40. [DOI] [PubMed] [Google Scholar]
- [31].Renehan A, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174–83. [DOI] [PubMed] [Google Scholar]
- [32].Gérard J, Chamorey E, Gourgou-Bourgade S, et al. Clinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the ACCORD 12/PRODIGE 2 randomized trial. Radiother Oncol 2015;115:246–52. [DOI] [PubMed] [Google Scholar]
- [33].Maas M, Beets-Tan R, Lambregts D, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633–40. [DOI] [PubMed] [Google Scholar]
- [34].Cercek A, Roxburgh C, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018;4:e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hupkens BJP, Maas M, Martens MH, et al. Organ preservation in rectal cancer after chemoradiation: should we extend the observation period in patients with a clinical near-complete response? Ann Surg Oncol 2018;25:197–203. [DOI] [PubMed] [Google Scholar]


