Supplemental Digital Content is available in the text
Keywords: enteral nutrition, meta-analysis, probiotics, severe head injury, systematic review
Abstract
Background:
The role of early enteral nutrition (ENN) supplemented with probiotics (<48 hours) in improving clinical outcomes of patients with severe head injury (SHI) remains controversial. We performed this meta-analysis to investigate the efficacy of EEN supplemented with probiotics on clinical outcomes in these patients.
Methods:
Systematic searches were performed in PubMed, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure, Wanfang database, and Chinese Biomedical Literature to identify potential studies. Two investigators checked citations, extracted data, appraised risk of bias, and then STATA 12.0 was used to perform statistical analysis.
Results:
A total of 18 trials were eventually included in the present study. Meta-analysis indicated that EEN supplemented with probiotics was associated with decreased risk of infection (risk ratio [RR], 0.53; 95% confidence interval [CI], 0.44–0.65), decreased risk of mortality (RR, 0.56; 95% CI, 0.38–0.82), decreased risk of gastrointestinal complications (RR, 0.19; 95% CI, 0.13–0.25), and shortened stays in intensive care unit (ICU) (mean difference [MD], −4.55; 96% CI, −5.91 to −3.19).
Conclusion:
EEN supplemented with probiotics may be a promising alternative for patients with SHI because it effectively decreased the risk of infection, mortality, and gastrointestinal complications, as well as shortened the stays in ICU.
1. Introduction
Severe head injury (SHI) refers to a pathologic condition where coma persists at least 6 hours or happens again after head trauma.[1] Published data illustrated that the mortality of patients with SHI was eventually up to 60% when they underwent infection.[2] Because this condition can accelerate body catabolism and impair immunity and intestinal barrier function. It is also important that the introduction of invasive treatments increases the risk of these patients to suffer from infection and septicopyemia.
Early enteral nutrition (EEN) is an important alternative of rectifying the metabolic disturbance, enhancing immunity of the organism and improving clinical outcomes in patients with SHI.[3] Several Clinical Practice Guidelines (CPGs) recommended that this nutrition support should be started early within the first 24 to 48 hours following admission for the fully resuscitated, hemodynamically stable critical patients.[4] It must be noted that; however, approximate 50% to 80% patients with SHI were intolerant to enteral nutrition within 2 weeks after head injury. They will suffer from several gastrointestinal symptoms such as abdominal cramps, bloating and gastric retention, which all significantly worsen nutritional status and prognosis.[5] In this process, it is important to keep the intestinal microbial balance which plays a critical role of maintaining the normal intestinal function, promoting the absorption of nutrients, and keeping normal nutritional status.[6] Probiotics are generally defined as “live microbial feed supplements.”[7] When administered in adequate amounts, they are beneficial to the human host by restoring the intestinal microbiome diversity and modulating the damaged intestinal function.[8] Hence, many trials[9–11] have investigated the efficacy of EEN supplemented with probiotics on clinical outcomes in patients with SHI; however, the role of this approach in reducing mortality and infection rate, improving the intestinal function and shortening the length of stay in ICU among patients with SHI remains controversial. Recently, 1 meta-analysis[12] investigated the role of probiotics in trauma patients, which indicated improvement in overall health when use of probiotics compared to not use in those critically ill patients. However, substantial heterogeneity was tested among these studies, which was not surprising given the differences in the features of the study populations, regimen and composition of probiotics, and study designs. All elements stated above affected the validity and reliability of this meta-analysis and cannot reflect the effect of probiotics exactly.
To address these issues and accelerate the informed decision making in this filed eventually; we performed this systematic review and meta-analysis to objectively and comprehensively assess the efficacy and safety of EEN supplemented with probiotics for the treatment of patients with SHI through calculating the summary estimates based on individual result reported by all published studies.
2. Materials and methods
We designed and performed this systematic review and meta-analysis in accordance with recommendations proposed by the Cochrane Handbook for Systematic Reviews of Interventions[13] and reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) Statement.[14] We also registered the protocol at the PROSPRO platform, and a unique identifier of CRD42017079865 has been approved. Moreover, the protocol has been published in TMR Integrative Nursing.[15] We performed all statistical analyses based on published studies, and thus no ethical approval or patient informed consent was required.
2.1. Inclusion and exclusion criteria
We prespecified the inclusion criteria for our study. The following inclusion criteria were determined according to the PICOS acronym (abbreviation of participant, intervention, comparison, outcomes of interest, and study design): Population (P): all the patients were diagnosed as SHI, who had a Glasgow coma scale (GCS) score on admission between 5 and 8 and lasted more than 12 hours, and the estimated survival time of more than 7 days[16]; Intervention (I) and comparison (C): these trials investigated the comparative efficacy and safety of EEN enriched with probiotics vs standard enteral nutrition; Outcomes of interest (O): we assessed the following outcomes including infection rate and mortality (which were all regarded as primary outcome), the length of stay in the intensive care unit (ICU) and the incidence of the gastrointestinal complications (which were all viewed as secondary point); and Study design (S): only randomized controlled trials with or without blind method were considered. Moreover, we also imposed the language restrictions, that is to say, only articles published in English and Chinese language were permitted.
A study will be excluded if met the following criteria: pregnant women, patients with cancer, or those participants underlying severe multiple trauma such as extremity fractures and chest or abdominal trauma, gastrointestinal injuries and cardiopulmonary insufficiency[16]; the essential information was not available to be obtained or we cannot acquire primary data from authors; and the article with the most strict methodology and most complete data would be chosen in terms of duplicate literatures.
2.2. Searching strategy
Two independent investigators electronically searched several databases including PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure (CNKI), the Chinese Biomedical Literature Database (CBM) and Chinese Wanfang Data in order to capture RCTs comparing the efficacy and safety of EEN supplemented with probiotics vs the standard EN until the end of June, 2016. Search results were updated weekly to timely capture any recent studies, the date that it was last updated was August 10, 2017. We used following search terms to perform procedures by using combination of medical subject heading (MeSH) and text word embedded in specific files involving title, keywords and abstract: “Craniocerebral Trauma,” “Head Injur∗,” “Craniocerebral Injur∗,” “Head Trauma∗,” “Probiotics,” “Prebiotics,” “Synbiotics,” “Lactobacillus,” “Bifidobacterium,” “Akkermansia muciniphila,” “Escherichia,” and “random∗.” These all search strings were constructed by Boolean operator. To guarantee the precision and recall ratio, we also manually checked the reference lists of eligible studies and relevant reviews for including any potential studies. Two independent reviewers critically examined citations identified by 2 steps of reading the titles/abstracts and full texts.
2.3. Data extraction
Essential data were independently extracted from each included study by 2 investigators using the predesigned data extraction table,[17] which included first author, publication year, sample size (male/female), average age, GCS, interventions (study group/control group), outcomes of interest, and information of risk of bias. We contacted the leading author when essential data were missing. All information will be rechecked mutually. Any discrepancies were resolved by consulted a 3rd investigator.
2.4. Quality assessment
Two independent investigators critically appraised the methodology quality of all eligible studies using the Cochrane risk of bias assessment tool.[18] Seven indexes were rated independently and successively and the evaluation results were cross-checked: randomization sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other biases. The risk of each incorporated study was rated as “high bias risk,” “unclear bias risk,” or “low bias risk” according to the adequate level of information extracted. Any divergences concerning risk of bias were dissolved by consulted a 3rd investigator.
2.5. Statistical analysis
All statistical analyses were performed by using STATA software version 12.0 (Stata Corp., College Station, TX). The estimates of dichotomous data (this study includes infection rate, mortality and the incidence of the gastrointestinal complications) were expressed as relative risk (RR) with 95% confidence interval (CI). Because only 1 continuous outcome was involved in this study (the ICU length of hospital stay) and used the same unit of measure, and thus mean difference (MD) with 95% CI were calculated. This study adopted the difference between end point and baseline as effect of parameters, the specific calculation formula was cited from the Davidson study.[19] If a study only reported median and range, we will perform transformation with the method reported by Hozo study.[20]
We firstly analysis the clinical similarity and methodologic comparability of every eligible study according to the characteristics of the participants, research design and method, intervention regimes, and measurement and statistical analysis of outcomes, if the clinical characteristic and methodology was consider to have heterogeneity, we will select a qualitative analysis to describe the studies. If not, we will use the Chi-squared test and associated with P value to qualitatively evaluated the heterogeneity across studies of each outcome measure.[21] Moreover, the level of heterogeneity was quantified by the I2 statistic. If a P of ≥.1 and I2 of <50% were calculated, the eligible studies were considered to be homogeneous, and a fixed-effects model based on Mantel–Haenszel (MH) or inverse variance (IV) statistical approach was selected; conversely the pooled results will be affected by substantial heterogeneity and the random-effects model based on MH or IV statistical approach was selected. If the accumulated number of eligible studies for single outcome of interest was >10,[22] we will perform Egger linear test to detect publication bias.[23]
3. Results
3.1. Identification and selection of studies
We identified 140 citations at the initial literature search stage, and no additional citation was added. Sixty duplicate articles were removed by using EndNote bibliography management software version 7.2.1. After a detailed assessment of the remaining records, 18 eligible articles,[2,9–11,16,24–36] involving 1016 participants met our inclusion criteria finally. A flow chart of the literature retrieval and selection is depicted in Figure 1.
Figure 1.
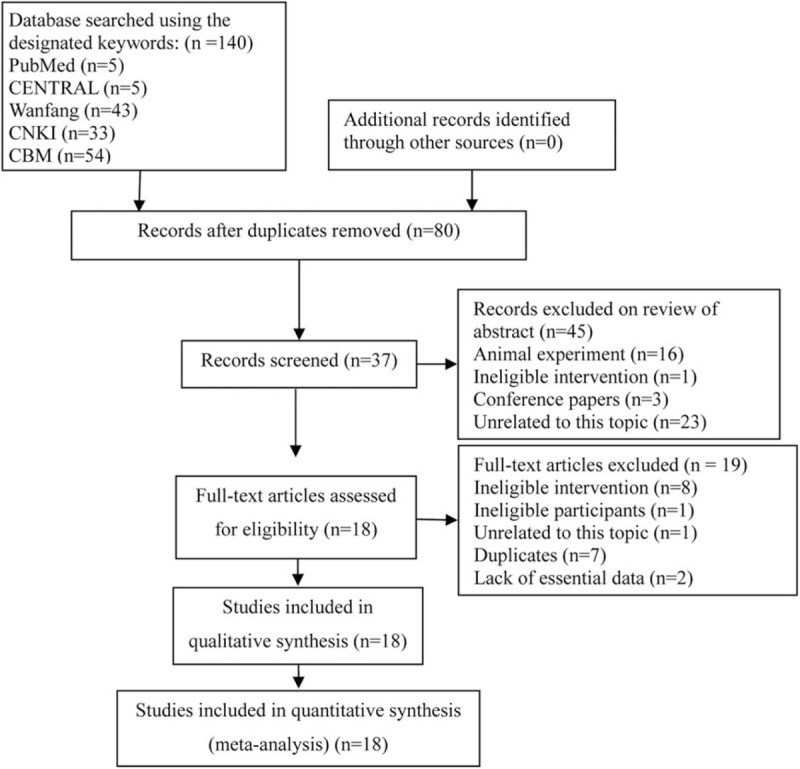
Flow diagram of retrieval and screen. CBM = China Biomedical Literature database; CENTRAL = Cochrane Central Register of Controlled Trials; CNKI = China National Knowledge Infrastructure.
3.2. Study characteristics
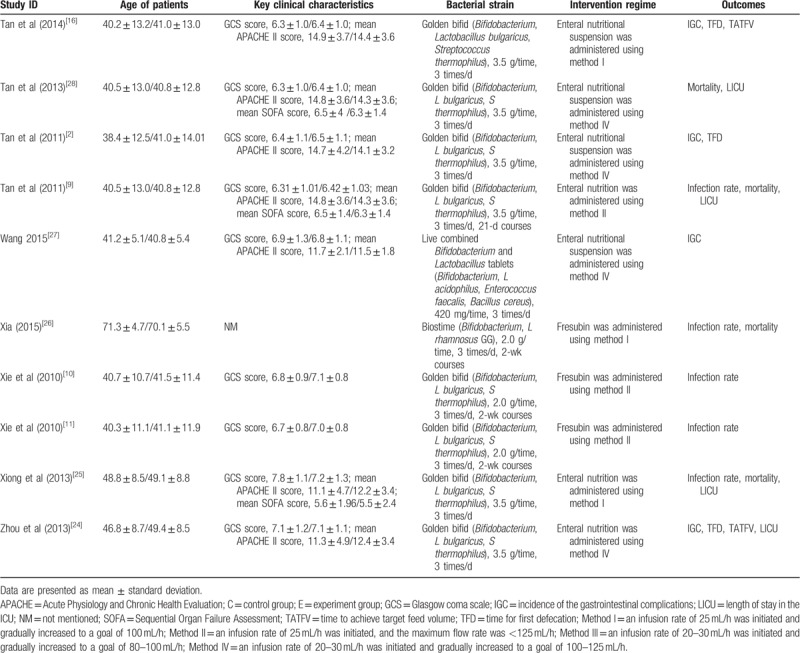
The basic characteristics of all eligible studies[2,9–11,16,24–36] are documented in Table 1. These studies were published between 2010 and 2016. All eligible studies were conducted in mainland China and 1 of them was published in English language. Sample size of each eligible study was ranged from 35 to 110 with a total of 1016 patients. Moreover, we documented the URLs of accessing abstracts of all eligible studies in Supplementary Material 1.
Table 1.
Basic characteristics of all the eligible trials.
3.3. Quality of individual study
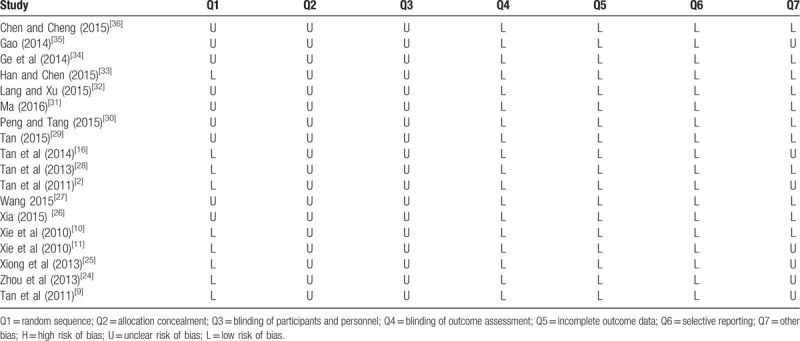
A total of 18 eligible studies[2,9–11,16,24–36] were incorporated into the present systematic review and meta-analysis. Randomization were mentioned in all eligible studies, and 11 of them adopted appropriate methods to generate random sequences (i.e., random number table used in 5,[11,16,25,27,33] computer random number generator applied in 4,[2,9,24,28] and order of admission adopted in remaining 2).[31,32] No study adequately described whether allocation concealment was actually used and whether blinding of participants and personnel was performed; however, because of all outcomes evaluated in each study were objective measures and thus the reliability of results cannot be impaired whether blinding method was applied or not. Based on the reason stated above, this domain was regarded as "low risk of bias” for all studies. Sixteen studies were at low risk for attrition bias as no patient was lost to follow-up. The remaining 2 studies[9,16] stated details of dropout, withdrawal and loss to follow-up during the research process. Among them, 1 study[9] was based on intention-to-treat analyses, and missing outcome data were balanced in numbers across intervention groups in the remaining study. All studies adequately reported all outcomes of interest. Seven studies[2,9,11,16,24,25,35] existed the problems of small sample size, which might impair the power of this study. The methodologic quality assessment of included trials is revealed in Table 2.
Table 2.
Risk of bias summary.
3.4. Meta-analysis on all outcomes of interest
3.4.1. Infection rate
Ten studies[9–11,25–27,32–34,36] estimated the efficacy of EEN supplemented with probiotics on infection rate of patients with SHI. In these 10 studies, 643 patients were analyzed with 318 patients in the observation group and 325 patients in the control group, respectively. The clinical characteristic and methodology are homogeneous in all studies, no evidence of heterogeneity was found among them (P = .804, I2 = 0.0%), and thus we selected the fixed-effect model to pool data. The summarized result showed that EEN supplemented with probiotics was associated with decreased risk of infection events (RR, 0.53; 95% CI, 0.44–0.65; Z, 6.27; P < .001) (Fig. 2).
Figure 2.
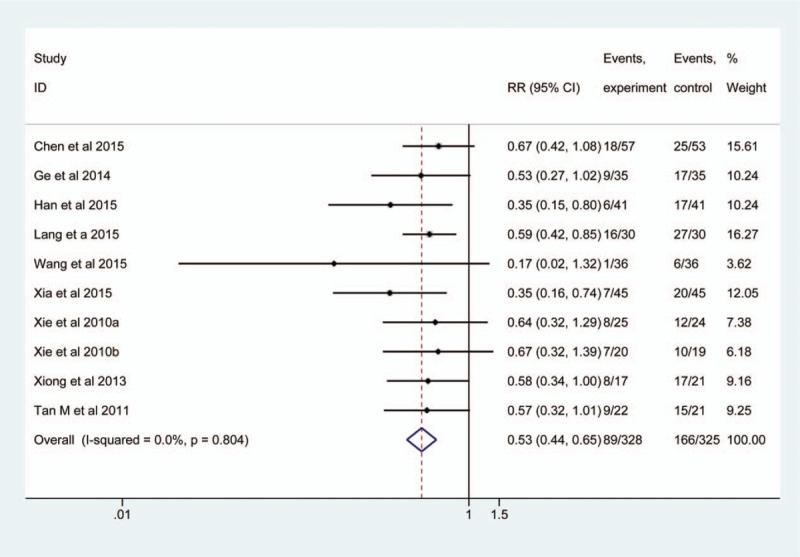
Meta-analysis of infection rate. The horizontal line, square, and diamond represent 95% CI, point estimate, and pooled effect size, respectively. CI = confidence interval; RR = relative risk.
Of these 10 studies,[9–11,25–27,32–34,36] 9[9–11,25,27,32–34,36] reported pulmonary infections, 5[25,27,33,34,36] reported intracranial infections, 5 analyzed urinary tract infections, 3[25,32,36] reported operative incision infections, and 5[10,11,25,32,34] described other infection events. Therefore, we separately assessed the efficacy of EEN supplemented with probiotics on the risk of individual infection event adopting the subgroup analysis method. Evidence on heterogeneity was not detected among them (I2 = 0.0%), and thus a fixed-effect model was adopted. The result suggested that the targeted intervention decreased the risk of pulmonary infections (RR, 0.56; 95% CI, 0.43–0.74; P < .001), but did not other events (Fig. 3).
Figure 3.
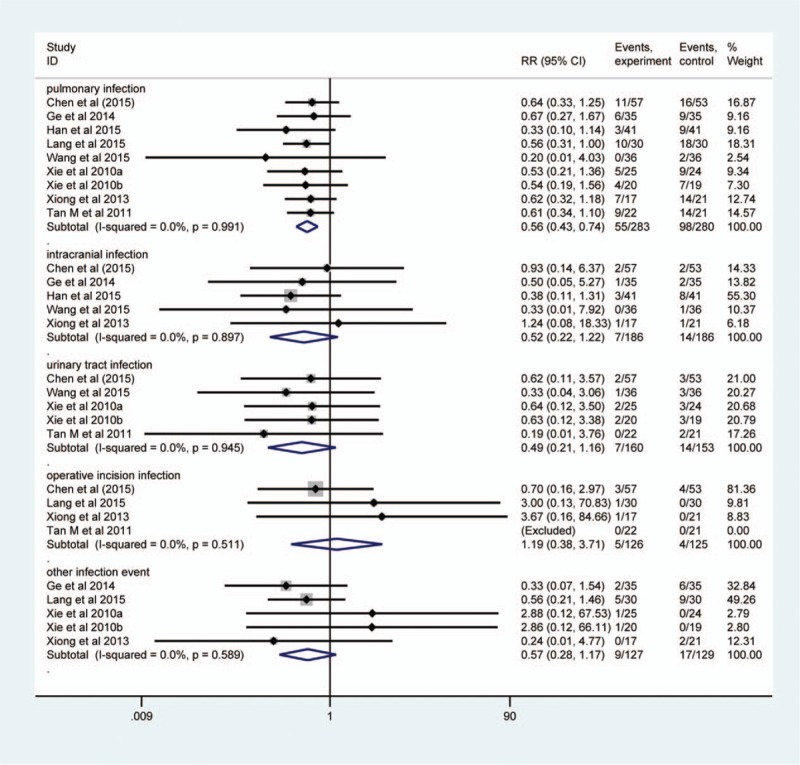
Subgroup analysis of infection rate. The horizontal line, square, and diamond represent 95% CI, point estimate, and pooled effect size, respectively. CI = confidence interval; RR = relative risk.
3.4.2. Mortality
Of all eligible studies, 8[9,25,26,28,32–34,36] calculated the estimate of EEN supplemented with probiotics on mortality in patients with SHI. A total of 545 patients were included with 273 in the observation group and 272 in the control group. No heterogeneity was found among them (P = .136, I2 = 11.07%), we consequently used a fixed-effect model to calculate the pool estimate. The result revealed that the EEN supplemented with probiotics reduced mortality related to standard EN (RR, 0.56; 95% CI, 0.38–0.82; Z, 2.95; P = .003) (Fig. 4).
Figure 4.
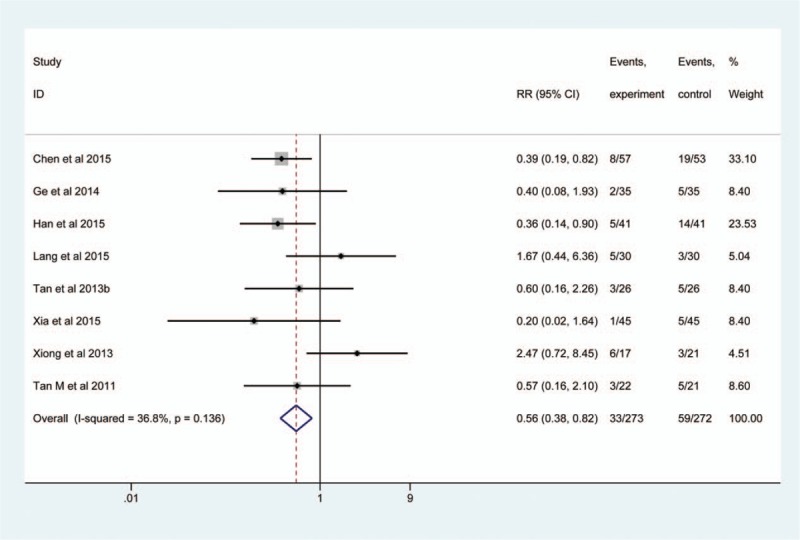
Meta-analysis of mortality. The horizontal line, square, and diamond represent 95% CI, point estimate, and pooled effect size, respectively. CI = confidence interval; RR = relative risk.
3.4.3. Gastrointestinal complications
A total of 7 studies[2,16,24,29–31,35] investigated the incidence of the gastrointestinal complications when EEN supplemented with probiotics vs standard EN, in which 322 patients were included. Significant heterogeneity was not detected (I2 = 20.6%, P = .278), and a fixed-effects model was consequently applied. The pooled result suggested that EEN supplemented with probiotics was associated with decreased incidence of gastrointestinal complications (RR, 0.19; 95% CI, 0.13–0.25; Z, 6.38; P < .001) (Fig. 5). Furthermore, this study also analyzed EEN supplemented with probiotics on the risk of incidence of individual gastrointestinal complication based on subgroup analysis, and the pooled result suggested that targeted intervention decreased bloating, diarrhea, vomit, gastric retention, constipation, and regurgitation (Fig. 5).
Figure 5.
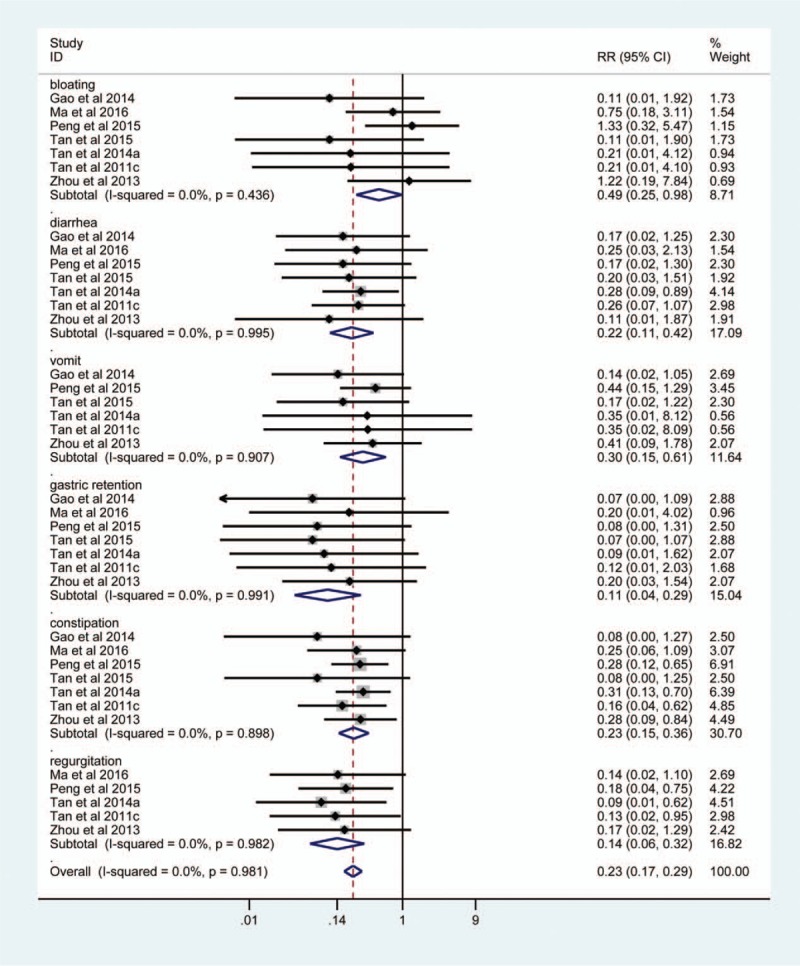
Meta-analysis of gastrointestinal complications. The horizontal line, square, and diamond represent 95% CI, point estimate, and pooled effect size, respectively. CI = confidence interval; RR = relative risk.
3.4.4. Length of ICU stay
Eight studies[9,24,25,28,30,32,33,36] investigated the length of ICU stay when EEN vs standard EN, and 418 participants were analyzed finally. Because we identified significant heterogeneity across studies (I2 = 69.4%, P = .002), and we selected consequently a random-effects model to calculate the estimate. The pooled result showed a shorten length of ICU stay in the EEN group (MD, −4.55; 95% CI, −5.91 to −3.19; Z, 6.57; P < .001) (Fig. 6).
Figure 6.
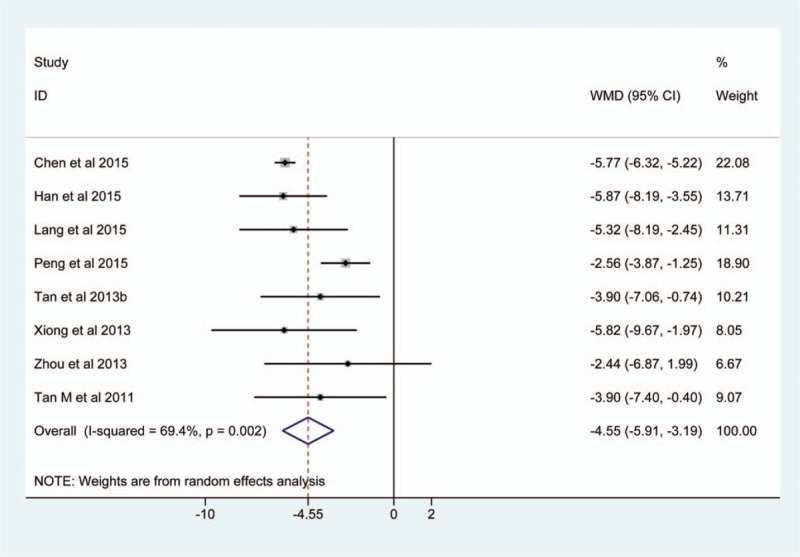
Meta-analysis of the length of ICU stay. The horizontal line, square, and diamond represent 95% CI, point estimate, and pooled effect size respectively. CI = confidence interval; ICU = intensive care unit; RR = relative risk.
3.5. Publication bias
According to our prespecified criteria, we drawn the Egger plot to detect the publication bias for infection rate.[23] The Egger test generated a t value of −2.61 with a P value of .03, which indicated that publication bias may impair the pooled result for this given outcome.
4. Discussion
Severe head injury is a common but acute and critical neurosurgical condition.[1] Systematic metabolic disorders resulted from stress and invasive operations can be rectified through early introducing the EN, and thus improving clinical outcome of these patients.[37] However, it is noted that following the SHI, intestinal microbial imbalance and bacterial translocation will be caused, which are all play critical role in destructing intestinal ecologic barrier,[38] damaging the normal gastroenteric function, and weakening immunity.[39] These changes can not only seriously impair the absorption and negatively affect the actual effect of EN, but also notably increase the risk of several infectious complications and mortality.[40] The findings from published experimental studies showed that exogenous probiotics may have the potential of balancing the intestinal flora. That is to say, intestinal microecology and the integral intestinal mucosa can be recovered and maintained, and the gastrointestinal immunity can be enhanced eventually through administration of exogenous probiotics.[41] And thus, it is necessary to investigate the efficacy and safety of EEN supplemented with exogenous probiotics in patients with SHI.
4.1. Summary of main results
In this meta-analysis, we included 18 eligible RCTs enrolling 1016 patients. After completed all analyses, we found that patients with SHI can be benefit from the administration of EEN supplemented with probiotics.
It is noted that patients with SHI usually undergoing destroyed immunity. Impaired cellular immunity which is characterized by an obvious shift from Th1 response to Th2 may occur in several hours and last for several weeks.[42–44] Issued data suggested that the incidence of infection can be up to 40% to 80% in inpatients with SHI, and of these infectious events, pulmonary infection which is a main determinant of the clinical process of patients with SHI[45] can reach up to 60%. Therefore, the intervention regimes which have the potential of effectively decreasing the infection risk can improve the clinical outcome and lower the mortality of these given patients. The present study showed that the EEN combined with probiotics obviously reduced the risk of infectious events and mortality in patients with SHI. Meanwhile, the results based on subgroup analyses further revealed that the EEN supplemented with probiotics can also lower the risk of pulmonary infection. Some studies also shown, it is noted that, a decreased risk of infection and increased survival time when EEN supplemented with probiotics compared to standard EN alone in critical ill patients underwent burn and trauma.[46,47] Based on the published evidences, it was supposed that the mechanisms of probiotics to reduce the risk of infection including the following three aspects: improving the expression of epithelial transmembrane proteins and immune globulin and reducing the gastric mucosal permeability by enhancing tight junctions, correcting the imbalance in intestinal microecology, maintaining the integrity of intestinal mucosal, and cutting down or holding the translocation of bacteria and endotoxin[48]; increasing the sIgA in intestinal mucosa, enhancing the recovery of intestine mucosal immunity and relieving early inflammation[49]; strengthening the contractile activities of the small intestine so as to accelerate nutrient absorption, further enhancing patients’ immunity.[50]
The present meta-analysis also demonstrated that the EEN supplemented with probiotics effectively reduced gastrointestinal complications, and improved the intestinal function in patients with SHI. Under physiologic status, a certain number of normal floras exist on intestinal tracts as a biologic barrier for the healthier digestion. With these floras absorbing on enterocyte, it can prevent pathogenic bacterium from appearing on the one hand, and adjust intestinal secretion function to a normal level on the other, reinforcing regional immune competence of intestinal tract. In this way, the inflammatory response at early state can be controlled.[51] Husebye and his colleagues[52] discovered a disturbance of intestinal motility if rat intestinal was in aseptic condition. Not only does the settles in Escherichia coli not to rectify the condition of gastric motility disorder, it intensifies the degree of disorder. However, planting in the beneficial intestinal microbial communities such as Lactobacillus or Bifidobacterium can correct intestinal movement pattern. At the early status, intestinal microbial imbalance was accompanied by the decreased number of the beneficial intestinal communities such as Lactobacillus or Bifidobacterium, while pathogenic bacterium (e.g., Escherichia coli) would increase sharply, which critically affecting the gastrointestinal motility in patients with SHI. It may results from large amounts of applied antibacterial agents during surgeries.[40] Besides, dysfunction of central nervous system for gastrointestinal and metabolic complications may happen, which manifested a downfall of ability on gastric emptying and intestinal tract movement, destroying the absorbing ability of intestines.[53] A series of studies manifested that exogenous probiotics might recover gastric emptying function[54] with Helicobacter pylori, rectify intestinal tonic contraction[55] and facilitate the gastric emptying and speed of intestinal transmission with SHI,[38] eventually, enhancing the absorption function of intestines. Hence, exogenous probiotics is conducive to the intestinal microbial balance in patients with SHI, improving gastrointestinal motility disorder.
The SHI may lead to an increase in hormonal readiness for noradrenaline and cortisol irritably, resulting in a high metabolic status in a human body.[56] A study[57] showed that the energy expenditure on metabolism can reach to a normal level of 160% for patients with SHI, leading to the exhaust of reserve nutrient in a body in a short time and reduction of weight. This status of high metabolism and low nutrition can harm the immune function in a body as well, resulting in weak physique and the ability to be infected, even to the disadvantage of prognosis.[58] In our study, it also showed that the enteral nutritional intervention in the early period by replenishing probiotics has obviously shortened the length of ICU stay for patients with SHI. The main reason for the intervention may lie in the condition where probiotics can contribute to a shorter of time for patients being nursed at a requisite amount as well as a decrease of fatality rate. At the same time, it can promote nutritive absorption through increasing the surface area of villi in the small intestine as well as the recovery of nutritional status in patients.
4.2. Limitations of included studies and implications for future studies
As the included 18 studies have not been blinded to participants and personnel, the results could be affected by subjective bias; in these qualified studies included, only 1 provides a high sample capacity (N = 110), while others were largely influenced by the bias producing lower creditable results. Therefore, blind method shall be set in future studies and the best sample size shall be estimated based on the previous evidences. In this way, enough credibility can be ensured from the results in current studies. In the included studies, it is different in probiotic strains and intervention periodicity, so the comparisons for different strains or the same strain under different intervention periodicity are the good research contents in the future.
As patients with SHI are widely distributed, it is necessary for further study to research the effects for EEN combined with probiotics on patients in different age period. In addition, specific probiotics mechanism of action has not yet been obtained in spite of a number of relevant studies. For this reason, to further define the mechanism and provide theoretical support for practical clinical care, extra fundamental researches shall be carried out.
4.3. Limitations of the present study
In the present study, we only searched two English language database including PubMed and CENTRAL; however, other relevant databases such as Embase and CINAL (cumulative index to nursing and allied health literature) were not considered, which may introduce the selection bias. Only articles published in Chinese and English language were considered, which may also introduce selection bias. Most of the included researches were with small samples, and thus the results were largely affected by random error, which may have an effect on the reliability of summarized results. Trial sequential analysis for all clinical outcomes and performance statistics merged by all indicators after calculating were not implemented in this study, so the robustness of all combined results cannot be determined. Besides, we performed the bias test for overall infection rate, which showed that the publication bias may affect the reliability of results. When patients are suffered from SHI, support from EEN would be a big challenge; as probiotics is capable of improving intestinal microbial balance in critically ill patients, protecting the integrity of the intestinal mucosal barrier, enhancing the intestinal mucosal immune system and strengthening intestinal motility and the effect of EEN. It gradually becomes an effective treatment option for patients with SHI. Even though limitations existed in 10 present study as well as the included qualified ones, each 1 was highly homogeneous; meanwhile, for the identical clinical results, the objects of effect size were highly consistent; thus, it was deemed that the results from our study was reliable to some extent.
5. Conclusion
In general, EEN supplemented with probiotics are effective for reducing risk of infection and mortality, improving their gastrointestinal dysfunction and shortening the length of ICU stay in patients with SHI. Further studies with large scale and well designed were warranted to establish this conclusion because some limitations impaired the power of these results of our study.
Acknowledgment
The authors express their warm appreciation to all authors who listed in eligible studies which were included in the present study.
Author contributions
Li-Juan Yi, Xu Tian, Bing Shi, Yuan-Ping Pi and Wei-Qing Chen conceived and designed this study; Li-Juan Yi and Xu Tian searched and selected studies; Li-Juan Yi and Xu Tian extracted essential information; Xu Tian and Wei-Qing Chen assessed the risk of bias; Li-Juan Yi, Xu Tian and Yuan-Ping Pi performed statistical analyses; and Li-Juan Yi, Xu Tian and Bing Shi interpreted the pooled results; Li-Juan, Xu Tian and Wei-Qing Chen drafted the manuscript.
Conceptualization: Li-Juan Yi, Xu Tian, Bing Shi, Yuan-Ping Pi, Wei-Qing Chen.
Data curation: Xu Tian.
Formal analysis: Li-Juan Yi, Xu Tian.
Investigation: Xu Tian.
Methodology: Xu Tian, Bing Shi.
Resources: Yuan-Ping Pi.
Software: Li-Juan Yi, Xu Tian, Bing Shi.
Supervision: Xu Tian, Yuan-Ping Pi, Wei-Qing Chen.
Validation: Xu Tian, Wei-Qing Chen.
Writing – original draft: Li-Juan Yi, Xu Tian.
Writing – review & editing: Xu Tian, Wei-Qing Chen.
Supplementary Material
Footnotes
Abbreviations: CBM = Chinese Biomedical Literature Database, CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, CINAHL = cumulative index to nursing and allied health literature, CNKI = China National Knowledge Infrastructure, CPGs = Clinical Practice Guidelines, EEN = early enteral nutrition, GCS = Glasgow coma scale, ICU = intensive care unit, IV = inverse variance, MD = mean difference, MeSH = medical subject heading, MH = Mantel–Haenszel, PRIMSA = preferred reporting items for systematic reviews and meta-analysis, RR = relative risk, SHI = severe head injury.
LJY, XT, BS, and YPP have contributed equally to this work as joint first authors.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Lobato RD, Alen JF, Perez-Nunez A, et al. Value of serial CT scanning and intracranial pressure monitoring for detecting new intracranial mass effect in severe head injury patients showing lesions type I-II in the initial CT scan. Neurocirugia (Asturias, Spain) 2005;16:217–34. [PubMed] [Google Scholar]
- [2].Tan M, Zhu JC, Wang HJ. Effects of early enteral nutrition combined with probiotics on nutritional status in severe traumatic brain-injured patients. Chin J Traumatol 2011;27:316–9. [Google Scholar]
- [3].Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24Suppl 1:S1–06. [DOI] [PubMed] [Google Scholar]
- [4].Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med 2009;37:1757–61. [DOI] [PubMed] [Google Scholar]
- [5].Tan M, Zhu JC, Yin HH. Enteral nutrition in patients with severe traumatic brain injury: reasons for intolerance and medical management. Br J Neurosurg 2011;25:2–8. [DOI] [PubMed] [Google Scholar]
- [6].Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol 2009;587(Pt 17):4169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verdu EF, Bercik P, Collins SM. Effect of probiotics on gastrointestinal function: evidence from animal models. Therap Adv Gastroenterol 2009;2:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J 2004;80:516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Critical Care (London, England) 2011;15:R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xie CX, Zhu JC, Huang GF, et al. Influence of early enteral nutrition combined with synbiotics agents on intestinal flora and SIgA of patients with severe craniocerebral injury. J Nursing Res 2010;2557–60. [Google Scholar]
- [11].Xie CX, Zhu JC, Yin HH, et al. Early enteral nutrition combined with synbiotics agents in enhancing cellular immune function in patients with severe head injury. Nurs J Chin People's Liberation Army 2010;404–7. [Google Scholar]
- [12].Gu WJ, Deng T, Gong YZ, et al. The effects of probiotics in early enteral nutrition on the outcomes of trauma: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr 2013;37:310–7. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol 2009;210:S38. [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London, England) 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [15].Yi LJ, Tian X, Chen WQ, et al. Early enteral nutrition supplemented with probiotics improved the clinical outcomes in patients with severe head injury: protocol for a meta-analysis of randomized controlled trials. TMR Integrat Nurs 2017;1:6–11. [Google Scholar]
- [16].Tan M, Duan JW, Peng H. Effects of probiotics on nasogastric feeding intolerance in patients with severe traumatic brain injury. J North Sichuan Medical College 2014;567–70. [Google Scholar]
- [17].Tian X, Chen WQ, Huang JL, et al. Effects of polyethylene glycol 2 L alone or with ascorbic acid compared with polyethylene glycol 4 L alone for bowel preparation before colonoscopy: protocol for a systematic review and network meta-analysis. BMJ Open 2017;7:e018217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Davidson MB, Castellanos M, Kain D, et al. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med 2005;118:422–5. [DOI] [PubMed] [Google Scholar]
- [20].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- [23].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou YY, Xiong XW, Dong L, et al. Effect of early enteral nutrition with probiotics on gastrointestinal motility disturbance and nutritional status of patients with severe craniocerebral trauma. Chin J Traumatol 2013;29:320–4. [Google Scholar]
- [25].Xiong XW, Zhou YY, Dong L, et al. Probiotics as supplement for early enteral nutrition decreases infection in severe brain injury. J Third Military Med Univ 2013;35:536–9. [Google Scholar]
- [26].Xia W. Effect of Symbiotic combined with early enteral nutrition in treatment of severe head injury elderly patients with infections. Zhe Jiang Clin Med J 2015;17:2160–1. [Google Scholar]
- [27].Wang W. Live combined bifidobacterium combined with early enteral nutrition in treatment of severe head injury patients. Chin J Rural Med Pharmacy 2015;8–9. [Google Scholar]
- [28].Tan M, Lu XF, Duan JW, et al. Effects of probiotics on blood glucose levels and clinical outcomes in patients with severe craniocerebral trauma. Chin Crit Care Med 2013;25:627–30. [DOI] [PubMed] [Google Scholar]
- [29].Tan JK. Effect of probiotics in treatment of severe head injury patients with early nutritional support. Contemporary Med Forum 2015;135–6. [Google Scholar]
- [30].Peng HJ, Tang DD. Effects of difference early nutrition programs on the gastrointestinal function and nutritional status of patients with severe traumatic brain injury. Chin J Practic Nerv Dis 2015;3–5. [Google Scholar]
- [31].Ma XR. Effect of different enteral nutrition on nutritional status of severe craniocerebral injury patients. World Latest Med Inform 2016;155–6. [Google Scholar]
- [32].Lang SW, Xu JM. Effect of bifidobacterium on inflammatory response in traumatic brain injury patients. Chin J Primary Med Pharm 2015;22:337–40. [Google Scholar]
- [33].Han DJ, Chen WF. Effect of symbiotic combined with early enteral nutrition in treatment of severe head injury elderly patients with infections. Chin J Gerontol 2015;1819–21. [Google Scholar]
- [34].Ge HJ, Wang Q, Guo Y, et al. Effect of probiotics combined with early enteral nutrition in treatment of severe traumatic brain injury patients with infections. Chin J Neurosurg 2014;4324–6. [Google Scholar]
- [35].Gao WF. Effect of probiotics in treatment of severe brain injury patients. Contemporary Med 2014;59–60. [Google Scholar]
- [36].Chen LPQ, Cheng LWJ. Influence of early enteral nutrition combined with probiotics on postoperative infections in patients with severe traumatic brain injury. Chin J Neurosurg 2015;1357–9. [Google Scholar]
- [37].Sun P, Li SC, Chen LZ. Effect of different nutrition modes on intracranial infections in severe brain injury patients. Chin J Nosocomiol 2014;24:173–5. [Google Scholar]
- [38].Yu XY, Yin HH, Zhu JC. Increased gut absorptive capacity in rats with severe head injury after feeding with probiotics. Nutrition 2011;27:100–7. [DOI] [PubMed] [Google Scholar]
- [39].Singh G, Harkema JM, Mayberry AJ, et al. Severe depression of gut absorptive capacity in patients following trauma or sepsis. J Trauma 1994;36:803–8. [DOI] [PubMed] [Google Scholar]
- [40].Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol 2012;67:287–94. [DOI] [PubMed] [Google Scholar]
- [41].Verma E, Lucak S. Use of probiotics in gastrointestinal disorders: what to recommend? Therap Adv Gastroenterol 2010;3:307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dziedzic T, Slowik A, Szczudlik A. Nosocomial infections and immunity: lesson from brain-injured patients. Critical Care 2004;8:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg 1995;130:1159–62. [DOI] [PubMed] [Google Scholar]
- [44].Woiciechowsky C, Asadullah K, Nestler D, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med 1998;4:808–13. [DOI] [PubMed] [Google Scholar]
- [45].LaPar DJ, Rosenberger LH, Walters DM, et al. Severe traumatic head injury affects systemic cytokine expression. J Am Coll Surg 2012;214:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Spindler-Vesel A, Bengmark S, Vovk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr 2007;31:119–26. [DOI] [PubMed] [Google Scholar]
- [47].Han CM, Yu JX, Fu SZ. Effect of early enteral nutrition with synbiotics on plasma endotoxin levels in serious burned patients. Acta Nutri Sinica 2005;66–9. [Google Scholar]
- [48].Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology 2008;123:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mengheri E. Health, probiotics, and inflammation. J Clin Gastroenterol 2008;42 Suppl 3 Pt 2:S177–8. [DOI] [PubMed] [Google Scholar]
- [50].Xie CX, Zhu JC, Wen XX, et al. Influence of early enteral nutrition supplemented with probiotics on TNF-( and IL- 6 of patients with severe traumatic brain injury. Chin Nurs Res 2012;26:2702–5. [Google Scholar]
- [51].Hillier R, Everett B. Evaluating culture practices used to identify infection in patients with brain injury. Crit Care Nurs Q 2012;35:228–33. [DOI] [PubMed] [Google Scholar]
- [52].Husebye E, Hellstrom PM, Sundler F, et al. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol 2001;280:G368–80. [DOI] [PubMed] [Google Scholar]
- [53].Chapman MJ, Nguyen NQ, Deane AM. Gastrointestinal dysmotility: clinical consequences and management of the critically ill patient. Gastroenterol Clin N Am 2011;40:725–39. [DOI] [PubMed] [Google Scholar]
- [54].Verdu EF, Bercik P, Huang XX, et al. The role of luminal factors in the recovery of gastric function and behavioral changes after chronic Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol 2008;295:G664–70. [DOI] [PubMed] [Google Scholar]
- [55].Verdu EF, Bercik P, Bergonzelli GE, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 2004;127:826–37. [DOI] [PubMed] [Google Scholar]
- [56].Bochicchio GV, Bochicchio K, Nehman S, et al. Tolerance and efficacy of enteral nutrition in traumatic brain-injured patients induced into barbiturate coma. J Parent Enteral Nutrit 2006;30:503–6. [DOI] [PubMed] [Google Scholar]
- [57].Bratton S, Chestnut R, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. XII nutrition. J Neurotr 2006;24:S77–82. [DOI] [PubMed] [Google Scholar]
- [58].Foley N, Marshall S, Pikul J, et al. Hypermetabolism following moderate to severe traumatic acute brain injury: a systematic review. J Neurotr 2008;25:1415–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


