Supplemental Digital Content is available in the text
Keywords: clinicopathologia, colorectal cancer, meta-analysis, micin-1, prognosis
Abstract
Background:
Accumulating evidence supports the overexpression of mucin 1 (MUC1) in colorectal cancer (CRC), but the value of elevated MUC1 expression remains controversial. Here, we evaluated the prognostic and clinicopathological value of MUC1 expression in CRC.
Materials and methods:
The Web of Science, PubMed, Embase, Cochrane Library, and Wanfang databases, as well as the China Biology Medicine disc (CBMdisc) and China National Knowledge Infrastructure (CNKI) were searched for studies on MUC1 expression and prognosis of CRC through July 20, 2018. The pooled relative risks (RRs) and hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated to evaluate the prognostic and clinicopathological value of MUC1 expression in CRC. The Revman version 5.3 package and STATA, version 12 were employed for pooled analysis and analysis of publication bias.
Results:
This meta-analysis included 16 published studies. The combined analysis showed that CRC patients with high MUC1 expression had a worse clinical outcome in overall survival (OS) (HR = 1.51, 95% CI = 1.30–1.75, P <.00001). In addition, high MUC1 expression was associated with higher TNM stage (RR = 1.44, 95% CI = 1.17–1.77, P = .0007), greater depth of invasion (RR = 1.30, 95% CI = 1.10–1.53, P = .002), and lymph node metastasis (RR = 1.47, 95% CI = 1.20–1.80, P = .0002) of CRC. However, the elevated MUC1 expression was not related to disease-free survival/recurrence-free survival (DFS/RFS) (HR = 1.51, 95% CI = 0.78–2.89, P = .22), histological grade (RR = 1.15, 95% CI = 0.96–1.38, P = .12), gender (RR = 0.95; 95% CI = 0.83–1.08, P = .44), tumor size (RR = 1.11, 95% CI = 0.85–1.44, P = .44), tumor site (RR = 1.01, 95% CI = 0.88–1.16, P = .84), or mucinous component (RR = 0.83, 95% CI = 0.60–1.14, P = .24) in CRC.
Conclusion:
Our findings indicated that high MUC1 expression represents a marker of poor prognosis in CRC. Meanwhile, elevated MUC1 expression was associated with advanced TNM stage, greater depth of invasion, and lymph node metastasis.
1. Introduction
Colorectal cancer (CRC) is a frequently diagnosed cancer worldwide.[1] In addition, CRC is the most common cause of cancer-related death.[2,3] Studies have shown that only 40% of CRC cases are diagnosed at an early stage.[4] Although surgery is the primary means of treating cancer, 30% to 40% of patients have metastatic disease that cannot be treated by surgery.[5] In addition, patients with CRC have a high risk of recurrence.[3,6]
The classic tumor, node, and metastasis (TNM) staging system were considered to be the most standard prognostic parameter, providing a basis for CRC treatment.[7] However, the TNM system does not reflect the inherent biological heterogeneity of CRC, and about 50% of recently diagnosed cases will progress to metastatic cancer.[8,4] There are no suitable markers for predicting the progression, metastasis, and response to treatment of CRC.[9] Thus, it is necessary to find better biomarkers for predicting the outcome of CRC.
The mucin family members are high-molecular-weight glycosylated proteins[10] that form a barrier to protect the epithelial cells.[11–13] To date, about 20 human mucins have been identified and categorized into secreted gel-forming mucins and transmembrane mucins according to their structure and function.[14] Among them, MUC1 has a heavily glycosylated extracellular domain. It is normally expressed in secretory epithelial cells and hematopoietic cells,[15] but abnormally overexpressed in lung cancer, pancreatic tumors, prostate cancer, epithelial ovarian tumors, breast cancer, and colon cancers.[13,16–20] MUC1, as a master regulator of the metabolic program, facilitates metabolic alterations to help tumor cells survive and proliferate.[21] MUC1 activates antiapoptotic proteins and induces drug resistance via upregulation of multidrug resistance genes in the treatment and development of CRC.[22,23] These observations identify MUC1 as an attractive marker for the diagnosis, immunotherapy, and prognosis of cancer.[24,25]
Currently, the association between MUC1 expression and malignancy has been indicated in many reports.[26–56] In CRC, MUC1 as a novel biomarker is highly expressed. However, Research findings regarding MUC1 expression and the prognosis of CRC are still conflicting, with some studies indicating that high MUC1 expression is a potential predictor of poor outcome in CRC patients[40,56–58] and other studies reporting contradictory evidence.[37,51,59] Thus, the prognostic value of MUC1 expression remains inconclusive. This quantitative meta-analysis aimed to determine the correlation of MUC1 with prognosis and clinicopathological features in CRC.
2. Methods
This meta-analysis was based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (File S1). Our meta-analysis was based on previously published studies and does not contain any studies with human or animal subjects, thus no ethical approval and patient consent were required.
2.1. Search strategy
The PubMed, Web of Science, Embase, Cochrane Library, Wanfang databases as well as the China Biology Medicine disc (CBMdisc) and China National Knowledge Infrastructure (CNKI) were searched for studies on MUC1 expression and its prognostic value in CRC through July 20, 2018. All studies were analyzed to explore the association between MUC1 level and CRC prognosis. The primary terms used for literature retrieval included (CRC or colorectal neoplasms or colorectal cancer or colorectal carcinoma or colon cancer or rectal cancer) and (mucin 1 [MUC1]) and (survival or outcome or prognosis or prognostic factor) (File S2). To obtain additional eligible studies, conference summaries and references cited in these papers were surveyed. All literature searches were conducted by 2 independent reviewers.
2.2. Inclusion and exclusion criteria
Inclusion criteria:
-
(1)
the patients were diagnosed with CRC according to pathological findings;
-
(2)
clinicopathological parameters, MUC1 expression, and survival rate were investigated;
-
(3)
values of relative risk (RR) and/or hazard ratio (HR) with 95% confidence interval (CI) were calculated; and
-
(4)
publication in English or Chinese.
Exclusion criteria:
-
(1)
repeated publication of data or poor quality data, lacking raw data, or presenting incomplete information; and
-
(2)
review articles, case reports, conference abstracts, commentary, or letters to editors. The most recent paper was selected when several studies were published on the same trial.
2.3. Data extraction and quality assessment
All search results were screened and extracted by 2 authors (Chao Li and Tao Liu), and cases of inconsistency and disagreement were submitted to a third investigator (Libin Yin) for further review. The following information, including author, country, time of publication, number of patients, methods, antibodies, cut-off for MUC1 expression, mean or median age, follow-up time, and pathological outcome were systematically obtained from the charts and article contents. For studies providing HRs, we extracted data directly. We obtained the necessary data by using Engauge Digitizer, version 4.1 when patients’ survival data were provided in the form of a Kaplan–Meier curve. Furthermore, the quality of the studies was assessed by 2 independent authors according to the Newcastle Ottawa Scale (NOS).[60] NOS score ranges from 0 to 9 and studies with a NOS score of 7 or more were considered to be high-quality studies.
2.4. Statistical analysis
HRs (95% CIs) were used to evaluate the relationship between MUC1 expression and survival (OS and disease-free survival [DFS]/recurrence-free survival [RFS]). An observed HR >1 suggested a worse prognosis in patients with high MUC1 expression and an HR ≤1 indicated a better prognosis. The association between MUC1 expression and the clinicopathological status of CRC, including gender, tumor size, tumor site, mucinous expression, histological grade, TNM stage, depth of invasion, and lymph node metastasis were assessed using RRs and 95% CIs. We assessed the heterogeneity among studies by calculating relevant P values and I2 values. If the I2 was >50%, indicating the presence of heterogeneity in studies, a random effects model was applied in the pooled analysis. Otherwise, a fixed effects model was selected.[61,62] The potential for publication bias was assessed using Begg funnel plots and the Egger linear regression test, in which a P value <.05 was considered to indicate significant potential publication bias. Sensitivity analysis was performed to investigate the robustness of the results. The meta-regression analysis was searched for the sources of heterogeneity.
Meta-analyses were performed using Review Manager 5.3 software (Cochrane Collaboration, Copenhagen, Denmark) and STATA, version 12.0 (Stata Corporation, College Station, TX). The significance of pooled data was further tested, and P <.05 was considered statistically significant.
3. Results
3.1. Study selection
We retrieved 462 articles through the initial database searches. Among them, 82 duplicates were retrieved. After rough screening of the titles and abstracts of all studies, 380 articles were further excluded according to the predefined criteria. Thirty articles were excluded after full review of the remaining 46 articles, due to the following reasons: review article (6) and no study endpoint (24). Finally, 16 eligible studies involving 2614 patients were included.[29,40,48,50,55–58,63–70]Figure 1 details the selection process.
Figure 1.
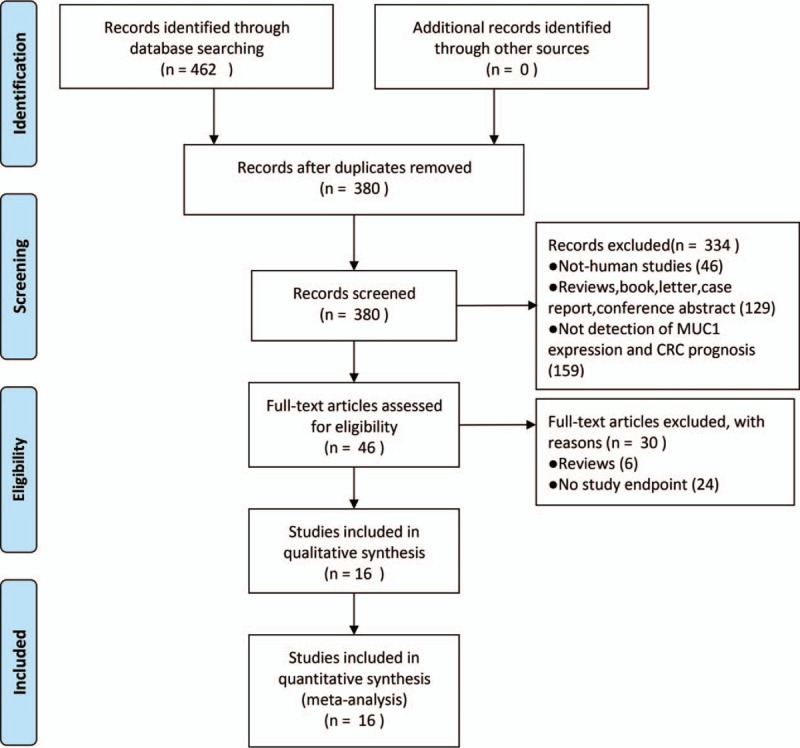
Flow diagram outlining the identification of retrieved publications.
3.2. Patients’ characteristics
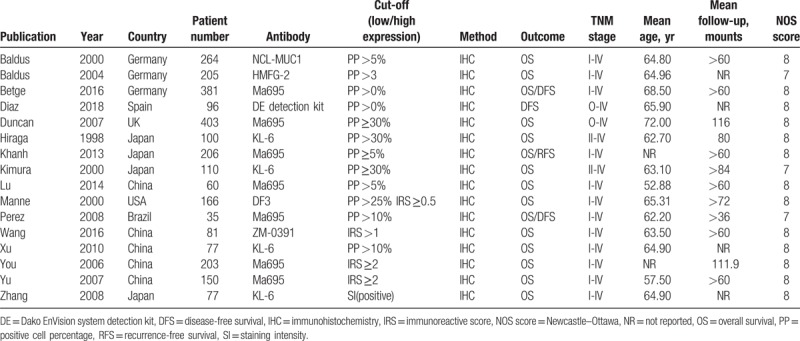
A total of 6 countries’ studies were included in this meta-analysis, with sample sizes ranging from 35 to 403. The mean age of patients in the 16 articles ranged from 52.88 to 72.00 years old, and the follow-up ranged from 36 to 116 months (shown in Table 1). The MUC1 expression was detected by immunohistochemical (IHC) staining using different anti-MUC1 monoclonal antibodies, including clone MA695, clone KL-6, clone HMFG-2, clone DF3, clone NCL-MUC1, Dako EnVision system detection kit, and clone ZM-0391, which impacted the rate of positive MUC1 expression. The cut-off values for IHC evaluation applied in the studies were not consistent, ranging from 0% to 35% according to the positive cell percentage. All the clinicopathological parameters showed a significant association with the expression of MUC1 in CRC. According to the qualities assessed with the NOS score (Table S1), all studies were of high quality.
Table 1.
Main characteristics of the included publications.
3.3. Outcomes
3.3.1. MUC1 and survival in CRC
Fifteen studies provided data on overall survival (OS). Figure 2 shows the forest plot from the analysis of OS. The results showed that, compared with the group with lower MUC1 expression, the OS was worse in higher MUC1 expression group (HR = 1.51, 95% CI = 1.30–1.75, P <.00001). A fixed effects model was used in view of small heterogeneity (P = .69, I2 = 0%). In addition, the DFS/RFS among CRC patients was reported in 4 studies. The combination of HRs suggested that positive MUC1 expression was not associated with DFS/RFS in CRC (HR = 1.51, 95% CI = 0.78–2.89, P = .22, Fig. S1). A random-effects model was applied due to significant heterogeneity (P = .01, I2 = 72%) between the studies.
Figure 2.
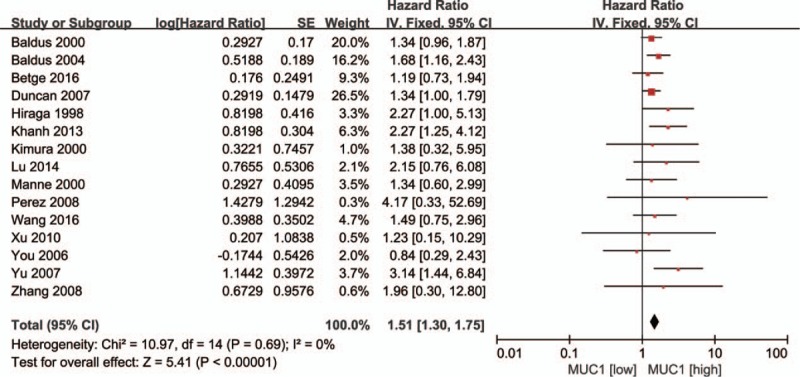
Forest plots of HRs for MUC1 expression and colorectal cancer OS. HRs = hazard ratios, MUC1 = mucin 1, OS = overall survival.
3.3.2. MUC1 and CRC TNM stage
Twelve articles were analyzed for the association between MUC1 expression and CRC TNM state. Pooled analysis showed significant heterogeneity among these studies (P <.00001, I2 = 81%), and thus a random effects model was used. Further analysis showed MUC1 overexpression was associated with TNM stage (III/IV vs I/II: RR = 1.44, 95% CI = 1.17–1.77, P = .0007; Fig. 3). This suggests that MUC1 expression is closely related to the clinicopathological parameters of CRC.
Figure 3.
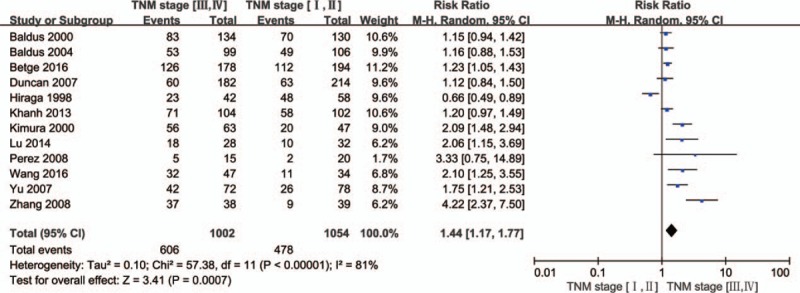
Forest plots of RRs for MUC1 expression and TNM stage in CRC. CRC = colorectal cancer, MUC1 = mucin 1, RRs = relative risks, TNM = tumor, node, and metastasis.
3.3.3. MUC1 and CRC invasion
Eleven studies on the depth of invasion showed that the pooled RR (positive vs negative) was 1.30 (95% CI = 1.10–1.53, P = .002). A random effects model was applied (P = .02, I2 = 53%, Fig. 4).
Figure 4.

Forest plots of RRs for MUC1 expression and depth of invasion in CRC. CRC = colorectal cancer, MUC1 = mucin 1, RRs = relative risks.
3.3.4. MUC1 and CRC lymph node metastasis
Eleven articles reported lymph node metastasis, and a significant relationship was revealed between MUC1 expression and lymph node metastasis in CRC (HR = 1.47, 95% CI = 1.20–1.80, P = .0002, Fig. 5). The random effects model was applied because the heterogeneity was obvious among these studies (P <.00001, I2 = 77%).
Figure 5.
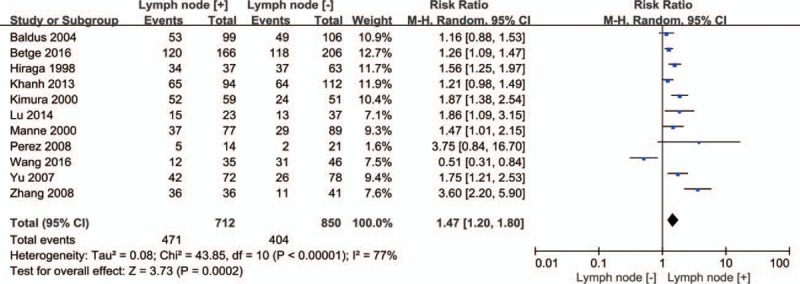
Forest plots of RRs for MUC1 expression and lymph node metastasis in CRC. CRC = colorectal cancer, MUC1 = mucin 1, RRs = relative risks.
3.3.5. MUC1 and CRC clinicopathological features
Elevated MUC1 was not significantly associated with histological grade (3 vs 1/2: RR = 1.15, 95% CI = 0.96–1.38, P = .12, Fig. S2), gender (female vs male: RR = 0.95, 95% CI = 0.83–1.08, P = .44, Fig. S3), tumor size (small vs large: RR = 1.11, 95% CI = 0.85–1.44, P = .44, Fig. S4), tumor site (rectum vs colon: RR = 1.01, 95% CI = 0.88–1.16, P = .84, Fig. S5), and mucinous component (≥50% vs <50%: RR = 0.83, 95% CI = 0.60–1.14, P = .24, Fig. S6).
3.4. Publication bias
Begg funnel plots and Egger linear regression test were used to evaluate the publication bias in our meta-analysis. No evidence of publication bias was found for OS (P = .553, 0.219; Fig. S7A), DFS/RFS (P = .308, 0.336; Fig. S7B). We also performed Begg test and Egger test for clinicopathological features (Fig. S8, S9) and found no evidence of publication bias.
3.5. Sensitivity analysis
Sensitivity analysis was applied to test the robustness of the results by omitting each single study. The results suggested no significant changes in the pooled HRs for OS (Fig. S10A). However, for DFS/RFS (Fig. S10B), the results are not reliable, probably because an article[70] uses antibodies that are different from other articles.[50,56,57] The data were robust and reasonable in the sensitivity analysis for clinicopathological status (Fig. S11, S12).
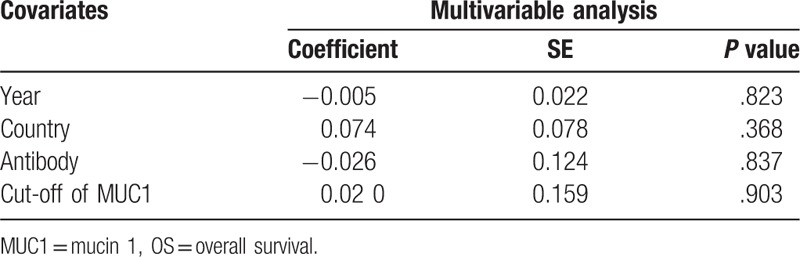
3.6. Meta-regression analysis
Table 2 shows the results of a meta-regression analysis. None of the covariates listed including year (P = .823), country (P = .368), antibody (P = .837), and cut-off (P = .903) contributed to the heterogeneity in our study. Due to insufficient observations, meta-regression analysis for DFS/RFS and tumor size were not performed. In addition, none of the above covariates led to inconsistencies in the clinicopathological characteristics (Table, S2).
Table 2.
Meta-regression analysis for OS.
4. Discussion
Evidence suggests that MUC1 is highly expressed in CRC tissues and MUC1 plays roles in the development and progression of CRC. However, its prognostic value in CRC is still inconclusive. Previous research has reported the prognostic significance of MUC1 in various human epithelial cancers, gastric cancer, and cholangiocarcinoma, but not in CRC.[71–73] Zeng et al[74] demonstrated a correlation between MUC1 expression and CRC metastasis; however, they did not study the prognostic value of MUC1 expression in CRC. This is the first comprehensive meta-analysis assessing and systematically reviewing the correlation of MUC1 expression with CRC prognosis and clinicopathological characteristics based on published studies.
The present meta-analysis showed that MUC1 expression in CRC tissue was strongly related to worse OS, and HR for OS was 1.51 (95% CI = 1.30–1.75, P <.00001). Furthermore, an elevated MUC1 expression level did not contribute to reduced DFS/RFS (HR = 1.51, 95% CI = 0.78–2.89, P = .22) compared with that in the lower MUC1 expression group. The possible mechanisms may be that MUC1, a ligand of cell adhesion molecules, induces circulating tumor cells (CTCs) to conglutinate at endothelial cells, and CTCs are then transported to distant sites to establish secondary tumors.[75] Furthermore, MUC1 can induce the expression of multiple growth factors that play roles in the survival and proliferation of tumor cells and induce the production of the proangiogenic factors that promote the formation of new blood vessels in tumor tissues.[76–81] In addition, MUC1 also promotes epithelial-mesenchymal transition (EMT) and cell invasion.[82] Thus, the above results and mechanisms indicate that MUC1 is a promising protein target in future clinical trials and can serve as a novel, valuable biomarker for predicting survival in CRC. To date, several anti-MUC1 monoclonal antibodies have entered into preclinical studies, including PankoMab-GEX, AS1402 antibody, BrevaRex, mAb-AR20.5, and muHMFG1 labeled with Yttrium-90.[83–87] However, a MUC1 antibody has not been marketed yet. However, the MUC1 expression level did not contribute to improved OS (HR = 1.51, 95% CI = 0.78–2.89, P = .22). The reason may be that the number of studies included is small. If we exclude studies[70] that affect stability, the results indicated that elevated MUC1 was strongly related to worse DFS/RFS.
As clinicopathological features are closely correlated with the prognosis of CRC patients, our study investigated the relationship between MUC1 expression and its features in CRC. Previous evidence supports that MUC1 expression is significantly associated with advanced TNM stage (III/IV) in CRC.[40,56–58] Likewise, some studies have reported that MUC1 expression is significantly and strongly correlated with advanced tumor grade, depth of invasion, and lymph node involvement.[29,58,65,67,88] In our meta-analysis, elevated MUC1 was associated with advanced TNM stage (RR = 1.44), depth of invasion (RR = 1.30), and lymph node involvement (RR = 1.42), but no significant association was found with other clinicopathological characteristics, including histological grade, gender, tumor size, tumor site, and mucinous component. According to TNM stage, advanced TNM stage, lymph node metastasis, and lymphatic invasion are predictors of poor prognosis in CRC. These studies also demonstrated that high expression of MUC1 indicates a poor prognosis in CRC.
In clinical work, high expression of MUC1 in colorectal tumor tissue may contribute to the diagnosis of CRC and help in the assessments of predicted survival and risk of recurrence. For patients with tumors overexpressing MUC1 in an early stage, it is worthwhile to perform a more detailed examination to find the existing small transition, especially for those without signs of metastasis or symptoms. Because CRC patients with high MUC1 expression have a higher risk of metastasis, the expression level of MUC1 is important for guiding the development of treatment plans. In addition, detection of MUC1 expression in CRC by immunohistochemical methods may be important for being able to determine a treatment strategy in clinical situations.
We acknowledge that there are some limitations in this study. First, the type of CRC, antibody, cut-off expression, detection method, and treatment regimen varied among included studies, which increased the heterogeneity and affected the stability of the results. Second, 16 studies were included for meta-analysis, and the case numbers in each were relatively small, which may cause bias due to variation. Third, some of the original articles did not provide HRs, which were then extracted from the Kaplan–Meier curves, potentially affecting the robustness of the results. Finally, because the current prognosis of patients with CRC is improved through regular treatment and follow-up, the role of MUC1 as a prognostic marker is weakened. Thus, more updated studies are required to confirm our findings.
Taken together, our findings indicate that high expression of MUC1 predicts a poor prognosis and survival outcome in CRC. Meanwhile, high expression of MUC1 correlates with TNM stage, depth of invasion, and lymph node metastasis, but not with histological grade, gender, tumor size, tumor site, and mucinous component. These findings indicate MUC1 expression is a promising prognostic factor for CRC and may serve as a novel, valuable biomarker of CRC.
Author contributions
Conceptualization: Lei Wang.
Data curation: Chao Li, Tao Liu.
Formal analysis: Tao Liu, Libin Yin, Didi Zuo.
Funding acquisition: Lei Wang.
Investigation: Chao Li, Tao Liu, Libin Yin.
Methodology: Chao Li.
Project administration: Lei Wang.
Resources: Chao Li.
Software: Didi Zuo, Yuyang Lin.
Supervision: Lei Wang.
Validation: Chao Li, Libin Yin.
Visualization: Lei Wang.
Writing - original draft: Chao Li.
Writing - review & editing: Lei Wang.
Chao li orcid: 0000-0003-2857-5714.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CRC =colorectal cancer, DFS = disease-free survival, HR = hazard ratio, MUC1 = mucin 1, RFS = recurrence-free survival, RR = relative risk.
All authors state no potential conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Jorgensen ML, Young JM, Solomon MJ. Optimal delivery of colorectal cancer follow-up care: improving patient outcomes. Patient Relat Outcome Meas 2015;6:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Henley SJ, Singh SD, King J, et al. Invasive cancer incidence and survival—United States, 2012. MMWR Morb Mortal Wkly Rep 2015;64:1353–8. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [4].Gonzalez-Pons M, Cruz-Correa M. Colorectal cancer biomarkers: where are we now. Biomed Res Int 2015;2015:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deschoolmeester V, Smits E, Peeters M, et al. Status of active specific immunotherapy for stage II, stage III, and resected stage IV colon cancer. Curr Colorectal Cancer Rep 2013;9:380–90. [Google Scholar]
- [6].Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263–70. [DOI] [PubMed] [Google Scholar]
- [7].Compton CC. Optimal pathologic staging: defining stage II disease. Clin Cancer Res 2007;13:6862s–70s. [DOI] [PubMed] [Google Scholar]
- [8].Ueno H, Mochizuki H, Akagi Y, et al. Optimal colorectal cancer staging criteria in TNM classification. J Clin Oncol 2012;30:1519–26. [DOI] [PubMed] [Google Scholar]
- [9].Paul D, Kumar A, Gajbhiye A, et al. Mass spectrometry-based proteomics in molecular diagnostics: discovery of cancer biomarkers using tissue culture. Biomed Res Int 2013;2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta 2006;1765:189–222. [DOI] [PubMed] [Google Scholar]
- [11].Vandenhaute B, Buisine MP, Debailleul V, et al. Mucin gene expression in biliary epithelial cells. J Hepatol 1997;27:1057–66. [DOI] [PubMed] [Google Scholar]
- [12].Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 2004;4:45–60. [DOI] [PubMed] [Google Scholar]
- [13].Lakshmanan I, Ponnusamy MP, Macha MA, et al. Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J Thorac Oncol 2015;10:19–27. [DOI] [PubMed] [Google Scholar]
- [14].Itoh Y, Kamata-Sakurai M, Denda-Nagai K, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology 2008;18:74–83. [DOI] [PubMed] [Google Scholar]
- [15].Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 2008;70:431–57. [DOI] [PubMed] [Google Scholar]
- [16].Horm TM, Schroeder JA. MUC1 and metastatic cancer: expression, function and therapeutic targeting. Cell Adh Migr 2013;7:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roy LD, Sahraei M, Subramani DB, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011;30:1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cozzi PJ, Wang J, Delprado W, et al. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis 2005;22:565–73. [DOI] [PubMed] [Google Scholar]
- [19].Rahn JJ, Dabbagh L, Pasdar M, et al. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer 2001;91:1973–82. [DOI] [PubMed] [Google Scholar]
- [20].Deng J, Wang L, Chen H, et al. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev 2013;32:535–51. [DOI] [PubMed] [Google Scholar]
- [21].Chaika NV, Gebregiworgis T, Lewallen ME, et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA 2012;109:13787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nath S, Daneshvar K, Roy LD, et al. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem 2004;279:20607–12. [DOI] [PubMed] [Google Scholar]
- [24].Karsten U, von Mensdorff-Pouilly S, Goletz S. What makes MUC1 a tumor antigen. Tumour Biol 2005;26:217–20. [DOI] [PubMed] [Google Scholar]
- [25].Hu Y, Duan J, Zhan Q, et al. Novel MUC1 aptamer selectively delivers cytotoxic agent to cancer cells in vitro. PLoS One 2012;7:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu XW, Rong W, Xu FL, et al. Expression and clinical significance of Mucin and E-cadherin in colorectal tumors. Ai Zheng 2007;26:1204–10. [PubMed] [Google Scholar]
- [27].Huang WB, Shi LH, Zhu XQ, et al. Expression of mucin MUC1 and MUC2 in colorectal carcinoma and their clinical significance. Ai Zheng 2002;21:1231–4. [PubMed] [Google Scholar]
- [28].Nagai S, Takenaka K, Sonobe M, et al. A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. J Thorac Oncol 2006;1:46–51. [PubMed] [Google Scholar]
- [29].Kimura T, Tanaka S, Haruma K, et al. Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int J Oncol 2000;16:55–64. [DOI] [PubMed] [Google Scholar]
- [30].Matsuda K, Masaki T, Watanabe T, et al. Clinical significance of MUC1 and MUC2 mucin and p53 protein expression in colorectal carcinoma. Jpn J Clin Oncol 2000;30:89–94. [DOI] [PubMed] [Google Scholar]
- [31].Guo Q, Tang W, Inagaki Y, et al. Clinical significance of subcellular localization of KL-6 mucin in primary colorectal adenocarcinoma and metastatic tissues. World J Gastroenterol 2006;12:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jang KT, Chae SW, Sohn JH, et al. Coexpression of MUC1 with p53 or MUC2 correlates with lymph node metastasis in colorectal carcinomas. J Korean Med Sci 2002;17:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baldus SE, Monig SP, Hanisch FG, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology 2002;40:440–9. [DOI] [PubMed] [Google Scholar]
- [34].Matsumura N, Yamamoto M, Aruga A, et al. Correlation between expression of MUC1 core protein and outcome after surgery in mass-forming intrahepatic cholangiocarcinoma. Cancer 2002;94:1770–6. [DOI] [PubMed] [Google Scholar]
- [35].Hamada T, Nomura M, Kamikawa Y, et al. DF3 epitope expression on MUC1 mucin is associated with tumor aggressiveness, subsequent lymph node metastasis, and poor prognosis in patients with oral squamous cell carcinoma. Cancer 2012;118:5251–64. [DOI] [PubMed] [Google Scholar]
- [36].Westgaard A, Schjolberg AR, Cvancarova M, et al. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology 2009;54:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oshima T, Kawasaki T, Ohashi R, et al. Downregulated P1 promoter-driven hepatocyte nuclear factor-4alpha expression in human colorectal carcinoma is a new prognostic factor against liver metastasis. Pathol Int 2007;57:82–90. [DOI] [PubMed] [Google Scholar]
- [38].Situ D, Wang J, Ma Y, et al. Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol 2011;suppl 1:S596–604. [DOI] [PubMed] [Google Scholar]
- [39].Retterspitz MF, Monig SP, Schreckenberg S, et al. Expression of {beta}-catenin, MUC1 and c-met in diffuse-type gastric carcinomas: correlations with tumour progression and prognosis. Anticancer Res 2010;30:4635–41. [PubMed] [Google Scholar]
- [40].Wang X, Zhan P, Guo W. Expression of MUC1 and Gal-3 in colorectal carcinoma and its relation to prognosis. J Pract Oncol 2016;31:250–4. [Google Scholar]
- [41].Akyurek N, Akyol G, Dursun A, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: their relationship with clinicopathologic parameters and prognosis. Pathol Res Pract 2002;198:665–74. [DOI] [PubMed] [Google Scholar]
- [42].Kim SM, Oh SJ, Hur B. Expression of MUC1 and MUC4 in gallbladder adenocarcinoma. Korean J Pathol 2012;46:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Park SY, Roh SJ, Kim YN, et al. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep 2009;22:649–57. [DOI] [PubMed] [Google Scholar]
- [44].Togami S, Nomoto M, Higashi M, et al. Expression of mucin antigens (MUC1 and MUC16) as a prognostic factor for mucinous adenocarcinoma of the uterine cervix. J Obstet Gynaecol Res 2010;36:588–97. [DOI] [PubMed] [Google Scholar]
- [45].Kaneko I, Tanaka S, Oka S, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol 2007;13:3829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu S, Ruan M, Li S, et al. Increased expression of MUC1 predicts poor survival in salivary gland mucoepidermoid carcinoma. J Craniomaxillofac Surg 2014;42:1891–6. [DOI] [PubMed] [Google Scholar]
- [47].Karamitopoulou E, Zlobec I, Patsouris E, et al. Loss of E-cadherin independently predicts the lymph node status in colorectal cancer. Pathology 2011;43:133–7. [DOI] [PubMed] [Google Scholar]
- [48].Baldus SE, Monig SP, Huxel S, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res 2004;10:2790–6. [DOI] [PubMed] [Google Scholar]
- [49].Lee HS, Lee HK, Kim HS, et al. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer 2001;92:1427–34. [DOI] [PubMed] [Google Scholar]
- [50].Perez RO, Bresciani BH, Bresciani C, et al. Mucinous colorectal adenocarcinoma: influence of mucin expression (Muc1, 2 and 5) on clinico-pathological features and prognosis. Int J Colorectal Dis 2008;23:757–65. [DOI] [PubMed] [Google Scholar]
- [51].Lugli A, Zlobec I, Baker K, et al. Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol 2007;60:534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ando H, Aihara R, Ohno T, et al. Prognostic significance of the expression of MUC1 and collagen type IV in advanced gastric carcinoma. Br J Surg 2009;96:901–9. [DOI] [PubMed] [Google Scholar]
- [53].Woenckhaus M, Merk J, Stoehr R, et al. Prognostic value of FHIT, CTNNB1, and MUC1 expression in non-small cell lung cancer. Hum Pathol 2008;39:126–36. [DOI] [PubMed] [Google Scholar]
- [54].Wang JY, Chang CT, Hsieh JS, et al. Role of MUC1 and MUC5AC expressions as prognostic indicators in gastric carcinomas. J Surg Oncol 2003;83:253–60. [DOI] [PubMed] [Google Scholar]
- [55].Duncan TJ, Watson NF, Al-Attar AH, et al. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol 2007;5:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Khanh DT, Mekata E, Mukaisho K, et al. Transmembrane mucin MUC1 overexpression and its association with CD10(+) myeloid cells, transforming growth factor-beta1 expression, and tumor budding grade in colorectal cancer. Cancer Sci 2013;104:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Betge J, Schneider NI, Harbaum L, et al. MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: expression profiles and clinical significance. Virchows Archiv 2016;469:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lu ML, Jin H, Weidong Y. Combined expression and clinicopathological correlation study of MUC1 and MUC2 in colorectal adenomas and colorectal carcinoma. J Colorectal Anal Surg 2014;20:158–63. [Google Scholar]
- [59].Zwenger A, Rabassa M, Demichelis S, et al. High expression of sLex associated with poor survival in Argentinian colorectal cancer patients. Int J Biol Markers 2014;29:e30–9. [DOI] [PubMed] [Google Scholar]
- [60].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [61].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Baldus SE, Zirbes TK, Hanisch FG, et al. Thomsen-Friedenreich antigen presents as a prognostic factor in colorectal carcinoma: a clinicopathologic study of 264 patients. Cancer 2000;88:1536–43. [PubMed] [Google Scholar]
- [64].Hiraga Y, Tanaka S, Haruma K, et al. Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology 1998;55:307–19. [DOI] [PubMed] [Google Scholar]
- [65].Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res 2000;6:4017–25. [PubMed] [Google Scholar]
- [66].You JF, Hsieh LL, Changchien CR, et al. Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res 2006;12:4244–50. [DOI] [PubMed] [Google Scholar]
- [67].Yu XW, Rong W, Xu FL, et al. Expression and clinical significance of Mucin and E-cadherin in colorectal tumors. Ai Zheng = Aizheng = Chin J Cancer 2007;26:1204–10. [PubMed] [Google Scholar]
- [68].Zhang W, Tang W, Inagaki Y, et al. Positive KL-6 mucin expression combined with decreased membranous beta-catenin expression indicates worse prognosis in colorectal carcinoma. Oncol Rep 2008;20:1013–9. [PubMed] [Google Scholar]
- [69].Xu HL, Fengshan W, Ziyao T. Cliniacal significance and possible mechanism of KL-6 mucin expression in digestive system cancers. Microbiol Biochem Shandong Univ 2010. [Google Scholar]
- [70].Diaz DAC, Garre P, Molina RE, et al. MUC1 expression in colorectal carcinoma: clinicopathological correlation and prognostic significance. Rev Esp Patol 2018;51:204–9. [DOI] [PubMed] [Google Scholar]
- [71].Xu F, Liu F, Zhao H, et al. Prognostic Significance of Mucin antigen MUC1 in various human epithelial cancers: a meta-analysis. Medicine (Baltimore) 2015;94:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang XT, Kong FB, Mai W, et al. MUC1 immunohistochemical expression as a prognostic factor in gastric cancer: meta-analysis. Dis Markers 2016;2016:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ruys AT, Groot KB, Wiggers JK, et al. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:487–500. [DOI] [PubMed] [Google Scholar]
- [74].Zeng Y, Zhang Q, Zhang Y, et al. MUC1 predicts colorectal cancer metastasis: a systematic review and meta-analysis of case controlled studies. PLoS One 2015;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hayashi T, Takahashi T, Motoya S, et al. MUC1 mucin core protein binds to the domain 1 of ICAM-1. Digestion 2001;suppl 1:87–92. [DOI] [PubMed] [Google Scholar]
- [76].Singh PK, Wen Y, Swanson BJ, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res 2007;67:5201–10. [DOI] [PubMed] [Google Scholar]
- [77].Hattrup CL, Gendler SJ. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res 2006;8:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sahraei M, Roy LD, Curry JM, et al. MUC1 regulates PDGFA expression during pancreatic cancer progression. Oncogene 2012;31:4935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Behrens ME, Grandgenett PM, Bailey JM, et al. The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene 2010;29:5667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Besmer DM, Curry JM, Roy LD, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res 2011;71:4432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kitamoto S, Yokoyama S, Higashi M, et al. MUC1 enhances hypoxia-driven angiogenesis through the regulation of multiple proangiogenic factors. Oncogene 2013;32:4614–21. [DOI] [PubMed] [Google Scholar]
- [82].Rajabi H, Alam M, Takahashi H, et al. MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene 2014;33:1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ibrahim NK, Yariz KO, Bondarenko I, et al. Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clin Cancer Res 2011;17:6822–30. [DOI] [PubMed] [Google Scholar]
- [84].de Bono JS, Rha SY, Stephenson J, et al. Phase I trial of a murine antibody to MUC1 in patients with metastatic cancer: evidence for the activation of humoral and cellular antitumor immunity. Ann Oncol 2004;15:1825–33. [DOI] [PubMed] [Google Scholar]
- [85].Verheijen RH, Massuger LF, Benigno BB, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol 2006;24:571–8. [DOI] [PubMed] [Google Scholar]
- [86].Oei AL, Verheijen RH, Seiden MV, et al. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer 2007;120:2710–4. [DOI] [PubMed] [Google Scholar]
- [87].Fiedler W, DeDosso S, Cresta S, et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur J Cancer 2016;63:55–63. [DOI] [PubMed] [Google Scholar]
- [88].Yu XW, Cheng H, Wang JF. Associated expression mucin mUC1, mUC2 and matrix metalloproteinase(MMP-3) in colorectal carcinoma and their clinical significance. J Med Res 2008;37:23–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


