Abstract
Background:
Biomechanical studies have demonstrated that cortical bone trajectory (CBT) screw can provide a 30% increase in uniaxial yield pullout load than pedicle screw (PS). In addition, the insertion torque of CBT screw is 1.71 times higher than that of PS. A meta-analysis was conducted to evaluate clinical results between CBT screw technique and PS technique in lumbar fusion surgery.
Methods:
An extensive search of literature was performed in PubMed, Embase, the Cochrane library. The following outcomes were extracted: visual analog scale (VAS), Oswestry disabilities index (ODI), Japanese Orthopaedic Association (JOA) score, complications, fusion rates, hospital stay, incision length, blood loss, and operation time. Data analysis was conducted with RevMan 5.3 and STATA 12.0.
Results:
A total of 12 studies were included in the final analysis. The results indicated that CBT group with less blood loss [P < .01], less hospital stay [P < .01], and less incision length [P < .01] than PS group. There were no significant differences between 2 groups in other clinical parameters and outcomes.
Conclusion:
CBT technique provided similar clinical outcomes and fusion rates compared to PS technique in lumbar fusion surgery. However, CBT technique provided additional benefits of less blood loss, less hospital stay, and less incision length.
Keywords: cortical bone trajectory, fusion, lumbar, meta-analysis, pedicle screw
1. Introduction
Lumbar fusion has been widely used to address certain lumbar pathologies including lumbar stenosis, degenerative disk disease, trauma, deformities, and neoplasms.[1,2,3,4] Pedicle screw (PS) technique has been recognized as an irreplaceable instrument in fusion surgery of lumbar spine due to its biomechanical advantage including three-dimensional fixation and short-segment fixation.[5,6,7] The traditional insertion pathway of PS is transpedicular lateral to medial trajectory. The initial insertion point of PS is the junction of the transverse process and lateral wall of the facet.[8,9] However, several complications are associated with PS technique including long incisions, significant muscle dissection, neurovascular injury, superior facet joint violation, and screw loosening particularly in patients with osteopenia or osteoporosis.[10,11]
Cortical bone trajectory (CBT) screw technique was first described by Santoni et al.[12] The initial insertion point of CBT is the junction of the superior articular process and pars, which allows for minimal soft tissue dissection and may reduce the risk of neurovascular injury. CBT involves caudocephalad path and medial-to-lateral path with the objective of maximizing cortical bone contact through the pedicle to the vertebral body and minimizing the risk of instrumentation failure.[13]
Since the introduction of CBT, several biomechanical studies have supported its viability for pedicle fixation.[14,15] Furthermore, several clinical studies have compared outcomes and complications between 2 techniques.[11,16,17,18,19,20,21,22,23,24,25,26,27,28] However, there is no clear conclusion on which technique, CBT or PS, is better. Therefore, we performed a meta-analysis to evaluate the clinical results between CBT screw technique and PS technique in lumbar fusion surgery.
2. Materials and methods
2.1. Ethics statement
As all analyses were based on previously published studies, ethical approval was not necessary in this review.
2.2. Search strategy and study selection
An extensive search of literature was performed in PubMed, Embase, and the Cochrane library up to February 1, 2019. The language was restricted to English and only the published articles were included. The following terms were used in our search: “cortical bone trajectory”, “CBT”, “medial-lateral superior trajectory”, “traditional trajectory”, “pedicle screw”, and “lumbar”. The reference lists of included studies were also hand-searched for additional qualified studies.
2.3. Inclusion and exclusion criteria
Studies were included if they met the following criteria:
-
1.
Participants: patients with spinal disease which need lumbar fusion.
-
2.
Interventions: the intervention in the experimental group was CBT screw technique.
-
3.
Comparisons: the intervention in the control group was PS technique.
-
4.
Outcomes: JOA, ODI, VAS, complications, fusion rates, hospital stay, incision length, blood loss, and operation time were collected as the outcomes.
-
5.
Study design: prospective comparative study, retrospective comparative study, and randomized controlled study.
The exclusion criteria were as follow:
-
1.
case reports, reviews, or letters;
-
2.
the same data had been published repeatedly;
-
3.
outcomes of interest did not be reported.
Two reviewers independently selected the potentially qualified studies according to the inclusion and exclusion criteria. Any disagreement was resolved by discussion and the conformity was reached.
2.4. Data extraction and management
Two reviewers extracted data independently. The data includes the following categories: study design, patients demographic data (sample size, age, and sex), duration of follow-up, clinical evaluation (JOA, ODI, and VAS), complications, fusion rates, hospital stay, incision length, blood loss, and operation time.
2.5. Quality assessment
As most included studies were non-randomized controlled studies, the Newcastle–Ottawa Scale was used to assess the quality of each study. This scale for non-randomized case controlled studies and cohort studies was used to allocate a maximum of nine points for the quality of selection, comparability, exposure, and outcomes for study participants.[29]
3. Statistical analysis
We calculated relative risk (RR) with 95% confidence interval (CI) for dichotomous outcomes and mean difference (MD) with 95% CI for continuous outcomes. P values less than .05 denoted significant differences. I2 statistic was used to quantify heterogeneity, which I2 greater than 50% implied significant heterogeneity. The random-effect model was used if there was significant heterogeneity. Otherwise, the fixed-effect model was used. Sensitivity analysis was conducted to examine the influence of excluding each study. Publication bias was assessed using Egger and Begg tests. The trim and fill method was used to estimate the number of studies that might have been missing and to adjust the pooled estimate. Review Manager 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) and STATA 12.0 (Stata Corporation, College Station, TX) were used for the statistical analyses.
4. Results
4.1. Search results
A total of 345 records were identified through initial database search. Finally, 12 studies were included in this meta-analysis after removing duplicates and reviewing title/abstracts/full-text. The literature search procedure was shown in Figure 1.
Figure 1.
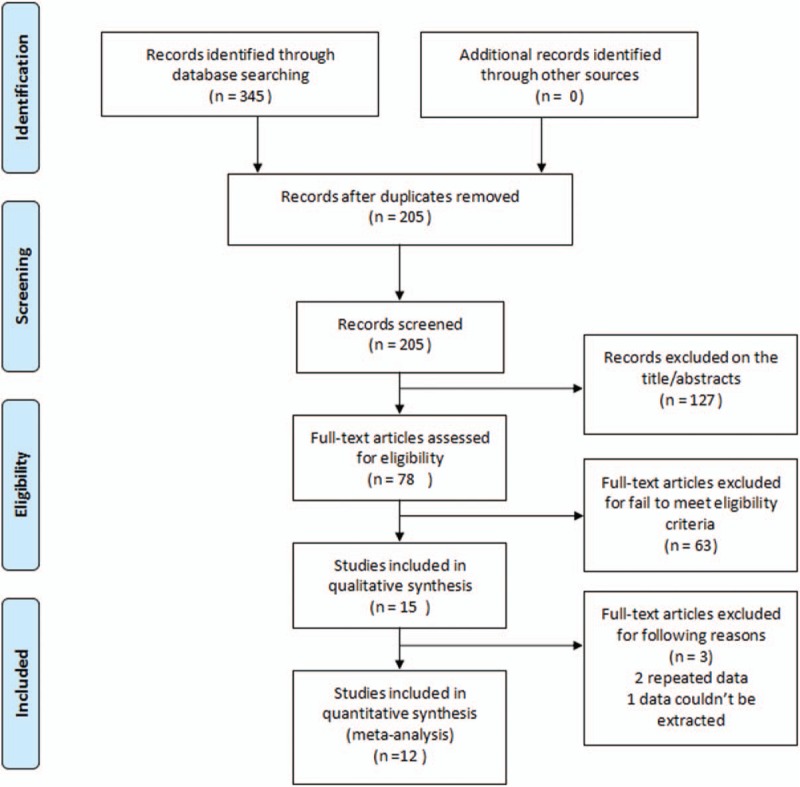
Flow diagram of study selection.
4.2. Baseline characteristics and quality assessment
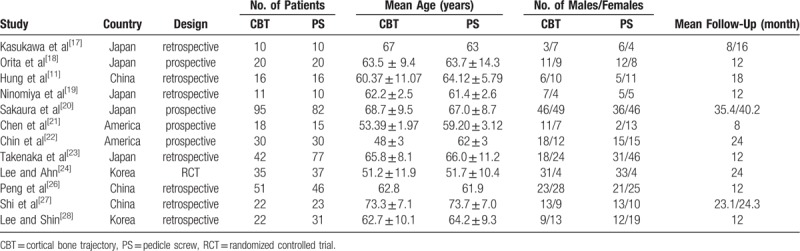
All 12 studies were published between 2015 and 2018. The number of patients in CBT and PS group ranged from 10 to 95 (total = 372) and 10 to 82 (total = 397) respectively. Characteristics of included studies were presented in Table 1.
Table 1.
Characteristics of included studies.
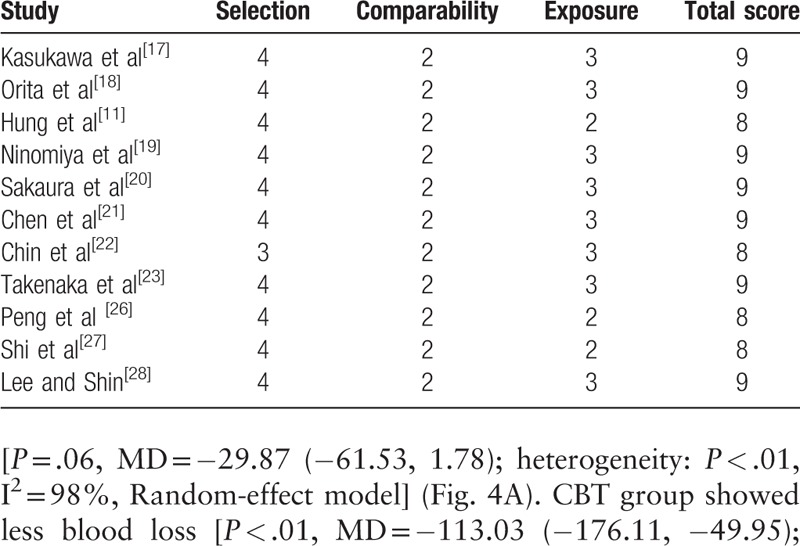
As most studies included were non-randomized controlled studies (4 in prospective and 7 in retrospective), the Newcastle–Ottawa Scale was used to assess the quality of each study. Of these, 4 studies scored 8 points and 7 studies scored 9 points. Therefore, the quality of each study was high (Table 2).
Table 2.
The quality assessment of non-randomized controlled studies according to the Newcastle–Ottawa Scale.
4.3. Clinical evaluation
Preoperative and postoperative VAS (final follow-up) of lower back pain were analyzed in 6 studies (164 patients in CBT group and 168 patients in PS group). There was no significant difference between 2 groups in preoperative VAS [P = .25, MD = 0.14 (−0.10, 0.38); heterogeneity: P = 0.51, I2 = 0%, Fixed-effect model] (Fig. 2A) and in postoperative VAS [P = 0.32, MD = 0.41 (−0.40, 1.22); heterogeneity: P < .01, I2 = 94%, Random-effect model] (Fig. 2B).
Figure 2.
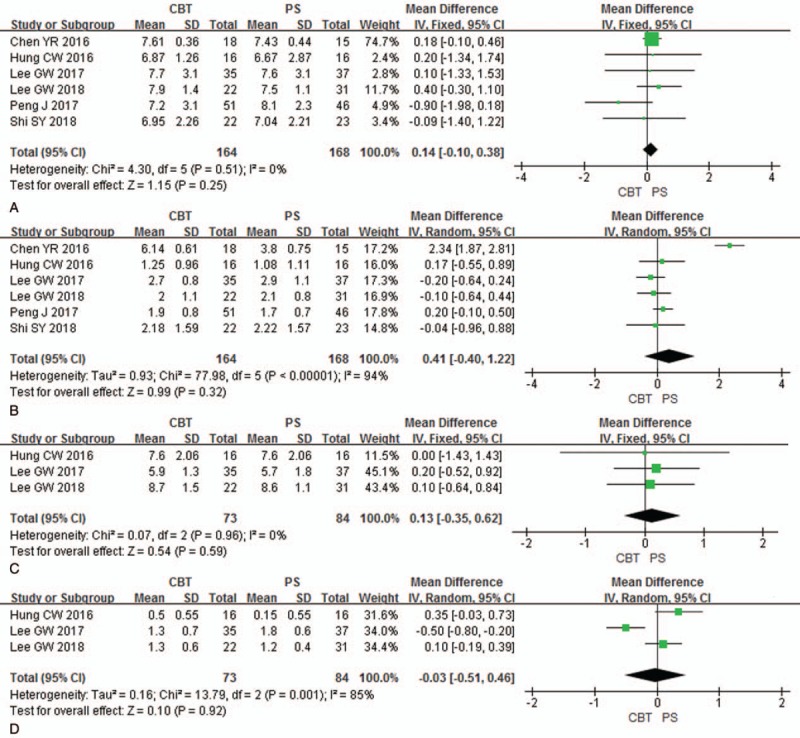
Forest plots of preoperative VAS of lower back pain (A), postoperative VAS of lower back pain (B), preoperative VAS of radiating pain (C), and postoperative VAS of radiating pain (D) in CBT group and PS group. CBT = cortical bone trajectory, PS = pedicle screw, VAS = visual analogue score.
Preoperative and postoperative VAS (final follow-up) of radiating pain were analyzed in three studies (73 patients in CBT group and 84 patients in PS group). There was no significant difference between 2 groups in preoperative VAS [P = .59, MD = 0.13 (−0.35, 0.62); heterogeneity: P = .96, I2 = 0%, Fixed-effect model] (Fig. 2C) and in postoperative VAS [P = .92, MD = −0.03 (−0.51, 0.46); heterogeneity: P < .01, I2 = 85%, Random-effect model] (Fig. 2D).
Preoperative and postoperative JOA scores (final follow-up) were analyzed in 2 studies (111 patients in CBT group and 98 patients in PS group). There was no significant difference between 2 groups in preoperative JOA scores [P = .27, MD = −3.25 (−9.01, 2.51); heterogeneity: P = .01, I2 = 83%, Random-effect model] (Fig. 3A) and in postoperative JOA scores [P = .07, MD = 0.87 (−0.06, 1.81); heterogeneity: P = .51, I2 = 0%, Fixed-effect model] (Fig. 3B).
Figure 3.
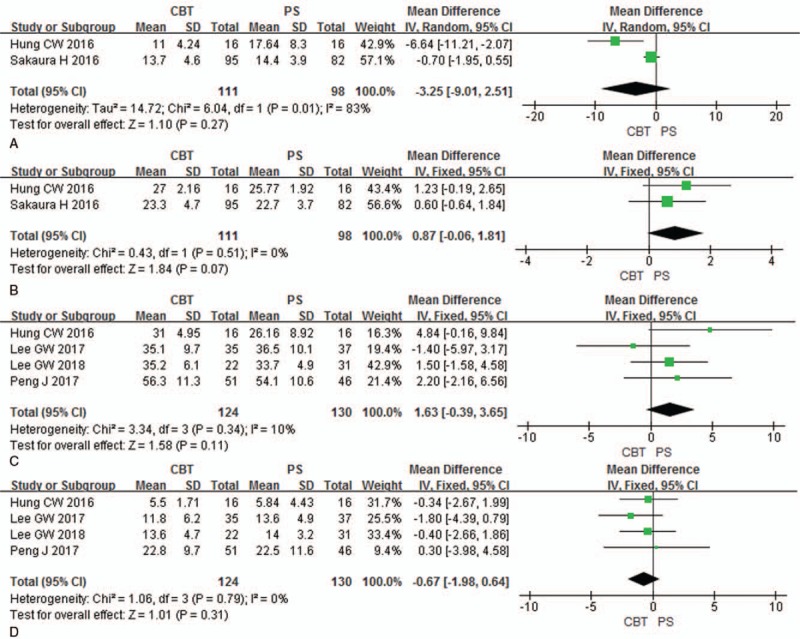
Forest plots of preoperative JOA (A), postoperative JOA (B), preoperative ODI (C) and postoperative ODI (D) in CBT group and PS group. CBT = cortical bone trajectory, JOA = Japanese Orthopedic Association, ODI = Oswestry disabilities index, PS = pedicle screw.
Preoperative and postoperative ODI (final follow-up) were analyzed in 4 studies (124 patients in CBT group and 130 patients in PS group). There was no significant difference between 2 groups in preoperative ODI [P = .11, MD = 1.63 (−0.39, 3.65); heterogeneity: P = .34, I2 = 10%, Fixed-effect model] (Fig. 3C) and in postoperative ODI [P = .31, MD = −0.67 (−1.98, 0.64); heterogeneity: P = .79, I2 = 0%, Fixed-effect model] (Fig. 3D).
4.4. Operation time and blood loss
Operation time and blood loss were analyzed in 9 studies (308 patients in CBT group and 335 patients in PS group). There was no significant difference between 2 groups in operation time [P = .06, MD = −29.87 (−61.53, 1.78); heterogeneity: P < .01, I2 = 98%, Random-effect model] (Fig. 4A). CBT group showed less blood loss [P < .01, MD = −113.03 (−176.11, −49.95); heterogeneity: P < .01, I2 = 95%, Random-effect model] than PS group (Fig. 4B).
Figure 4.
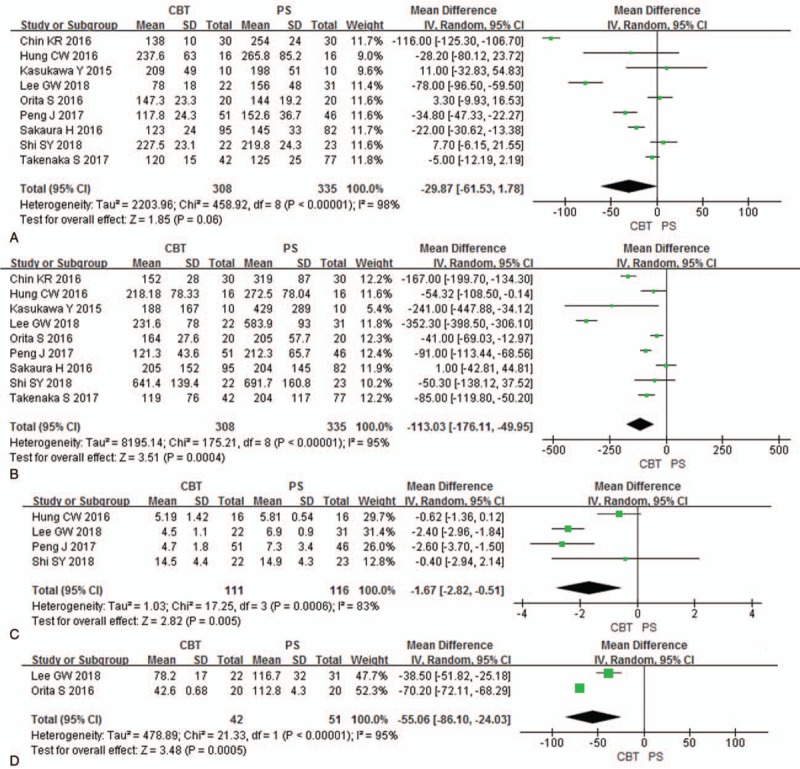
Forest plots of operation time (A), blood loss (B), hospital stay (C), and incision length (D) in CBT group and PS group. CBT = cortical bone trajectory, PS = pedicle screw.
4.5. Hospital stay and incision length
Hospital stay was analyzed in 4 studies (111 patients in CBT group and 116 patients in PS group). CBT group showed less hospital stay [P < .01, MD = −1.67 (−2.82, −0.51); heterogeneity: P < .01, I2 = 83%, Random-effect model] than PS group (Fig. 4C).
Incision length was analyzed in 2 studies (42 patients in CBT group and 51 patients in PS group). CBT group showed less incision length [P < .01, MD = −55.06 (−86.10, −24.03); heterogeneity: P < .01, I2 = 95%, Random-effect model] than PS group (Fig. 4D).
4.6. Complications
Intraoperative complications were analyzed in 4 studies (198 patients in CBT group and 215 patients in PS group). There was no significant difference between 2 groups in intraoperative complications [P = .49, RR = 0.73 (0.31, 1.76); heterogeneity: P = .31, I2 = 17%, Fixed-effect model] (Fig. 5A).
Figure 5.
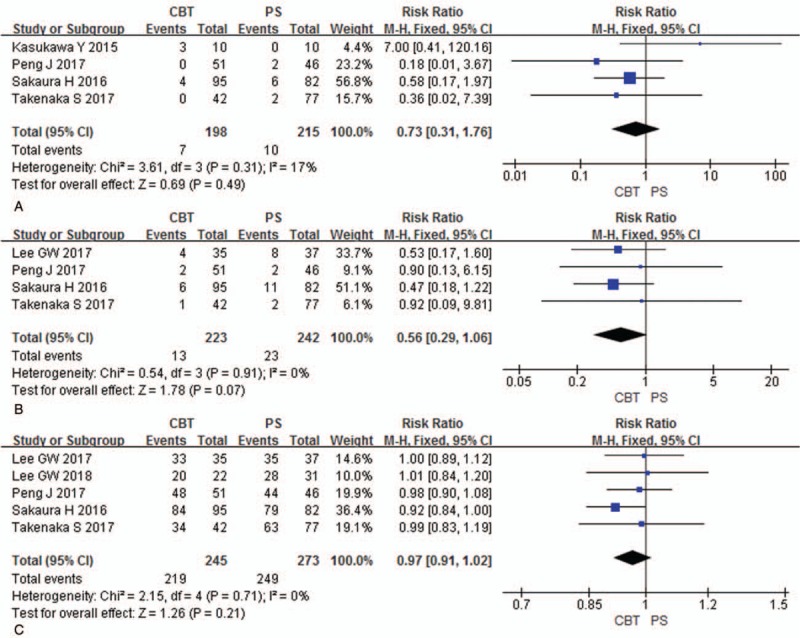
Forest plots of intraoperative complications (A), postoperative complications (B), and fusion rates (C) in CBT group and PS group. CBT = cortical bone trajectory, PS = pedicle screw.
Postoperative complications were analyzed in 4 studies (223 patients in CBT group and 242 patients in PS group). There was no significant difference between 2 groups in postoperative complications [P = .07, RR = 0.56 (0.29, 1.06); heterogeneity: P = .91, I2 = 0%, Fixed-effect model] (Fig. 5B).
4.7. Fusion rates
Fusion rates were analyzed in 5 studies (245 patients in CBT group and 273 patients in PS group). There was no significant difference between 2 groups in fusion rates [P = .21, RR = 0.97 (0.91, 1.02); heterogeneity: P = .71, I2 = 0%, Fixed-effect model] (Fig. 5C).
5. Sensitivity analysis
To confirm the stability of the meta-analysis, sensitivity analysis was performed by sequentially omitting individual eligible studies.[29] The pooled results were not materially changed after any single study was excluded which indicated the stability of the results.
5.1. Publication bias
For 9 studies analyzed operation time, both Egger tests (P = .983) and Begg tests (P = .602) showed no publication bias (Fig. 6A). For 9 studies analyzed blood loss, both Egger tests (P = 0.678) and Begg tests (P = .754) showed no publication bias (Fig. 6B). As included studies were few, publication bias was not assessed for other parameters.
Figure 6.
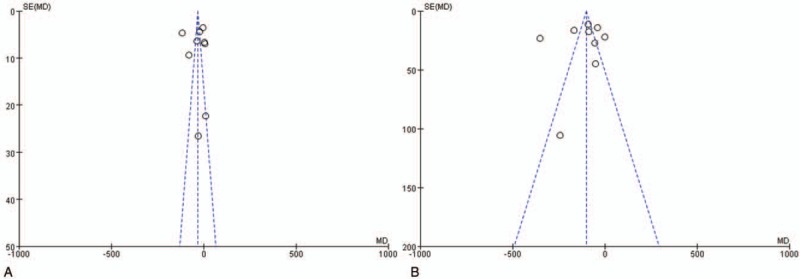
Funnel plots of operation time (A) and blood loss (B).
6. Discussion
The key elements of screw fixation are pullout strength and stability. Biomechanical studies have demonstrated that CBT screw can provide a 30% increase in uniaxial yield pullout load than PS. In addition, the insertion torque of CBT screw is 1.71 times higher than that of PS.[9,12] However, whether or not CBT can provide similar clinical outcomes compared to PS has not yet been fully evaluated. Thus, we performed a meta-analysis to evaluate clinical results (JOA, ODI, and VAS), complications, fusion rates, hospital stay, incision length, blood loss, and operation time between 2 groups in lumbar fusion surgery.
This study suggests that there were no significant difference between two groups in VAS of lower back pain, VAS of radiating pain, JOA, ODI, intraoperative complications, postoperative complications, fusion rates, and operation time. However, CBT technique provided the additional benefits of less blood loss [P < .01], less hospital stay [P < .01], and less incision length [P < .01].
VAS, JOA, and ODI were often used to evaluate the improvement of clinical outcomes. The pooled data showed that there were no significant difference between the 2 groups in VAS, JOA, and ODI. Meanwhile, there were no significant differences between 2 groups in fusion rates. Intervertebral fusion and effective decompression were key points of lumbar fusion surgery. Strong internal fixation was the prerequisite for intervertebral fusion. Biomechanical studies have demonstrated that CBT screw can provide similar or better pullout strength and stability than PS.[12] Thus, both CBT and PS could provide well intervertebral fusion and satisfactory clinical outcomes.
Complications reported in the literature include intraoperative complications (dural tear, pedicle fracture, and misplacement) and postoperative complications (hematoma, wound infection, and adjacent segment disease). The pooled data showed that there were no significant differences between the 2 groups in intraoperative complications and postoperative complications. Sakaura et al demonstrated that the incidence of symptomatic adjacent segment disease was significantly higher in PS group than CBT group (P < .05).[20] Subgroup analysis was not possible due to the small number of included studies. So, further large-scale studies are needed in the future.
Operation time, blood loss, hospital stay, and incision length were important factors for assessing surgical trauma. The pooled data showed that CBT group with less blood loss, less hospital stay, and less incision length. As a novel technique, CBT has several advantages over PS. First, the insertion point of CBT is far from superior facet joint which may minimize the violation of superior facet joint.[4] Second, CBT has a medial starting point enables less muscle disruption and less tissue retraction which means less tissue trauma, less incision length, less blood loss, and less recovery time. For obese and morbid obese patients, the advantage will be more obvious.[5] Furthermore, its medial-to-lateral directed trajectory may also reduce risk of violation of pedicle medial wall and related dural injury.[10] Finally, CBT involves caudocephalad path and medial-to-lateral path with the objective of maximizing cortical bone contact through the pedicle to the vertebral body which can minimize the risk of instrumentation failure even in osteoporotic bone. So CBT screw may be applied to osteopenic or osteoporotic patients which may avoid the complications associated with bone cement.[13]
7. Study limitations
There are several limitations in this study. First, in all 12 included studies, only one study is randomized controlled trial and the other 11 included studies are non-randomized controlled studies (4 in prospective and 7 in retrospective). Second, the language of included studies was restricted to English. Third, follow-up times of included studies were different which may influence results.
8. Conclusions
CBT technique provided similar clinical outcomes (JOA, VAS, ODI), fusion rates, complications (intraoperative, postoperative), and operation time compared to PS technique in lumbar fusion surgery. However, CBT technique provided the additional benefits of less blood loss, less hospital stay, and less incision length.
Author contributions
Conceptualization: Yun-Zhi Ding.
Data curation: Jing-Nan Hu, Xiao-Feng Yang.
Formal analysis: Jing-Nan Hu.
Investigation: Jing-Nan Hu, Chuan-Ming Li.
Methodology: Chuan-Ming Li.
Software: Xin-Xin Li.
Validation: Xiao-Feng Yang, Xin-Xin Li.
Visualization: Xiao-Feng Yang, Xin-Xin Li.
Writing – original draft: Jing-Nan Hu, Xiao-Feng Yang.
Writing – review & editing: Xiao-Feng Yang, Yun-Zhi Ding.
Footnotes
Abbreviations: CBT = cortical bone trajectory, CI = confidence interval, JOA = Japanese Orthopedic Association, MD = mean difference, ODI = Oswestry disabilities index, PS = pedicle screw, RR = relative risk, VAS = visual analogue score.
JNH and XFY were contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1]. Kaiser MG, Eck JC, Groff MW, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine 2014;21:2–6. [DOI] [PubMed] [Google Scholar]
- [2]. Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF, and ALIF. J Spine Surg 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Wu AM, Zou F, Cao Y, et al. Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment. AME Med J 2017;2:63. [Google Scholar]
- [4]. Phan K, Ramachandran V, Tran TM, et al. Systematic review of cortical bone trajectory versus pedicle screw techniques for lumbosacral spine fusion. J Spine Surg 2017;3:679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Song T, Hsu WK, Ye T. Lumbar pedicle cortical bone trajectory screw. Chin Med J (Engl) 2014;127:3808–013. [PubMed] [Google Scholar]
- [6]. Wang Z, Fu S, Wu ZX, et al. Pedicle screw system augmentation for posterior lumbar interbody fusion. Spine 2013;38:2008–15. [DOI] [PubMed] [Google Scholar]
- [7]. Athanasakopoulos M, Mavrogenis AF, Triantafyllopoulos G, et al. Posterior spinal fusion using pedicle screws. Orthopedics 2013;36:e951–957. [DOI] [PubMed] [Google Scholar]
- [8]. Inceoglu S, Montgomery WH, Jr, St Clair S, et al. Pedicle screw insertion angle and pullout strength: comparison of 2 proposed strategies. J Neurosurg Spine 2011;14:670–6. [DOI] [PubMed] [Google Scholar]
- [9]. Matsukawa K, Yato Y, Kato T, et al. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976) 2014;39:E240–5. [DOI] [PubMed] [Google Scholar]
- [10]. Phan K, Hogan J, Maharaj M, et al. Cortical bone trajectory for lumbar pedicle screw placement: a review of published reports. Orthop Surg 2015;7:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Hung CW, Wu MF, Hong RT, et al. Comparison of multifidus muscle atrophy after posterior lumbar interbody fusion with conventional and cortical bone trajectory. Clin Neurol Neurosurg 2016;145:41–5. [DOI] [PubMed] [Google Scholar]
- [12]. Santoni BG, Hynes RA, McGilvray KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009;9:366–73. [DOI] [PubMed] [Google Scholar]
- [13]. Delgado-Fernandez J, García-Pallero MÁ, Blasco G, et al. Review of cortical bone trajectory: evidence of a new technique. Asian Spine J 2017;11:817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Perez-Orribo L, Kalb S, Reyes PM, et al. Biomechanics of lumbar cortical screw–rod fixation versus pedicle screw–rod fixation with and without interbody support. Spine. 2013;38(8):635–641. [DOI] [PubMed] [Google Scholar]
- [15]. Calvert GC, Lawrence BD, Abtahi AM, et al. Cortical screws used to rescue failed lumbar pedicle screw construct: a biomechanical analysis. J Neurosurg Spine 2015;22:166–72. [DOI] [PubMed] [Google Scholar]
- [16]. Lee GW, Son JH, Ahn MW, et al. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J 2015;15:1519–26. [DOI] [PubMed] [Google Scholar]
- [17]. Kasukawa Y, Miyakoshi N, Hongo M, et al. Short-term results of transforaminal lumbar interbody fusion using pedicle screw with cortical bone trajectory compared with conventional trajectory. Asian Spine J 2015;9:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Orita S, Inage K, Kubota G, et al. One-year prospective evaluation of the technique of percutaneous cortical bone trajectory spondylodesis in comparison with percutaneous pedicle screw fixation: a preliminary reportwith technical note. J Neurol Surg A Cent Eur Neurosurg 2016;77:531–7. [DOI] [PubMed] [Google Scholar]
- [19]. Ninomiya K, Iwatsuki K, Ohnishi Y, et al. Radiological evaluation of the initial fixation between cortical bone trajectory and conventional pedicle screw technique for lumbar degenerative spondylolisthesis. Asian Spine J 2016;10:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Sakaura H, Miwa T, Yamashita T, et al. Posterior lumbar interbody fusion with cortical bone trajectory screw fixation versusposterior lumbar interbody fusion using traditional pedicle screw fixation for degenerativelumbar spondylolisthesis: a comparative study. J Neurosurg Spine 2016;25:591–5. [DOI] [PubMed] [Google Scholar]
- [21]. Chen YR, Deb S, Pham L, et al. Minimally invasive lumbar pedicle screw fixation using cortical bone trajectory-a prospective cohort study on postoperative pain outcomes. Cureus 2016;8:e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Chin KR, Pencle FJR, Coombs AV, et al. Clinical outcomes with midline cortical bone trajectory pedicle screws versus traditional pedicle screws in moving lumbar fusions from hospitals to outpatient surgery centers. Clin Spine Surg 2017;30:E791–7. [DOI] [PubMed] [Google Scholar]
- [23]. Takenaka S, Mukai Y, Tateishi K, et al. Clinical outcomes after posterior lumbar interbody fusion: comparison of cortical bone trajectory and conventional pedicle screw insertion. Clin Spine Surg 2017;30:E1411–8. [DOI] [PubMed] [Google Scholar]
- [24]. Lee GW, Ahn MW. Comparative study of cortical bone trajectory-pedicle screw (cortical screw) versus conventional pedicle screw in single-level posterior lumbar interbody fusion: a 2-year post hoc analysis from prospectively randomized data. World Neurosurg 2018;109:e194–202. [DOI] [PubMed] [Google Scholar]
- [25]. Sakaura H, Miwa T, Yamashita T, et al. Cortical bone trajectory screw fixation versus traditional pedicle screw fixation for 2-level posterior lumbar interbody fusion: comparison of surgical outcomes for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine 2018;28:57–62. [DOI] [PubMed] [Google Scholar]
- [26]. Peng J, Zhan Y, Liu Y, et al. Comparison of effectiveness of cortical bone trajectory screw fixation and pedicle screw fixation in posterior lumbar interbody fusion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017;31:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Shi S, Ying X, Zheng Q, et al. Application of cortical bone trajectory screws in elderly patients with lumbar spinal tuberculosis. World Neurosurg 2018;117:e82–9. [DOI] [PubMed] [Google Scholar]
- [28]. Lee GW, Shin JH. Comparative study of two surgical techniques for proximal adjacent segment pathology after posterior lumbar interbody fusion with pedicle screws: fusion extension using conventional pedicle screw vs cortical bone trajectory-pedicle screw (cortical screw). World Neurosurg 2018;117:e154–61. [DOI] [PubMed] [Google Scholar]
- [29]. Liu FY, Yang SD, Huo LS, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical compressive myelopathy: a meta-analysis. Medicine (Baltimore) 2016;95:e3588. [DOI] [PMC free article] [PubMed] [Google Scholar]


