Abstract
Rationale:
Current guidelines for advanced non-small cell lung cancer (NSCLC) recommend the use of targeted agents for specific driver genes after confirming genetic alterations. Although epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement are usually mutually exclusive, EGFR and ALK co-alterations have been reported increasingly in cases of NSCLC. However, the optimal treatment for these cases has not been established.
Patient concerns:
This case series describes three patients diagnosed with advanced non-squamous NSCLC who harbored EGFR and ALK co-alterations. The complaints for each case are as follows: 57-year-old woman with coughing and dyspnea in case 1, 32-year-old man with diplopia in case 2 and 77-year-old woman with chest discomfort in case 3.
Diagnoses:
Three never-smokers were diagnosed pathologically with stage IV adenocarcinoma of the lung. Subsequent molecular studies revealed the EGFR L858R mutation gene and ALK rearrangement, which were proven by real-time polymerase chain reaction and fluorescence in situ hybridization, respectively.
Interventions:
All 3 patients received first-line therapy with EGFR-tyrosine kinase inhibitors (TKIs). Cases 1 and 2 were treated with ALK-TKIs as second-line therapy and received additional EGFR-TKIs as third- and fourth-line regimens.
Outcomes:
The patients achieved partial responses to EGFR-TKIs according to radiologic findings. However, second-line ALK-TKI therapy was ineffective in cases 1 and 2.
Lessons:
Cases of NSCLC with concomitant EGFR mutation and ALK rearrangement are rare, and the selection of an optimal targeted therapy is challenging. Here, EGFR-TKI appeared to yield better outcomes than ALK-TKI in patients with NSCLC who harbored EGFR/ALK co-alterations.
Keywords: ALK, EGFR, non-small cell lung cancer, targeted therapy
1. Introduction
Although lung cancer remains the leading cause of cancer-related mortality worldwide,[1–3] molecular screening and detection of driver genes has yielded improvements in survival, especially among patients with non-squamous non-small cell lung cancer (NSCLC). A recent clinical guideline recommends the use of targeted therapy for specific driver genes after confirmation of genetic mutation or rearrangement.[4] Epidermal growth factor receptor (EGFR) mutation is the most frequently detected driver gene in NSCLC, and is detected in 10% and 50% of cases in Western and Asian countries, respectively.[5] Currently, EGFR-tyrosine kinase inhibitors (TKIs) are recommended as a first-line therapy in patients with sensitizing EGFR mutations.[4] Anaplastic lymphoma kinase (ALK) rearrangement is less frequent, occurring in approximately 5% of patients with NSCLC.[6] Accordingly, ALK-TKIs are recommended as a first-line therapy for patients with ALK rearrangement.
Previously, EGFR mutation and ALK rearrangement were thought to be mutually exclusive.[7] However, recent reports have described these events concomitantly in patients with NSCLC.[8–14] In this report, we present a series of patients with NSCLC who harbored simultaneous EGFR mutation and ALK rearrangement in the context of a review of the literature.
2. Methods
This study was approved by the Institutional Review Board of the Chonnam National University Hwasun Hospital (the number of approval: CNUHH-2018-168). The patients described herein provided written informed consent for the publication of this report and all accompanying images and tables.
3. Case descriptions
3.1. Case 1
A 57-year-old woman presented to our hospital in August 2016 with the complaints of coughing, sputum and dyspnea. She had no history of smoking and an unremarkable medical history. Chest computed tomography (CT) revealed a 4.2 cm × 3.8 cm mass in the right upper lobe, with a huge pleural effusion at the right hemithorax. A bronchoscopic biopsy and pleural cytology confirmed an adenocarcinoma. Following positron emission tomography (PET), the patient was diagnosed with stage IV lung adenocarcinoma with metastases to the pleura and sacrum. Brain magnetic resonance imaging (MRI) did not detect a brain metastasis. Molecular screening revealed an L858R point mutation in EGFR exon 21 by real-time polymerase chain reaction (PCR) and ALK rearrangement by fluorescence in situ hybridization (FISH).
Beginning in September 2016, the patient received 250 mg of gefitinib once daily, and the improvement in her tumor burden was considered a partial response (Fig. 1). She ceased gefitinib therapy after disease progression was confirmed in April 2017 [initial progression-free survival (PFS) = 7.7 months] (Table 1). At that time, crizotinib was initiated to target the ALK rearrangement. However, this therapy was terminated after 3 weeks because the patient complained of double vision and exhibited no radiologic response. Subsequently, she received third-line osimertinib therapy until February 2018 and achieved a partial response, with a second PFS of 9.5 months. After further disease progression was confirmed, she received 4 cycles of gemcitabine and cisplatin chemotherapy, followed by pembrolizumab for 6 weeks. She began receiving pemetrexed in July 2018 and remains on this therapy.
Figure 1.
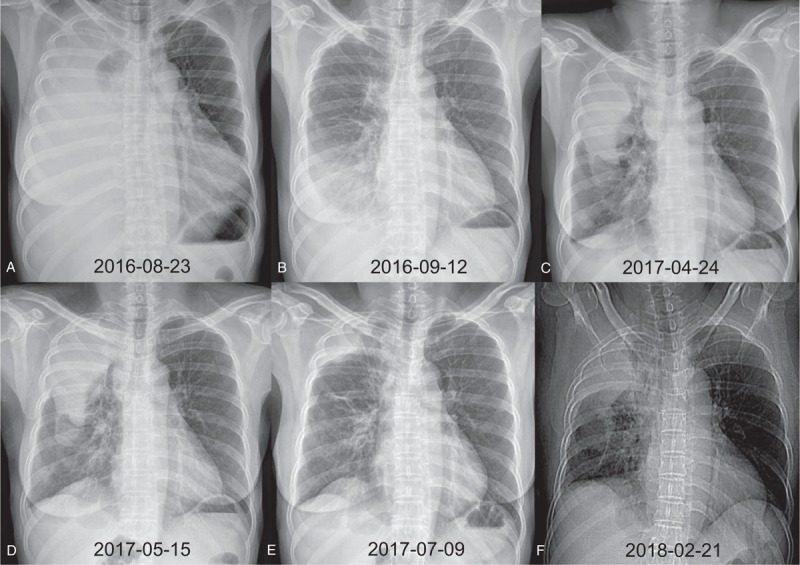
Serial chest radiography of case 1. (A) Initial chest radiography at diagnosis. (B) Chest radiography after 3 weeks of gefitinib therapy with a partial response. (C) Chest radiography after 8 months of gefitinib therapy with disease progression. (D) Chest radiography after 3 weeks of crizotinib therapy with subtle progression. (E) Chest radiography after 2 months of osimertinib therapy with a partial response. (F) Chest radiography after 9 months of osimertinib therapy with progressive disease.
Table 1.
Summary of clinicopathological characteristics.
3.2. Case 2
A 32-year-old man was admitted to the neurologic department with diplopia in February 2018. He had never smoked and had an unremarkable medical history. A chest CT scan revealed a 6.7 cm × 5.8 cm mass with total obstruction of the right middle lobe and multiple lung nodules (Fig. 2). A bronchoscopic biopsy revealed adenocarcinoma. PET and brain MRI revealed multiple bone metastases (left clavicle, right humerus, left scapula, thoracic spine, right ulnar, left ilium, right femur) and brain metastasis. Screening of the biopsy specimen revealed the EGFR L858R mutation by real-time PCR and ALK rearrangement by FISH.
Figure 2.
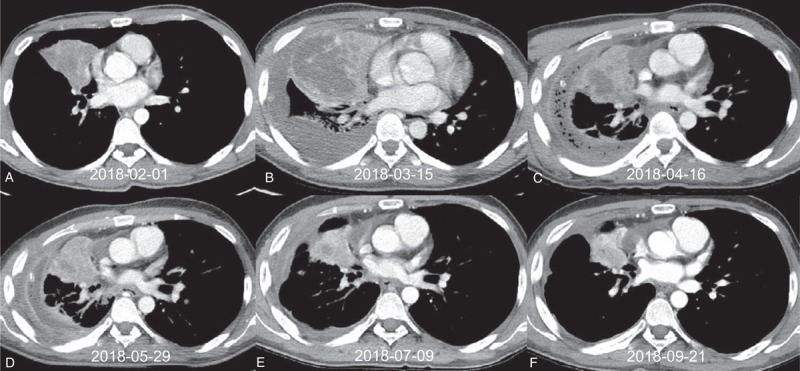
Serial computed tomography (CT) of case 2. (A) Baseline CT at diagnosis. (B) CT after 3 weeks of osimertinib therapy with progressive disease. (C) CT after 1 month of crizotinib therapy; although a decrease in the primary tumor is visible, multiple lung-to-lung metastases had developed (not shown in this figure). (D) Baseline CT before fourth-line erlotinib therapy. (E) CT after 5 weeks of erlotinib with a partial response. (F) CT after 4 months of erlotinib therapy with a partial response.
The patient underwent gamma-knife surgery for brain metastases and began receiving 80 mg of osimertinib once daily in February 2018. After 3 weeks, however, he ceased osimertinib therapy because of chest pain and fever. As a chest CT scan indicated rapid tumor progression, crizotinib therapy was initiated. However, a 1-month follow-up chest CT scan revealed a decrease in the primary tumor size with aggravated lung-to-lung metastases. After 2 cycles of pemetrexed chemotherapy, the patient has been receiving fourth-line erlotinib therapy since May 2018 and has achieved a partial response. His current PFS now exceeds 4 months.
3.3. Case 3
A 77-year-old woman visited our hospital in August 2017 with chest discomfort. She had never smoked and had an unremarkable medical history. A chest CT scan performed at another hospital revealed a 2.0 cm × 1.9 cm mass in the left upper lobe, as well as multiple lung nodules and enlarged mediastinal lymph nodes (Fig. 3). She was diagnosed with stage IV lung adenocarcinoma with lung-to-lung, lumbar spinal, and sacral metastases following endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and PET. Brain MRI led to a suspicion of brain metastases and leptomeningeal seeding. Screening of the EBUS-TBNA tumor specimen revealed the EGFR L858R mutation by real-time PCR and ALK rearrangement by FISH, with 60% positive tumor cells.
Figure 3.
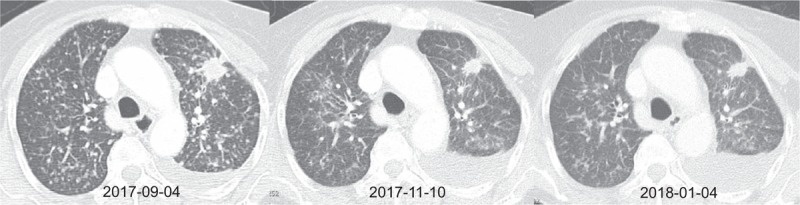
Serial computed tomography (CT) of case 3. (A) Baseline CT at diagnosis. (B) CT after 2 months of osimertinib therapy with a partial response. (C) CT after 4 months of osimertinib therapy with a partial response.
In September 2017, the patient began treatment with 80 mg of osimertinib once daily, and exhibited a tumor burden with a partial response. However, she presented to our emergency room with fever and dyspnea in February 2018, and a chest CT scan showed diffuse ground-glass opacities and consolidations in both lungs suggestive of drug-induced interstitial pneumonitis and/or atypical pneumonia. Despite treatment with broad-spectrum antibiotics and steroid pulse therapy, she died of respiratory failure after 4 days. Her PFS was 5.1 months.
4. Discussion
In this report, we describe a series of rare but clinically important lung adenocarcinomas harboring concomitant EGFR mutation and ALK gene rearrangement. To date, the clinical features of patients harboring this co-alteration have not been well characterized. Furthermore, it is difficult to select the best targeted drug in this situation, as no existing clinical guideline recommends the order of administration of EGFR-TKIs and ALK-TKIs.
Previous studies reported that ALK rearrangement and EGFR gene mutation are mutually exclusive.[7] Recently, however, co-alterations have been reported with increasing frequency as testing methods become more sensitive. Currently, the reported incidence of concomitant EGFR mutation and ALK rearrangement ranges from 1.3% to 1.6% in patients with NSCLC.[11,12] The frequency of concomitant EGFR mutation in patients with ALK rearrangement is 18.6%, whereas that of concomitant ALK rearrangement in patients with EGFR mutation ranges from 1.6% to 3.9%.[9,11] In a previous report of 6 patients with concomitant EGFR mutation and ALK rearrangement, 5 (83%) were female and 3 were never-smokers.[11] Compared to patients with single EGFR-mutant NSCLC, those harboring co-alterations were significantly younger (<60 years; P = .04).[9] Features including female and never-smoker in patients harboring co-alteration resembled those of single EGFR mutation or single ALK rearrangement in patients with NSCLC.[15,16]
In 1 case report, a patient with NSCLC with concomitant EGFR mutation and ALK rearrangement achieved clinical efficacy and a 2-year PFS with icotinib therapy.[13] However, Mao et al. demonstrated a reduced response to EGFR-TKI among patients harboring co-alterations, compared to those with a single EGFR mutation (median PFS: 6.6 months vs 10.7 months; P = .004).[9] However, Lo Russo et al reported that ALK-TKI appeared to be slightly more effective than EGFR-TKIs in a review of 100 cases.[8] Still, that report included only a small number of concomitant cases and did not report statistical results. Schmid et al reported that EGFR-TKIs may yield better outcomes than ALK-TKI in patients with EGFR/ALK co-alterations.[10] In our study, EGFR-TKI appeared to be more effective than ALK-TKI, as all three patients with EGFR/ALK co-alterations achieved partial responses with EGFR-TKI therapy, including 2 of 3 patients treated with EGFR-TKIs as a first-line therapy (Table 1). Two patients also achieved partial responses to third- and fourth-line EGFR-TKI therapy. Of particular interest is that despite poor responses to both first-line osimertinib and second-line crizotinib, a reintroduction of an EGFR-TKI (erlotinib) was effective in case 2. However, second-line crizotinib was not effective in either of 2 cases in our study. Further studies are needed to determine which TKIs are more effective in patients with NSCLC harboring EGFR/ALK co-alterations.
In Table 1, we include descriptions of 2 more cases of earlier-stage NSCLC. Case 4 involved a male patient with stage IIIA adenocarcinoma who underwent lobectomy of the left upper lobe after neoadjuvant chemotherapy. As adjuvant radiotherapy was impossible because of a poor performance status, he tried adjuvant afatinib therapy without an intrathoracic recurrence. However, this therapy was discontinued after he developed brain metastasis. Case 5 involved a female patient with stage IB adenocarcinoma with EGFR/ALK co-alterations. This patient did not experience a relapse after surgical resection.
There is a limitation in this case series. Because there was no patient who used ALK-TKIs as the first-line therapy, it was not a direct comparison of which targeted drugs are more effective between EGFR-TKIs and ALK-TKIs in patients with NSCLC who harbored EGFR/ALK co-alterations. Further randomized clinical trials are needed to know the answer.
5. Conclusion
Concomitant EGFR mutation and ALK rearrangement is a relatively rare event in patients with advanced-stage NSCLC, and the selection of optimal targeted therapy is challenging. However, EGFR-TKIs appeared to yield superior outcomes to ALK-TKIs in our case series of patients with NSCLC who harbored EGFR/ALK co-alterations.
Author contributions
Conceptualization: In-Jae Oh.
Data curation: Hong-Joon Shin, Bo Gun Kho, Min-Seok Kim, Ha Young Park, Tae-Ok Kim, Cheol-Kyu Park, Yoo-Duk Choi.
Formal analysis: Hong-Joon Shin, In-Jae Oh.
Funding acquisition: Young-Chul Kim, In-Jae Oh.
Resources: Hong-Joon Shin, Bo Gun Kho, Min-Seok Kim, Ha Young Park, Tae-Ok Kim, Cheol-Kyu Park, Young-Chul Kim, Yoo-Duk Choi, In-Jae Oh.
Supervision: Yu-Il Kim, Sung-Chul Lim, Cheol-Kyu Park, Young-Chul Kim.
Validation: Cheol-Kyu Park, Young-Chul Kim, Yoo-Duk Choi.
Writing – original draft: Hong-Joon Shin.
Writing – review & editing: Hong-Joon Shin, In-Jae Oh.
In-Jae Oh orcid: 0000-0003-4837-1321.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase, CT = computed tomography, EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration, EGFR = the epidermal growth factor receptor, FISH = fluorescence in situ hybridization, MRI = magnetic resonance imaging, NSCLC = non-small cell lung cancer, PCR = polymerase chain reaction, PET = positron emission tomography, PFS = progression-free survival, TKI = tyrosine kinase inhibitor.
This study was supported by a grant (HCRI 17907-1) from the Chonnam National University Hwasun Hospital Institute for Biomedical Science.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis 2016;79:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kweon SS. Updates on cancer epidemiology in Korea, 2018. Chonnam Med J 2018;54:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].National comprehensive cancer network. Non-small cell lung cancer (Version 6.2018). Available online at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf Accessed February 15, 2019. [Google Scholar]
- [5].Hirsch FR, Bunn PA., Jr EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432–3. [DOI] [PubMed] [Google Scholar]
- [6].Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6. [DOI] [PubMed] [Google Scholar]
- [8].Lo Russo G, Imbimbo M, Corrao G, et al. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget 2017;8:59889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mao Y, Wu S. ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. Onco Targets Ther 2017;10:3399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schmid S, Gautschi O, Rothschild S, et al. Clinical outcome of ALK-positive non-small cell lung cancer (NSCLC) patients with de novo EGFR or KRAS co-mutations receiving tyrosine kinase inhibitors (TKIs). J Thorac Oncol 2017;12:681–8. [DOI] [PubMed] [Google Scholar]
- [11].Ulivi P, Chiadini E, Dazzi C, et al. Non-small-cell lung cancer patients who carry a double mutation of EGFR, EML4-ALK or KRAS: frequency, clinical-pathological characteristics, and response to therapy. Clin Lung Cancer 2016;17:384–90. [DOI] [PubMed] [Google Scholar]
- [12].Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014;20:1383–92. [DOI] [PubMed] [Google Scholar]
- [13].Ye C, Wang J, Zheng S, et al. Effective treatment with icotinib in lung adenocarcinoma with EGFR and ALK co-alterations and brain metastasis. Onco Targets Ther 2016;9:6605–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yokoyama A, Tamura A, Miyakawa K, et al. Pulmonary adenocarcinoma, harboring both an EGFR mutation and ALK rearrangement, presenting a stable disease to erlotinib and a partial response to alectinib: a case report. Intern Med 2018;57:2377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kawaguchi T, Koh Y, Ando M, et al. Prospective analysis of oncogenic driver mutations and environmental factors: Japan molecular epidemiology for lung cancer study. J Clin Oncol 2016;34:2247–57. [DOI] [PubMed] [Google Scholar]


