Supplemental Digital Content is available in the text
Keywords: hsCRP, inflammatory marker, visceral fat, WBC
Abstract
Obesity is now considered a state of chronic low-grade inflammation. We investigated the relationship between several inflammatory markers and body composition for identifying patients with an increased risk of visceral obesity and compared the predictive values of inflammatory indices in visceral obesity.
Six hundred individuals who received health checkups for obesity-related risk factors in Severance Hospital between January 2008 and March 2017 were included in our study. Serum inflammatory markers, such as white blood cell (WBC), high-sensitivity C-reactive protein (hsCRP), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) levels were assessed. Intra-abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) areas were measured with computed tomography. We performed analysis of covariance, trend analysis, Steiger's Z tests, and multiple linear regression analysis to investigate associations between abdominal adiposity indices and inflammatory markers.
Pearson's correlation analysis revealed a stronger association of VAT with WBC counts (r = 0.157, P < .001) than with levels of NLR (r = 0.108, P = .11; Steiger's Z test, P‡ = .04) and PLR (r = 0.036, P = .39; Steiger's Z test, P‡ = .003). WBC and hsCRP levels linearly increased with VAT area (overall P < .001 and trend P < .001) and VAT/SAT ratio (overall P = .001 and trend P = .002; overall P < .001 and trend P < .001, respectively) but linearly decreased with SAT (overall P = .02 and trend P = .17; overall P = .03 and trend P = .01, respectively). Visceral adipose tissue area was more highly associated with WBC and hsCRP levels than with NLR and PLR. Only VAT area was significantly associated with WBC, hsCRP, and NLR levels after adjusting for confounding variables.
We found that VAT, but not SAT area is independently associated with several inflammatory markers. WBC and hsCRP are more strongly correlated with VAT compared with NLR and PLR. Thus, WBC and hsCRP could be useful parameters for identifying individuals at risk for visceral obesity and cardiometabolic diseases.
1. Introduction
Chronic low-grade inflammation has been documented to play regulatory roles in various metabolic diseases and cardiovascular disease (CVD) under both physiological and pathophysiological conditions.[1] Obesity is an important cause of chronic diseases and is also considered a state of chronic low-grade inflammation. The excessive accumulation of fat in adipose tissue recruits macrophages[1] and leads to increased production of many pro-inflammatory cytokines and chemokines, including tumor necrosis factor-α, monocyte-chemoattractant protein 1, and interleukin-6 that can attract inflammatory cells.[2] Finally, this obesity-mediated adipose tissue remodeling causes metabolic dysfunction such as insulin resistance[3] and obesity-related systemic diseases.[4] Therefore, the evaluation of an individual's inflammatory status could be helpful for predicting obesity-related health problems and decrease chronic disease burden in this population.
Several inflammatory biomarkers, such as high-sensitivity C-reactive protein (hsCRP) and white blood cell (WBC) count, have been used to predict the risk of coronary heart disease and other age-related degenerative diseases.[5,6] These markers[7,8] are also associated with the degree of obesity expressed as body mass index (BMI),[9] waist circumference (WC),[10,11] and waist-hip-ratio.[12] However, these body composition variables cannot distinguish visceral adipose tissue (VAT) from subcutaneous adipose tissue (SAT) and are limited for predicting CVD.[13] Visceral adipose tissue area has a stronger association with cardiometabolic risk than SAT area,[14] and although the pathological mechanisms linking VAT with its comorbidities are multifactorial, altered secretion of pro-inflammatory adipokines and a succession of inflammatory processes in VAT are considered primary contributing factors for CVD.[13]
Recently, neutrophil-lymphocyte and platelet-lymphocyte ratios (NLR[15,16] and PLR[17]) have emerged as reliable prognostic parameters in many cancers and inflammatory diseases. However, there has been controversy about the relationship between NLR, PLR, and body composition[18] and more studies are required.
Thus, we investigated the relationship between various serum inflammatory markers (hsCRP, WBC, NLR, and PLR) and body composition (VAT and SAT) accurately measured by abdominal computed tomography (CT) scans and compared the predictive values of inflammatory biomarker values in visceral obesity. We also used the VAT area to SAT area ratio (V/S ratio) at the L4–5 level in order to estimate the likelihood of visceral obesity in each subject (visceral V/S ratio ≥0.4; subcutaneous V/S ratio <0.4[19]) and evaluate the correlation with surrogate inflammatory marker levels.
2. Materials and methods
2.1. Study sample
Our study subjects were selected from 474,616 patients who visited the Severance Health promotion center or the Department of Family Medicine in Severance Hospital, in Seoul, South Korea, for health checkups between January 2008 and March 2017. Exclusion criteria were:
-
(1)
aged under 19 years or over 65 years;
-
(2)
non-Korean;
-
(3)
no CT scan to measure abdominal VAT;
-
(4)
diagnosis of hypertension, diabetes, dyslipidemia, thyroid dysfunction, or cancer; and
-
(5)
abnormal values for hsCRP (≥6.0 mg/L[20]) or WBC (≤4,000 or ≥10,000 cells/μL[21]).
After applying these criteria, 600 eligible adults were included in our study (Fig. 1), which complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Severance Hospital.
Figure 1.

Patient selection flow chart. hsCRP = high-sensitivity C-reactive protein, WBC = white blood cell.
Hypertension was defined as a history of taking anti-hypertensive medication, a resting systolic blood pressure (BP) ≥140 mm Hg, or a resting diastolic BP ≥90 mm Hg during at least two measurements. Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dL or a history of taking oral hypoglycemic agents or insulin. Dyslipidemia was defined as a total serum cholesterol level ≥240 mg/dL, low-density lipoprotein (LDL) cholesterol ≥160 mg/dL, or a history of taking lipid-lowering drugs.
2.2. Anthropometrics and biochemical variables
Body mass index was defined as body weight divided by the square of body height (kg/m2), and WC (cm) was measured at the umbilicus level at the narrowest point between the lower border of the rib cage and the uppermost border of the iliac crest during normal expiration while the patient was standing. Systolic and diastolic BP (mm Hg) was measured after 10 min of resting in a sitting position and was recorded as the average of three consecutive readings.
Intra-abdominal VAT and SAT areas were measured via fat measurement CT (Tomoscan 350, Philips; Mahwah, NJ, USA) as described previously.[22] Fat measurement CT is an imaging test widely used in clinical studies to assess the visceral and subcutaneous fat most accurately and conveniently with fewer slices and lesser radiation than typical abdomen and pelvis CT (APCT). The VAT and SAT areas were measured at the L4–5 level with 3 mm slice thickness in the supine position. We quantified the VAT area by defining the intra-abdominal cavity at the internal side of the abdominal and oblique muscle walls surrounding the cavity and the posterior aspect of the vertebral body. The V/S ratio (VAT area to SAT area ratio) at the L4–5[23] was then calculated. All measurements were verified by a skilled radiologist who was blinded to the patient data (see supplement figure, which illustrates the measurement of the VAT area, SAT area, and V/S ratio).
Lifestyle factors, including smoking status (pack-years) and alcohol consumption (average number of drinking days per week within the last year), were self-reported.
Blood samples were collected after an overnight fast (>12 h). Serum levels of glucose, total cholesterol, triglycerides (TG), high-density lipoprotein (HDL) cholesterol, LDL cholesterol, and hsCRP were measured with a Hitachi 7600 Automatic analyzer (High-Technologies Corporation, Hitachi; Tokyo, Japan). Total differential blood counts were recorded with an automatic blood counter system (ADVIA 120, Bayer; Whippany, NJ, USA). Patient NLR and PLR were calculated by dividing the total neutrophil count by the lymphocyte count and the total platelet count by the lymphocyte count, respectively.
Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria. Patients who met at least two of the following four criteria were diagnosed with metabolic syndrome:
-
(1)
abdominal obesity (WC >102 cm in men and >88 cm in women);
-
(2)
high TG levels (>150 mg/dL) or receiving treatment for dyslipidemia;
-
(3)
low HDL levels (<40 mg/dL for men and <50 mg/dL for women); and
-
(4)
high BP (systolic >130 mm Hg and/or diastolic >85 mm Hg) or using an anti-hypertensive medication.
2.3. Statistical analysis
Data were expressed as means and standard deviations. Normality of the variables was assessed with Kolmogorov–Smirnov tests. To examine the association among surrogate inflammatory markers (WBC, hsCRP, NLR, and PLR), metabolic parameters, and abdominal fat composition, analysis of covariance (ANCOVA) and trend analysis were performed after adjustments for age, sex, and BMI. The differences in absolute correlation coefficients between inflammatory markers and abdominal VAT area were determined using Steiger's Z tests while calculating the dependency for two correlation coefficients.[24] Additionally, multiple linear regression analysis with the Enter method was used to assess independent associations between abdominal adiposity indices and inflammatory markers. Statistical analysis was performed using SPSS version 20 (IBM Corp.; Armonk, NY, USA), and P values less than .05 indicated statistical significance.
3. Results
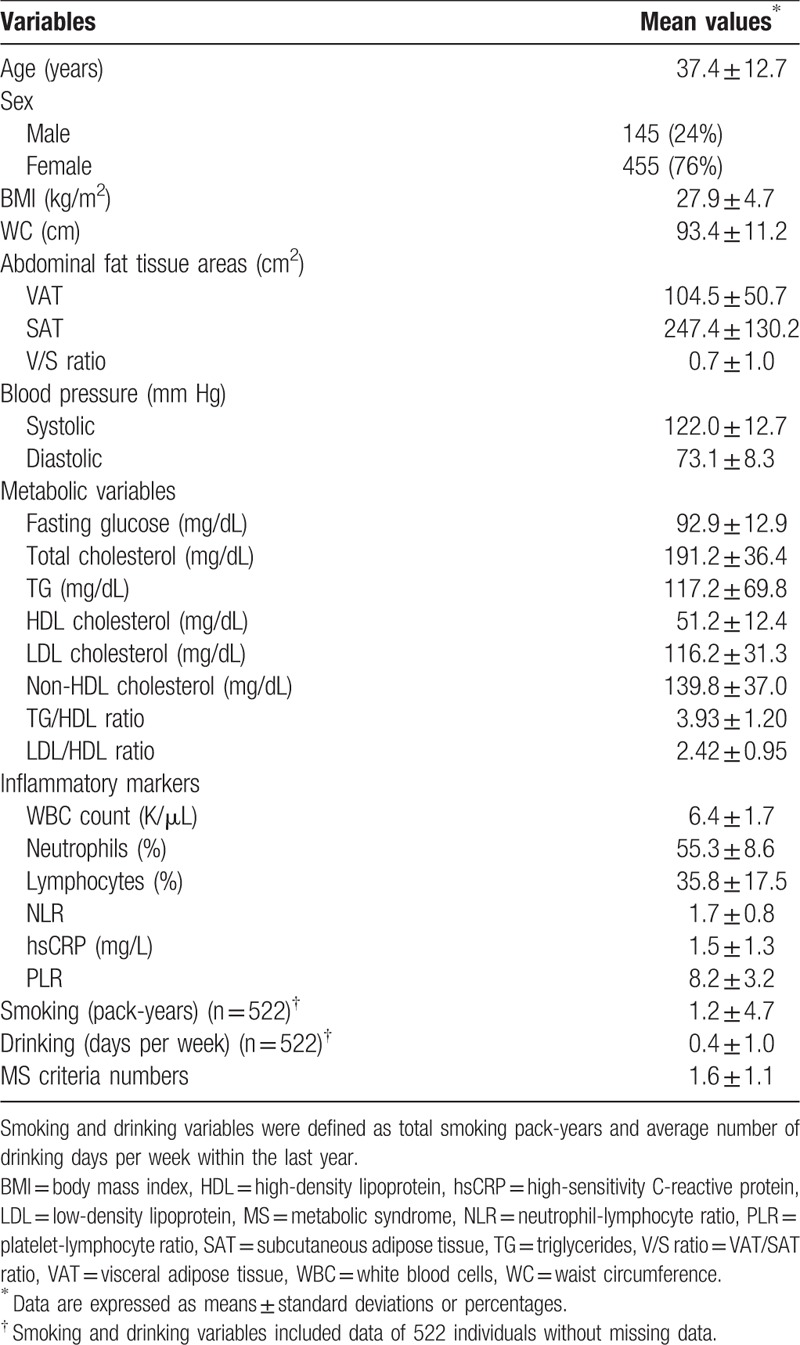
The patients’ demographic and clinical characteristics are shown in Table 1. The mean age was 37.4 years, and mean BMI was 27.9. The mean WBC count was 6400 cells/μL, and mean NLR and PLR were 1.7 and 8.2, respectively. The mean serum hsCRP concentration was 1.5 mg/L.
Table 1.
Demographic and clinical characteristics of study patients (n = 600).
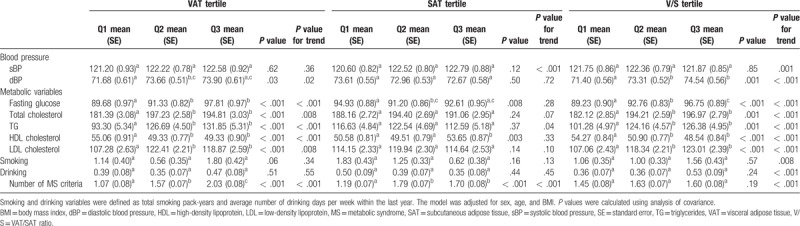
3.1. Associations between body composition and metabolic parameters
Table 2 shows the relationship between metabolic parameters and abdominal fat composition tertiles by ANCOVA and trend analysis. Diastolic BP, fasting glucose, total cholesterol, TG, LDL, and the mean numbers of metabolic syndrome criteria linearly increased with VAT and V/S values after adjusting covariates (overall P < .05 and trend P < .05). In contrast, HDL levels exhibited an inverse relationship with VAT area (overall P < .001 and trend P < .001) and V/S (overall P < .001 and trend P < .001).
Table 2.
Correlations between body composition and metabolic parameters.
3.2. Associations between abdominal fat composition and inflammatory markers
Figure 2 displays the mean levels of inflammatory markers by abdominal fat composition tertiles after adjusting for age, sex, and BMI. Covariate-adjusted mean WBC and hsCRP levels linearly increased with VAT area (overall P < .001 and trend P < .001) and V/S (overall P = .001 and trend P = .002; overall P < .001 and trend P < .001, respectively).
Figure 2.
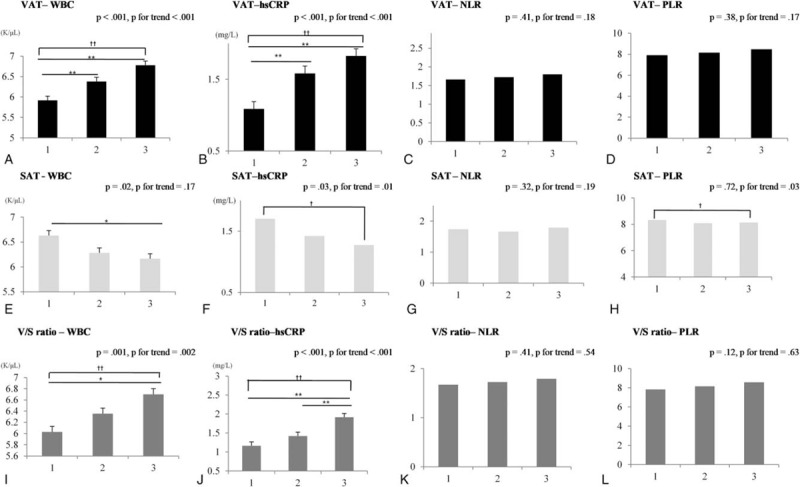
Inflammatory markers according to abdominal fat composition tertiles after adjusting for age, sex, and BMI. Mean (estimated) and standard error (indicated with error bars). ∗P < .05 and ∗∗P < .01 indicate significant differences among tertiles using analysis of covariance. †trend P < .05; ††trend P < .01. T, tertile. A, B, C, D: T1 (24.2–78.4), T2 (79.1–117.2), T3 (117.3–411) cm2; E, F, G, H: T1 (33–196.23), T2 (196.61–287.44), T3 (287.54–1189.11) cm2; I, J, K, L: T1 (0.08–0.3), T2 (0.3–0.48), T3 (0.48–10.82). hsCRP = high-sensitivity C-reactive protein, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio, SAT = subcutaneous adipose tissue, VAT = visceral adipose tissue, V/S ratio = VAT/SAT ratio; WBC, white blood cell.
However, covariate-adjusted mean hsCRP levels linearly decreased with SAT area (overall P = .03 and trend P = .01). Mean WBC counts showed similar results (overall P = .02 and trend P = .17), although no significant differences were noted for mean NLR and PLR levels in relation to VAT and V/S values.
3.3. Comparison of correlation coefficients of inflammatory markers and VAT area
We performed this comparison using Steiger's Z test with a model adjusted for age, sex, and BMI. Pearson's correlation analysis revealed a stronger association of VAT with WBC counts (r = 0.157, P < .001) than with levels of NLR (r = 0.108, P = .11; Steiger's Z test, P‡ = .04) and PLR (r = 0.036, P = .39; Steiger's Z test, P‡ = .003). However, the correlation coefficients for WBC and hsCRP levels (r = 0.159, P < .001, Steiger's Z test, P‡ = .97) and VAT area were not significantly different.
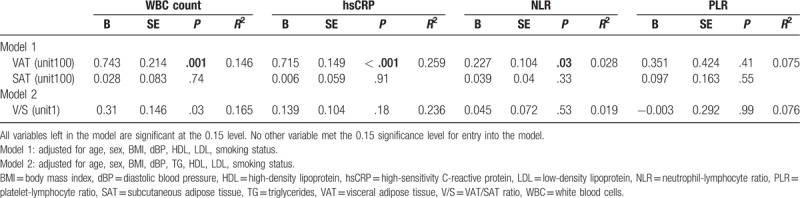
3.4. Independent associations between inflammatory markers and abdominal fat compositions
Table 3 shows the independent associations of inflammatory markers with abdominal fat compositions using multivariate-adjusted models from the Enter method for multiple linear regression analysis. VAT area showed significant associations with levels of WBC (P = .001), hsCRP (P < .001), and NLR (P = .03) after adjusting for confounding variables (age, sex, BMI, diastolic BP, HDL, LDL, and smoking status). V/S ratios were significantly associated with WBC (P = .03) but not with hsCRP levels (P = .18) after adjusting for the same confounding variables. We observed no significant association between SAT area and inflammatory markers after adjusting for covariates.
Table 3.
Multivariate linear regression analysis to determine relationships between abdominal fat parameters and inflammatory markers.
4. Discussion
We found that levels of certain surrogate inflammatory markers (WBC, hsCRP, and NLR) were independently associated with VAT, but not with SAT. Moreover, VAT area was more highly associated with WBC and hsCRP levels than with NLR or PLR after calculating correlation coefficients using Steiger's Z test.
A large body of evidence indicates that the regional distribution of body fat, rather than overall obesity, is linked to systemic inflammation,[25] insulin resistance, and oxidative stress.[26] Visceral fat is more metabolically active than subcutaneous fat[27] and affects the development of metabolic disturbances by contributing to the pro-inflammatory milieu (“meta-inflammation”[28]). In previous studies,[25,29] increased VAT showed a significant relationship with systemic inflammation. In line with the former study,[29] this study also assessed the association between various markers of systemic inflammation and visceral obesity.
Although the precise role of visceral fat in metabolic disturbance is unknown, various adipokines[30] and pro-inflammatory cytokines secreted by visceral adipocytes may be involved in altered metabolism.[31] Indeed, in vitro experiments have shown that VAT-derived adipocytes secrete more pro-inflammatory cytokines than SAT-derived adipocytes.[32] Also, as the central obesity level increases, the expression of IL-6 and MCP-1 expression has been manifested stronger in in vitro models.[33] Similar to these results, we found that abdominal VAT, but not SAT, area was independently associated with levels of WBC, hsCRP, and NLR.
Interestingly, mean hsCRP levels and WBC counts linearly decreased in relation to SAT in our study. Although the association between abdominal SAT area and inflammatory parameters has been controversial thus far, most previous studies have reported that SAT may have more protective[34,35] function in endocrine and inflammatory aspects than VAT. Further pathophysiological studies are required to elucidate the exact relationship between SAT and inflammatory markers, considering the anatomical division of subcutaneous fat between the superficial (sSAT) and deep (dSAT) layers.
The relationship between chronic low-grade inflammation, insulin resistance, and other obesity-associated metabolic disturbances has become increasingly recognized,[36] and various studies have tried to identify sensitive and reliable biomarkers of oxidative stress and systemic inflammation. Because tests for several serum inflammatory markers are inexpensive, widely available, and easy to interpret, they can serve as simple indicators of systemic inflammation, disease progression, and health outcomes.[37,38] Levels of WBC and hsCRP are well-known predictors of CVD,[39] and several epidemiological studies have linked these markers to various obesity parameters[40] and cardiovascular risk factors.[7] More recently, NLR and PLR have received attention as emerging inflammatory markers. High neutrophil and low lymphocyte counts represent the human physiologic immune response,[41] and platelet count increases during an acute inflammatory reaction.[42] To this end, NLR has been studied as a potential inflammatory biomarker in cardiac disorders,[43] gastrointestinal diseases, and malignancies,[44] while PLR has predicted mortality in patients with malignancies[45] and coronary artery disease.[46] However, the relationship between NLR and PLR and adiposity had not been investigated prior to our study. To our knowledge, no previous work has assessed which inflammatory markers are most closely correlated with abdominal visceral adiposity. We found that WBC and hsCRP levels are likely associated with visceral adiposity and are superior to NLR and PLR in this regard.
4.1. Limitations
Our study had several limitations. First, its observational cross-sectional design did not allow us to assess causality or temporality, and we could not exclude possible residual confounding factors. Second, selection bias may have influenced our results because the study sample only included data from a single hospital, which may not be representative of the general population or other races. Third, we did not assess various adipokines or pro-inflammatory mediators to clarify the relationship between visceral adiposity and inflammatory processes. Fourth, we did not distinguish dSAT from sSAT layers in the patients. Despite these limitations, this is the first study to investigate the relationship between multiple inflammatory biomarkers and abdominal adiposity precisely evaluated by CT to predict visceral obesity through comprehensive evaluation using various statistical approaches.
4.2. Future directions
Future investigations should clarify the possible mechanism between inflammatory markers, fat distribution, and chronic inflammation-related diseases. Also, longitudinal studies with larger datasets are needed in order to evaluate the best biomarker visceral obesity.
5. Conclusion
Visceral, but not subcutaneous, adipose tissue area is significantly and independently associated with levels of WBC, hsCRP, and NLR. In addition, VAT is more strongly correlated with WBC and hsCRP than with NLR and PLR.
Author contributions
Data curation: Ju-Yeon Yu, Hye-Sun Lee.
Formal analysis: Hye-Sun Lee.
Investigation: Ju-Yeon Yu, Won-Jun Choi, Hye-Sun Lee, Ji-Won Lee.
Methodology: Hye-Sun Lee.
Project administration: Ji-Won Lee.
Supervision: Won-Jun Choi, Hye-Sun Lee, Ji-Won Lee.
Writing – original draft: Ju-Yeon Yu.
Writing – review & editing: Won-Jun Choi, Hye-Sun Lee, Ji-Won Lee.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, HDL = high-density lipoprotein, hsCRP = high-sensitivity C-reactive protein, LDL = low-density lipoprotein, MS = metabolic syndrome, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio, SAT = subcutaneous adipose tissue, TG = triglycerides, VAT = visceral adipose tissue, V/S ratio = VAT/SAT ratio, WBC = white blood cells, WC = waist circumference.
This research was supported by the Bio and Medical Technology Development Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (NRF-2013M3A9B6046416, NRF-2018R1D1A1B07049223). This work was supported by the Technology Innovation Program (20002781, A Platform for Prediction and Management of Health Risk Based on Personal Big Data and Lifelogging) funded by the Ministry of Trade, Industry & Energy(MOTIE, Korea).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Johnson AR, Justin Milner J, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012;249:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011;300:E145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012;15:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Danesh J, Collins R, Appleby P, et al. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477–82. [DOI] [PubMed] [Google Scholar]
- [6].Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saijo Y, Kiyota N, Kawasaki Y, et al. Relationship between C-reactive protein and visceral adipose tissue in healthy Japanese subjects. Diabetes Obes Metab 2004;6:249–58. [DOI] [PubMed] [Google Scholar]
- [8].Farhangi MA, Keshavarz SA, Eshraghian M, et al. White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Health Popul Nutr 2013;31:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kitahara CM, Trabert B, Katki HA, et al. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev 2014;23:2840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ackermann D, Jones J, Barona J, et al. Waist circumference is positively correlated with markers of inflammation and negatively with adiponectin in women with metabolic syndrome. Nutr Res 2011;31:197–204. [DOI] [PubMed] [Google Scholar]
- [11].Rana JS, Arsenault BJ, Després J-P, et al. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur Heart J 2011;32:336–44. [DOI] [PubMed] [Google Scholar]
- [12].Faam B, Zarkesh M, Daneshpour MS, et al. The association between inflammatory markers and obesity-related factors in Tehranian adults: Tehran lipid and glucose study. Iran J Basic Med Sci 2014;17:577–82. [PMC free article] [PubMed] [Google Scholar]
- [13].Zeng Q, Dong S-Y, Sun X-N, et al. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz J Med Biol Res 2012;45:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr 2013;97:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen J, Deng Q, Pan Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio 2015;5:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Akboga MK, Canpolat U, Yuksel M, et al. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: a single center large-scale study. Platelets 2016;27:178–83. [DOI] [PubMed] [Google Scholar]
- [18].Richards CH, Roxburgh CS, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One 2012;7:e41883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- [20].Kablak-Ziembicka A, Przewlocki T, Sokołowski A, et al. Carotid intima-media thickness, hs-CRP and TNF-α are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis 2011;214:185–90. [DOI] [PubMed] [Google Scholar]
- [21].Liu KT, Lin TJ, Chan HM. Characteristics of febrile patients with normal white blood cell counts and high C-reactive protein levels in an emergency department. Kaohsiung J Med Sci 2008;24:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ryo M, Kishida K, Nakamura T, et al. Clinical significance of visceral adiposity assessed by computed tomography: a Japanese perspective. World J Radiol 2014;6:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sjöström L, Lönn L, Chowdhury B, et al. The sagittal diameter is a valid marker of the visceral adipose tissue volume. Prog Obes Res 1996;7:309–19. [Google Scholar]
- [24].Steiger JH. Tests for comparing elements of a correlation matrix. Psychol bull 1980;87:245–51. [Google Scholar]
- [25].Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010–3. [DOI] [PubMed] [Google Scholar]
- [26].Kelli HM, Corrigan FE, Heinl RE, et al. Relation of changes in body fat distribution to oxidative stress. Am J Cardiol 2017;120:2289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–8. [DOI] [PubMed] [Google Scholar]
- [28].Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obesity Rev 2012;13Suppl 2:30–9. [DOI] [PubMed] [Google Scholar]
- [29].Srinivasa S, Fitch KV, Torriani M, et al. Relationship of visceral and subcutaneous adipose depots to markers of arterial injury and inflammation among individuals with HIV. AIDS 2019;33:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Unamuno X, Gómez-Ambrosi J, Rodríguez A, et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest 2018;48:e12997. [DOI] [PubMed] [Google Scholar]
- [31].Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci 2010;1212:E1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004;145:2273–82. [DOI] [PubMed] [Google Scholar]
- [33].Ahluwalia A, Misto A, Vozzi F, et al. Systemic and vascular inflammation in an in-vitro model of central obesity. PLoS One 2018;13:e0192824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- [35].Pandzic Jaksic V, Sucic M. Multiple symmetric lipomatosis – a reflection of new concepts about obesity. Med Hypotheses 2008;71:99–101. [DOI] [PubMed] [Google Scholar]
- [36].Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 2008;14:1225–30. [DOI] [PubMed] [Google Scholar]
- [37].Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J 2013;40:17–29. [PMC free article] [PubMed] [Google Scholar]
- [38].Chmielewski PP, Strzelec B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol (Warsz) 2018;77:171–8. [DOI] [PubMed] [Google Scholar]
- [39].Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol Ther 2006;8:28–36. [DOI] [PubMed] [Google Scholar]
- [40].Dayal D, Jain H, Attri SV, et al. Relationship of high sensitivity C-reactive protein levels to anthropometric and other metabolic parameters in Indian children with simple overweight and obesity. J Clin Diagn Res 2014;8:PC05–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5–14. [PubMed] [Google Scholar]
- [42].Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol 2012;590:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther 2016;14:573–7. [DOI] [PubMed] [Google Scholar]
- [44].Bowen RC, Little NAB, Harmer JR, et al. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget 2017;8:32171–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, et al. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 2012;23:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Azab B, Shah N, Akerman M, et al. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis 2012;34:326–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


