Supplemental Digital Content is available in the text
Keywords: inflammation, Korean, migraine, psoriasis
Abstract
Both psoriasis and migraine are associated with inflammatory diseases. However, few studies have been conducted the increased risk of migraine in psoriasis patients. The aim of this study was to identify whether the psoriasis increases the risk of migraine. This study used the national cohort study data collected by the Korean Health Insurance Review and Assessment from 2002 to 2013. Patients with psoriasis (n = 11,071) and control participants (n = 44,284) were selected and matched 1:4 by age, sex, income, region of residence, and past medical history of hypertension, diabetes, and dyslipidemia. This study used Cox-proportional hazard model for calculating hazard ratio (HR) with crude and adjusted model. Stratification by age and sex was analyzed. Migraines occurred significantly more frequently in psoriasis patients than in control participants (adjusted HR = 1.16, 95% confidence interval (CI) = 1.04–1.31, P <.05). In the stratification analysis, migraines occurred significantly more frequently in psoriasis patients than in control participants only in the group of middle-aged males (adjusted HR = 1.62 95% CI = 1.22–2.13, P = .001). In conclusion, psoriasis might increase the risk of migraine.
1. Introduction
Psoriasis is a chronic multisystem inflammatory disease that mostly involves the skin and sometimes the joints. The clinical characteristics of psoriasis are symmetric erythematous plaques appearance with silvery scale of the skin.[1] The prevalence of psoriasis is approximately 2% to 3% in the worldwide population.[2,3] Because the occurrence of psoriasis varies depending on the distance from equator, the prevalence in Asian countries is relatively lower than that in Western countries.[4] The prevalence of psoriasis in Asian countries is reported to be 0.34% in Japan,[5] 0.3% in China,[6] and 0.44% to 0.45% in Korea.[7] Increasing evidence has suggested that psoriasis is highly associated with medical comorbidities including inflammatory diseases such as arthritis, cancer, and cardiovascular disease.[1,8,9]
Migraine is one of the common chronic disorders that affects approximately 10% to 20% of population.[10] The clinical symptoms of migraine are typically a unilateral, throbbing headache accompanied by photophobia, phonophobia, nausea, and disability.[11] Several studies have reported that migraine is associated with ischemic stroke and other cardiovascular diseases.[12,13] Recent studies have reported that psoriasis is also associated with an increased risk of migraine.[14,15]
The purpose of our study was to identify the association between the risk of migraine in psoriasis patients using data from a national cohort. We conducted an observational study, similar to previous studies.[14,15] In our study, we matched patients by their past medical history of hypertension, diabetes, and dyslipidemia as well as by other demographical factors, such as age, sex, income, and region of residence, in a ratio of 1 psoriasis patient to 4 control participants. We stratified the study population into age groups and sex groups to identify the specific association.
2. Methods
2.1. Data collection
The Ethics Committee of Hallym University (2014-I148) approved the use of these data. The Institutional Review Board exempted the requirement for written informed consent.
This national cohort study used data from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC). The Korean NHIS selects samples directly from the entire population database to prevent non-sampling errors. Approximately 2% of the samples (1 million) were selected from the entire Korean population (50 million). The selected data were classified into 1476 levels (age [18 categories], sex [2 categories], and income level [41 categories]) using randomized stratified systematic sampling methods via proportional allocation to represent the entire population. A previous study verified the appropriateness of the sample after data selection.[16] The National Health Insurance Sharing Service provides a detailed description of the methods used to perform these procedures (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do). This cohort database included
-
(i)
personal information,
-
(ii)
health insurance claim codes (procedures and prescriptions),
-
(iii)
diagnostic codes using the International Classification of Disease-10 (ICD-10),
-
(iv)
death records from the Korean National Statistical Office (using the Korean Standard Classification of disease),
-
(v)
socioeconomic data (residence and income), and
-
(vi)
medical examination data for each participant from 2002 to 2013 (11 years).
All Korean citizens are identified by a 13-digit resident registration number from birth to death. Therefore, exact population statistics can be determined using this database. All Koreans are required to enroll in the NHIS. All Korean hospitals and clinics use the 13-digit resident registration number to register individual patients in the medical insurance system. Therefore, the risk of overlapping medical records is minimal, even if a patient moves from 1 place to another. All medical treatments in Korea are tracked without exception using the Health Insurance Review & Assessment (HIRA) system. In Korea, the law states that a notice of death must be provided to an administrative entity before a funeral can be held. Medical doctors record the date and cause of death on a death certificate.
2.2. Definitions of psoriasis and migraine
The participants who had an ICD-10 code of L40 twice or more were defined as having psoriasis. The participants were defined as having migraines if they had an ICD-10 of G43 twice or more.
2.3. Setting and participants
To handle confounding factors in observational studies, data processing and analyses such as matching, adjustment, and stratification can be used.[17] Although we conducted an observational study, we not only used statistical adjustments but also used matching to examine the independent association between psoriasis and migraine. Through these study methods, we were able to determine an independent association with our findings.
Out of 1,125,691 participants with 114,369,638 medical claim codes, psoriasis patients (n = 12,921) were matched 1:4 with participants (control group) who never had a diagnosis of psoriasis from 2002 through 2013. We included 4 times of control group to increase the statistical power. The control group was selected from the overall population (n = 1,112,770). The participants were matched for age, sex, income group, region of residence, and past medical history (hypertension, diabetes, and dyslipidemia). To prevent selection bias when selecting the matched participants, the control group participants were sorted using a random number order, and they were then selected from top to bottom. It was assumed that the matched control participants were involved at the same time as each matched psoriasis patient (index date). Therefore, participants in the control group who died before the index date were excluded. Among both psoriasis patients and control participants, participants who had a history of migraine before the index date were excluded. In the psoriasis group, 376 participants were excluded. The psoriasis patients for whom we could not identify enough matching participants were also excluded (n = 33). We excluded participants who were younger than 20 years old (n = 1441). Finally, 1:4 matching resulted in the inclusion of 11,071 psoriasis patients and 44,284 control participants (Fig. 1). However, they were not matched for a history of ischemic heart disease or stroke.
Figure 1.
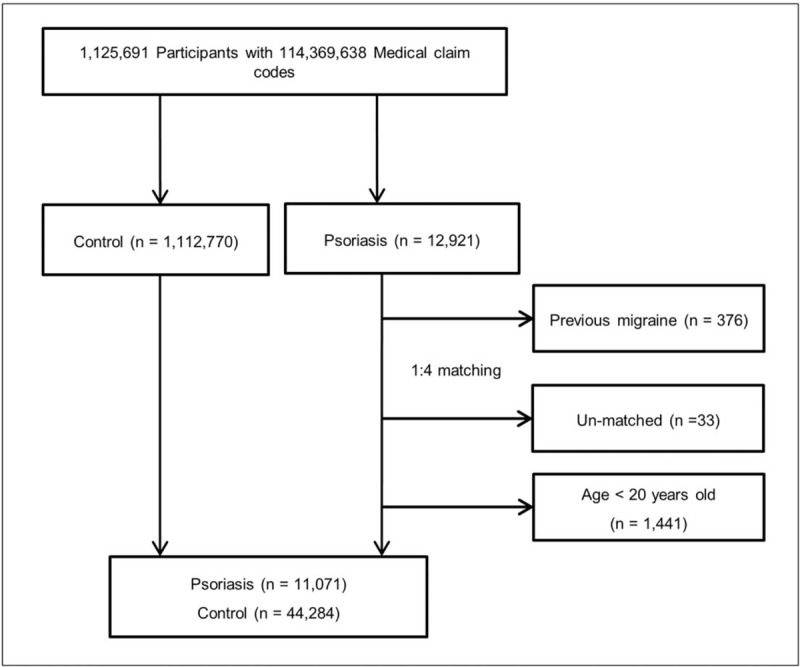
A schematic illustration of the participant selection process that was used in the present study. Out of a total of 1,125,691 participants, 11,071 psoriasis patients were matched with 44,284 control participants by age, sex, income group, region of residence, and past medical history.
2.4. Socioeconomic status and past medical history
The age groups were classified using 5-year intervals: 20 to 24, 25 to 29, 30 to 34…, and 85+ years old. A total of 14 age groups were designated. The income groups were initially divided into 41 classes (1 health aid class, 20 self-employment health insurance classes, and 20 employment health insurance classes). These groups were recategorized into 11 classes (class 1 [lowest income]-11 [highest income]). Participants’ regions of residence were divided into 16 areas according to administrative districts. These regions were regrouped into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju) areas.
Participants’ past medical history was evaluated using ICD-10 codes. For the accuracy of diagnosis, participants were considered to have hypertension (I10 and I15), diabetes (E10-E14), or dyslipidemia (E78) if they were diagnosed ≥2 times. Participants were considered to have ischemic heart disease (I24 and I25) or stroke (I60-I66) if they were diagnosed ≥1 time.
2.5. Statistical analysis
The chi-square test was used to compare the general characteristics between participants in the psoriasis and control groups.
To analyze the hazard ratio (HR) of psoriasis on migraine, a Cox-proportional hazard model was used. In this analysis, crude and adjusted (age, sex, income, region of residence, hypertension, diabetes, dyslipidemia, ischemic heart disease, and stroke) model were used to determine the independent association between psoriasis and migraine.
For the stratification analysis, we divided the participants by age (20–39 years old, 40–59 years old, and 60+ years old) and sex (male and female).
Two-tailed analyses were conducted, and P values less than .05 were considered to indicate significance. The results were statistically analyzed using SPSS v. 21.0 (IBM, Armonk, NY).
3. Results
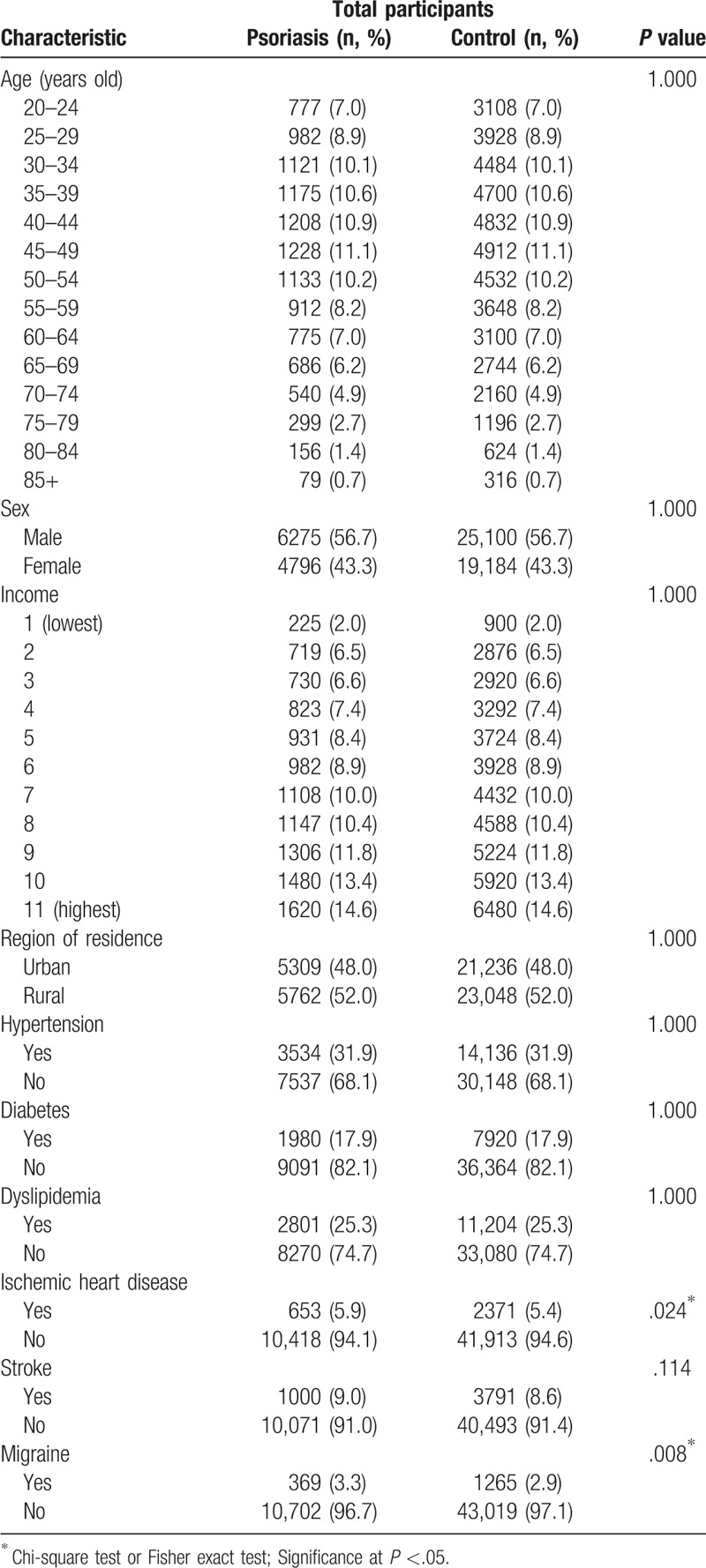
The rate of migraine was higher in psoriasis patients (3.3%, 369/11,071) than in control participants (2.9%, 1265/44,284). The distributions of age, sex, income, region of residence, and medical history of hypertension, diabetes, and dyslipidemia were comparably matched between the psoriasis and control groups (Each P = 1.000, Table 1). The rate of ischemic heart disease and migraine was higher in psoriasis patients than in control participants (Each P <.05, Table 1).
Table 1.
General participant characteristics.
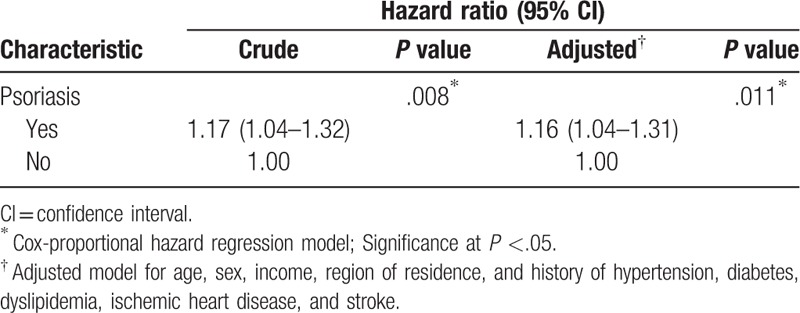
The crude HR of psoriasis for migraine was 1.17 (95% confidence interval (CI) = 1.04–1.32, P = .008, Table 2) and adjusted HR of psoriasis for migraine was 1.16 (95% CI = 1.04–1.31, P = .011, Table 2).
Table 2.
Crude and adjusted hazard ratios (95% confidence intervals) of psoriasis for migraine.
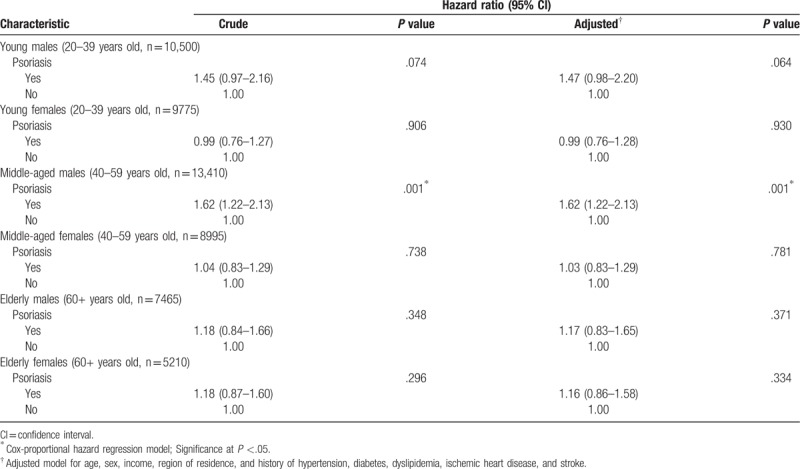
Through the stratification analysis by age and sex, only participants in the group of middle-aged (40–59 years old) male were found to have an association between psoriasis and an increased risk of migraine based on both crude and adjusted HRs (crude HR = 1.62, 95% CI = 1.22–2.13, P = .001; adjusted HR = 1.62, 95% CI = 1.22–2.13, P = .001, Table 3).
Table 3.
Stratification analysis of crude and adjusted hazard ratios (95% confidence intervals) of psoriasis for migraine according to age and sex.
4. Discussion
Higher proportion of migraines was shown in psoriasis patients in our study. Egeberg et al have suggested that psoriasis is associated with the risk of migraine in their cohort study.[14] Capo et al also found that migraines with aura occurred significantly more frequently in participants in a psoriasis with migraine group than in those in a migraine control group.[15] Unlike these previous studies, our study matched 4 control participants to each psoriasis patient by age, sex, income, region of residence, and cardiovascular risk factors and considered confounding factors to perform an appropriate statistical method. Moreover, we also stratified the subgroup not only by sex but also by age in the same proportions for each condition.
Although the definitive mechanism by which psoriasis increases the risk of migraine is unclear, endothelial inflammation may play a potential role in the link. One of the possible pathophysiologic mechanisms of psoriasis is that activation of plasmacytoid dendritic cells (DCs) triggers psoriasis. This activation produces tumor necrosis factor (TNF)-α, interleukin (IL)-12, and IL-23, which are proinflammatory cytokines that lead to systemic inflammation. The axis of TNF-α, IL-12, and IL-23 play a role in the development of psoriasis lesions.[18] In connection with this mechanism, increasing evidence has suggested that psoriasis patients may have endothelial dysfunction due to the effects of TNF-α.[19] Likewise, neuropeptide actions on the endothelium are a mechanism of migraine.[20] Therefore, inflammation-induced endothelial dysfunction may be the potential mechanism linking psoriasis and migraine.
Nervous system involvement may be another possible similarity between psoriasis and the risk of migraine. A number of clinical and histological observations suggest that neuropeptides contribute to the development of psoriasis.[21] Neuropeptides including substance P (SP) and calcitonin gene-related peptide (CGRP) modulate the immune system during the development of psoriasis.[22] In addition, according to recent evidence, CGRP, and SP are known triggers of migraine. These neuropeptides are released by cortical spreading depression. Eventually, these neuropeptides sensitize the trigeminal meningeal nociceptors in patients with migraines.[20] Thus, neuropeptides might be possible risk factors for migraine in psoriasis patients. Perhaps, psoriasis and migraine might be mutually associated if these neuropeptides are mediators involved in both mechanisms. Further study is warranted to determine a definitive association between these 2 diseases.
In the stratification analysis, psoriasis was associated with an increased risk of migraine only in the group of middle-aged males. In our study population, because the prevalence of psoriasis patients was higher in male patients and in the age 45 to 49 group, the higher proportion of migraines in patients in this subgroup may be the reason for this statistically significant result. In contrast to our study, a previous study reported conflicting results that psoriasis was associated with an increased risk of migraine in both males and females.[14] According to this prior study, the participants were adjusted by medication use such as cholesterol-lowering drugs. Males are more commonly exposed to unhealthy lifestyle factors, such as an unhealthy diet and substance use, than females.[23–25] Although we did not consider these unhealthy lifestyle factors in our study, these may be a potential confounding factors in participants with psoriasis and migraines. Additional larger and longitudinal randomized controlled studies are needed to confirm whether psoriasis is associated with an increased risk of migraine in particular sex and age groups.
The strengths of our study are as follows. First, we included a large population of psoriasis patients from a national cohort for higher statistical power. Second, our study had a long-term follow-up period so that we could identify the relationship between psoriasis and migraine. Third, we matched 1 psoriasis patient with 4 control participants by several confounding factors to independently identify the difference between psoriasis patients and control participants. Finally, we used an adjustment model with several confounding factors to investigate the independent association between psoriasis and migraine. For example, hypertension, diabetes, dyslipidemia, ischemic heart disease, and stroke were adjusted because both psoriasis and migraines are associated with inflammatory diseases.[12,18] Especially, we adjusted ischemic heart disease because the association between ischemic heart disease and migraine were reported in the previous study.[26]
Several limitations should be considered when interpreting the outcomes of our study. First, because our study was conducted by using a national cohort collected by NHIS, we could not consider certain variables for our purposes. For example, although psoriasis and migraine are closely associated with inflammatory condition such as obesity, smoking, alcohol, and exercise, these were not included in the data. Although various confounding factors were adjusted in the model, the study outcomes might be affected by the other confounding factors that we did not consider. Second, because of the lack of migraine patients in our study, migraine subtypes, such as migraine with aura and migraine without aura, were not distinguished. Third, our findings should be carefully interpreted because of the definition of migraine that was used. We defined migraine patients as those who had an ICD-10 code of G43 recorded twice or more. According to a previous Korean nationwide cohort study, the prevalence of migraine was approximately 6%.[27] Because only 24.4% of migraine patients are evaluated at the hospital,[28] the actual proportion of migraine patients is approximately 1.5%. In our study, the proportion of migraine patients was 1.2% because we defined migraine as a diagnosis of migraine that was recorded twice or more at a hospital. Thus, this proportion of migraine patients might be less than the actual prevalence of migraine patients. Finally, our study design was an observational study; thus, the ability to determine definitive causality was limited. Further intervention studies controlling for lifestyle factors should be examined.
We suggest that psoriasis might increase the risk of migraine, specifically in middle-aged males.
Table S1 and Table S2.
Author contributions
Conceptualization: Hyo Geun Choi.
Data curation: Hyoseob Lim.
Formal analysis: Jae-Sung Lim.
Methodology: Songyong Sim.
Writing – original draft: Chanyang Min, Hyo Geun Choi.
Writing – review & editing: Songyong Sim, Hyo Geun Choi.
Hyo Geun Choi orcid: 0000-0003-1655-9549.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, CGRP = calcitonin gene-related peptide, HIRA = Health Insurance Review & Assessment, HR = hazard ratio, ICD-10 = International Classification of Disease-10, IL = interleukin, NHIS-NSC = Korean National Health Insurance Service-National Sample Cohort, SP = substance P, TNF = tumor necrosis factor.
CM and HL equally contributed in this study.
This work was supported by the Hallym University Research Fund and in part by a research grant (NRF-2018-R1D1A1A02085328) from the National Research Foundation (NRF) of Korea and Hallym Research Fund (HURF-2018-35).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician 2017;63:278–85. [PMC free article] [PubMed] [Google Scholar]
- [2].Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014;70:512–6. [DOI] [PubMed] [Google Scholar]
- [3].Christophers E. Psoriasis− epidemiology and clinical spectrum. Clin Exp Dermatol 2001;26:314–20. [DOI] [PubMed] [Google Scholar]
- [4].Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–85. [DOI] [PubMed] [Google Scholar]
- [5].Kubota K, Kamijima Y, Sato T, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015;5:e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yip SY. The prevalence of psoriasis in the Mongoloid race. J Am Acad Dermatol 1984;10:965–8. [DOI] [PubMed] [Google Scholar]
- [7].Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: a population-based epidemiological study using the Korean National Health Insurance Database. Ann Dermatol 2017;29:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu SC-S, Lan C-CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci 2017;18:E2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Br J Dermatol 2011;165:1037–43. [DOI] [PubMed] [Google Scholar]
- [10].Lipton RB, Bigal ME. The epidemiology of migraine. Am J Med Suppl 2005;118:3–10. [DOI] [PubMed] [Google Scholar]
- [11].MacGregor EA. Migraine. Ann Intern Med 2017;166:Itc49–itc64. [DOI] [PubMed] [Google Scholar]
- [12].Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018;8:e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Egeberg A, Mallbris L, Hilmar Gislason G, et al. Increased risk of migraine in patients with psoriasis: a Danish nationwide cohort study. J Am Acad Dermatol 2015;73:829–35. [DOI] [PubMed] [Google Scholar]
- [15].Capo A, Affaitati G, Giamberardino MA, et al. Psoriasis and migraine. J Eur Acad Dermatol Venereol 2018;32:57–61. [DOI] [PubMed] [Google Scholar]
- [16].Lee J, Lee JS, Park SH, et al. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2016;46:e15. [DOI] [PubMed] [Google Scholar]
- [17].Gordis L. Epidemiology, 4th edition. United States of America: Saunders; 2009:253. [Google Scholar]
- [18].Furue M, Tsuji G, Chiba T, et al. Cardiovascular and metabolic diseases comorbid with psoriasis: beyond the skin. Intern Med 2017;56:1613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brezinski EA, Follansbee MR, Armstrong EJ, et al. Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 2014;20:513–28. [DOI] [PubMed] [Google Scholar]
- [20].Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol 2018;40:301–14. [DOI] [PubMed] [Google Scholar]
- [21].Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol 2006;155:876–82. [DOI] [PubMed] [Google Scholar]
- [22].Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol 2018;40:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dawson KA, Schneider MA, Fletcher PC, et al. Examining gender differences in the health behaviors of Canadian university students. J R Soc Promot Health 2007;127:38–44. [DOI] [PubMed] [Google Scholar]
- [24].Ek S. Gender differences in health information behaviour: a Finnish population-based survey. Health Promot Int 2015;30:736–45. [DOI] [PubMed] [Google Scholar]
- [25].Olson JS, Hummer RA, Harris KM. Gender and health behavior clustering among U.S. young adults. Biodemography Soc Biol 2017;63:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sacco S, Kurth T. Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep 2014;16:524. [DOI] [PubMed] [Google Scholar]
- [27].Kim BK, Chu MK, Lee TG, et al. Prevalence and impact of migraine and tension-type headache in Korea. J Clin Neurol 2012;8:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roh JK, Kim JS, Ahn YO. Epidemiological and clinical characteristics of migraine in Korea. J Korean Neurol Assoc 1997;15:1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


