Abstract
To investigate the age-sex-specific incidence and relative risk of pyogenic liver abscess (PLA) in patients with type 2 diabetes mellitus (T2DM), and to assess the joint effects of T2DM and other clinical risk factors for PLA on PLA incidence. We used a population-based cohort design with Taiwan's National Health Insurance claim data. Study subjects included 613,921 T2DM patients and 614,613 controls identified in 2000 and were followed to the end of 2010. Cox regression model was employed to calculate the hazard ratio (HR) and 95% confidence interval (CI) of PLA in relation to T2DM. Over an 11-year follow-up, 5336 T2DM and 1850 controls were admitted for PLA, representing a cumulative incidence of 0.87% and 0.30%, respectively. T2DM was significantly associated with increased hazard of PLA (HR, 2.88; 95% CI, 2.73–3.04). We also found that age and gender may significantly modify the relationship between T2DM and PLA, with a higher HR noted in males patients and those aged <45 years. Biliary tract diseases (HR, 8.60; 95% CI, 7.87–9.40) and liver cirrhosis (HR, 7.52; 95% CI, 6.58–8.59) may add substantially additional risk to the incidence of PLA in T2DM patients. The increased risk of PLA in T2DM was greater in male and younger patients. Careful management of biliary tract diseases and liver cirrhosis may also help reduce the incidence of PLA in T2DM patients.
Keywords: biliary tract diseases, diabetes, incidence, liver cirrhosis, pyogenic liver abscess
1. Introduction
Pyogenic liver abscess (PLA) is a devastating condition and usually results in a high case-fatality rate.[1] Previous studies reported that biliary tract disease, abdominal infections, surgical procedures, and liver cirrhosis were associated with increased risks of PLA.[2,3] Among the pathogens that could cause liver abscess, Escherichia coli has been found to be the most common one worldwide.[4,5] In Taiwan, on the other hand, Klebsiella pneumoniae is among the primary pathogens that contribute to PLA incidence, in which only a few strains exhibited resistance to the commonly used antibiotics. The use of ultrasound- or CT-guided percutaneous drainage combined with antibiotics was an appropriate way to treat the liver abscesses of these patients.[6] The infection of K pneumonia has been considered as an endemic disease in Taiwan over the past 3 decades since 1986when it was first noted.[6–8]
Liver abscess associated with K pneumoniaeis characterized by high rates of metastatic infections (9.5%–20.0%)[7,8], mortality (6.0%–11.3%).[8,9] A recent Taiwanese study also reported a significantly greater risk of delayed-onset primary liver cancer (PLC) including hepatocellular carcinoma (HCC) (1.5-fold) and intrahepatic cholangiocarcinoma (ICC) (11-fold) in patients with PLA.[10] A previous Taiwanese study found that liver abscesses caused by K pneumoniae were more strongly associated with diabetes mellitus (DM)or impaired fasting glucose than liver abscesses caused by non-K pneumoniae (70.2% vs 32.5%).[11] It is known that both cell-mediated and humoral immune responses in patients with DM may result in increased susceptibility to infectious diseases.[12,13] The association of DM with PLA has been well-recognized.[8,11,14,15] The prevalence of DM in patients with PLA was high at 33% to 58% in Taiwan[14,16], 30% in China[17], 31% in Canada[18], and 11.2% in Denmark.[19]
Despite DM has been considered as a risk factor for incidence and prognosis of PLA, little is known concerning whether age and sex may modify the relationships between DM and PLA. Additionally, risk of PLA incidence in DM patients who also suffered from other clinical risk factors for PLA has also not been fully illustrated.[20] Using Taiwan's National Health Insurance (NHI) database that included medical claims of the entire Taiwanese population, we carried out this population-based study to examine the risk of PLA in patients with type 2 diabetes mellitus (T2DM), according to various age and sex stratifications. We hypothesized that younger patients with type 2 diabetes are more vulnerable to increased risk of PLA than the older ones. Besides, we further assessed the risk of newly diagnosed PLA in relation to T2DM with and without selected clinical risk factors for PLA.
2. Methods
2.1. Source of data
A universal NHI program, which is administered by the National Health Insurance Administration (NHIA), Department of Health and Social Welfare, has been implemented in Taiwan since March 1995. Data analyzed in this study were retrieved from Taiwan's NHI database, a medical claim database that stores the medical records of beneficiaries that are uploaded by medical institutions to obtain reimbursement from NHIA. Taiwan's NHI program universally covers medical insurance for nearly all (>99%) Taiwanese citizens (prisoners and military personnel were exempted in our study period).[21] The National Health Research Institute (NHRI) cooperates with the NHIA to establish the NHI database. Access to the NHI database has been approved by the Review Committee of the NHRI. To ensure the accuracy of the claim files, the NHIA performs expert reviews on a random sample of every 50 to 100 ambulatory and inpatient claims in each hospital and clinic quarterly.[21] The approval informed consent from the patients was not needed because personal identification number for each study subject has been encrypted in the NHI database.
2.2. Design and study groups
This is a medical claim-based cohort study. Details of claim data analyzed and methods of selection of diabetes and control groups were described in detail in our previous reports.[22,23] Briefly, we identified a patient with T2DM if she or he had an initial diabetes diagnosis (International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM] code: 250. × 0 or 250. × 2) at any time in 2000, and then experienced another 1 or more diabetes diagnosis within the subsequent 12 months. To avoid accidental inclusion of miscoded patients, we further selected only those with the first and last outpatient visits at least 30 days apart.[22,24] The index date for case subjects was the date of first-time diagnosis of diabetes in 2000, while the index date for subjects in the control group was the first date of enrollment to NHI. If his/her first date of enrollment was before 1 January 2000, the index date was set as 1 January 2000.
The original study subjects consisted of 615,532 T2DM patients and 614,871 age- and sex-matched control subjects randomly selected from the registry of beneficiaries. In this study, diabetes and control subjects with a prior diagnosis of PLA ((ICD-9-CM): 572.0) from 1 January 1997 to the index date were excluded. The final cohort consisted of 613,921T2DMpatients and 614,613 control subjects. The flow chart of study subjects enrollment and follow-up is shown in Figure 1.
Figure 1.
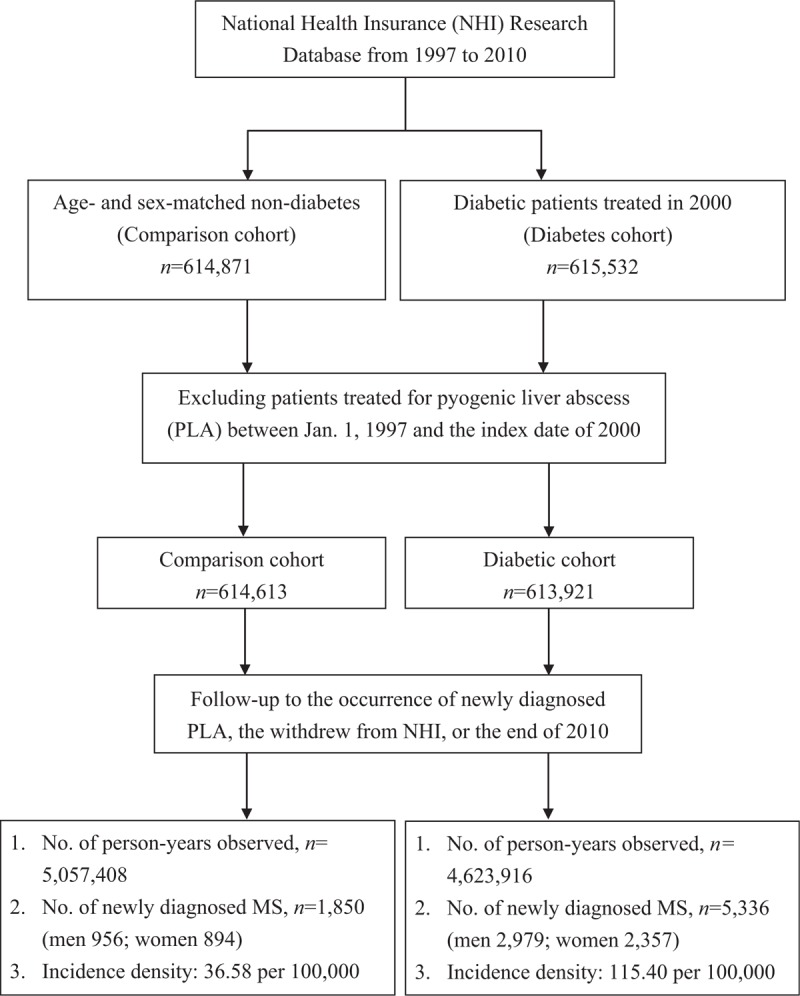
Flow chart of study subjects enrollment and follow-up.
We identified the first diagnoses of PLA from inpatient claims from 2000 to 2010 as the study end point. Only those with end point onset 1 year after the index date were counted in order to establish the temporal link between diabetes and PLA. All study subjects were followed from the index date to occurrence of end point, withdrawal from the NHI, or 31 December 2010, whichever date came first, and the later 2 dates were considered as censoring observations.
2.3. Clinical risk factors for PLA
A number of clinical risk factors for PLA were considered in the analysis, including diverticulitis (ICD-9-CM: 562.11, 562.13), acute appendicitis (ICD-9-CM: 540-542), biliary tract diseases (ICD-9-CM: 574-576), and liver cirrhosis (ICD-9-CM: 571.5). Information of the above clinical risk factors was identified from in-patient and ambulatory care claims for both T2DM and control subjects from 1997 to the onset of PLA or to the end of 2010, whatever came first. We counted the above clinical risk factors that occurred to individuals in both groups only when the dates of diagnosis for the selected clinical risk factors were noted before or on the day on which the study subjects’ endpoints or censoring took place.
2.4. Statistical analysis
The geographic area of each member's NHI unit, either the location of employment or residential area, was grouped into 4 geographic areas (North, Central, South, East) or 2 urbanization statuses (urban and rural) according to the National Statistics of Regional Standard Classification.[22,23]
In the statistical analyses, the age- and gender-specific incidence density was first calculated with person-years as the denominator under the Poisson assumption. To assess the independent effect of diabetes on the risk of PLA, we conducted Cox proportional hazard regression models with age, sex, geographic area, and urbanization statuses adjusted simultaneously in the model. The latter 2 geographic variables were adjusted for possible geographic variations of cancer incidence and mortality in Taiwan.[25] Moreover, we also assessed the relative hazards of PLA in relation to T2DM accompanied by each risk factor with Cox proportional hazard regression models with age, gender, geographic area, urbanization statuses simultaneously adjusted in the regression model. The study subjects who died in hospitals without a mention of PLA on the list of up to 5 discharge codes were censored in the survival analysis, and the date of censoring was the date of death. If a study subject did not encounter in-hospital mortality, the date of censoring was either the date of his/her last withdrawal from NHI or the date of termination of the study, that is, December 31, 2010. The interaction term of type 2 diabetes and age (or sex) was included in the model to assess the potential effect-modification by age and sex. No management of missing information on some variables was performed because it was very few. All statistical analyses were performed with SAS (version 9.2; SAS Institute, Cary, NC). A P value <.05 was considered statistically significant.
3. Results
3.1. Characteristics of study subjects
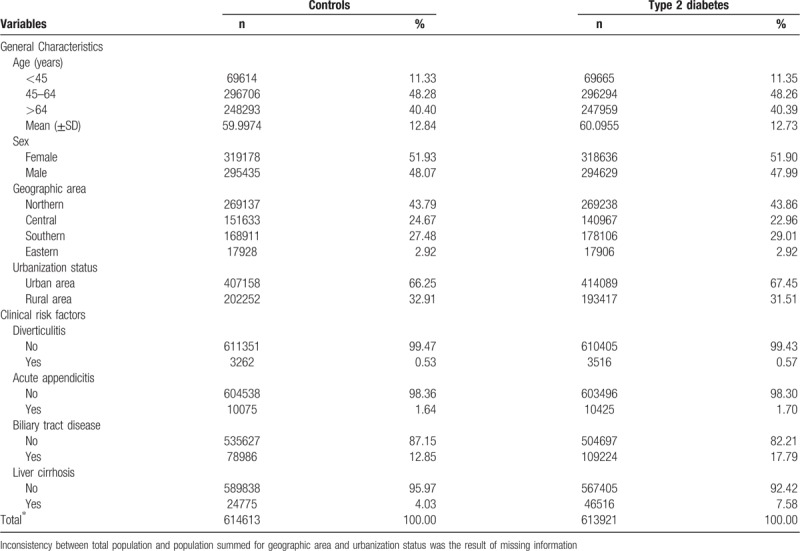
T2DM and control groups were comparable with respect to age, sex, geographic area, and urbanization status. In addition, patients with diabetes had higher prevalence rates of all selected clinical risk factors for PLA, with a notable difference for biliary tract disease (17.79% vs 12.85%) and liver cirrhosis (7.58% vs 4.03%) (Table 1).
Table 1.
Characteristics of the study subjects.
3.2. Overall and age-sex-specific incidence of PLA
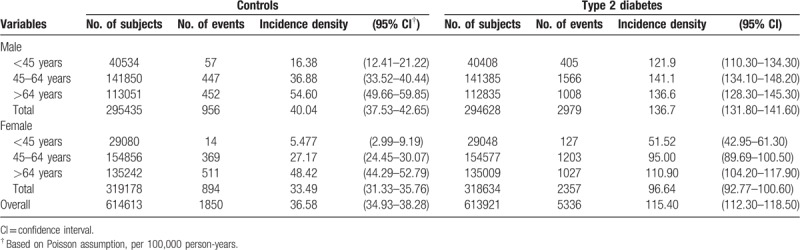
Over an11-year follow-up, 5336 T2DM patients and 1850 controls were treated for PLA, representing a cumulative incidence rate (CIR) of 0.87% and 0.30%, respectively. The overall incidence density of PLA for diabetes and controls was 115.40 per 100,000 person-years and 36.58 per 100,000 person-years, respectively. Additionally, patients with T2DM consistently experienced higher incidence density of PLA than controls, regardless of age and gender. The sex-specific incidence density showed a higher rate of PLA in men than in women irrespective of T2DM status. The incidence density also increased with age, with an exception that the incidence densities for male T2DM aged 45 to 64 years and those aged >64 years were similar (Table 2).
Table 2.
Overall and age- and sex-specific incidence density of pyogenic liver abscess in the diabetic and control groups.
3.3. Relative risk of PLA in association with T2DM and co-morbidity
Compared to controls, patients with T2DM were at a significantly increased hazard of PLA incidence by a moderate to high magnitude (HR = 3.13, 95% CI = 2.97–3.30). The increased HR sustained after the covariates were adjusted. The HR reduced to 2.88 (95% CI = 2.73–3.04) after further adjustment for selected clinical risk factors for PLA. The sex-specific adjusted HR of PLA incidence in association with T2DM was significantly higher in men than in women (3.11 vs 2.64; P for interaction = .0021). Moreover, there was also a significant interaction of age with T2DM on the risk of PLA in both men (P for interaction <.0001) and women (P for interaction <.0001). In men, the age-specific HR was highest for male T2DMpatients aged <45 years (HR = 6.74, 95% CI = 5.09–8.93), which declined to 3.43 (95% CI = 3.08–3.82) and 2.33 (95% CI = 2.09–2.61) for older men. Similar negative age gradient relationship was also noted for women with T2DM, with the corresponding figures of 8.39 (95% CI = 4.82–14.61), 3.12 (95% CI = 2.78–3.51), and 2.12 (95% CI = 1.90–2.36) (Table 3).
Table 3.
Overall and age- and sex-specific relative hazards of pyogenic liver abscess in the type 2 diabetes and control groups.
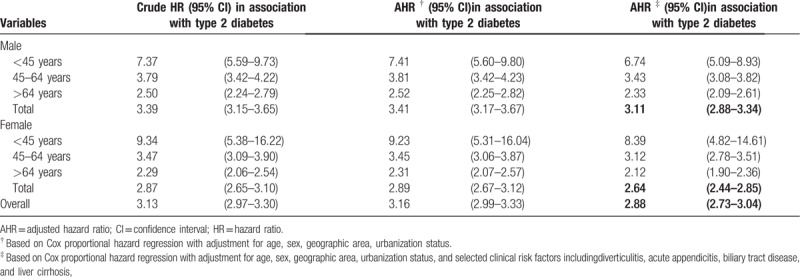
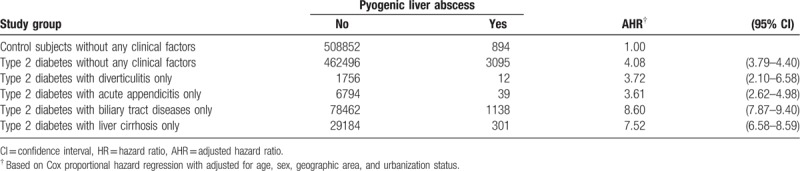
Table 4 shows the contribution of each clinical risk factor for PLA to the risk of PLA in T2DM patients. In the absence of those clinical risk factors, T2DM was associated with an elevated HR of PLA (4.08, 95% CI = 3.79–4.40). Certain factors including biliary tract diseases and liver cirrhosis were found to add substantially additional risk to the risk of PLA in patients with T2DM, with an HR of 8.60 (95% CI = 7.87–9.40), and 7.52 (95% CI = 6.58–8.59), respectively.
Table 4.
Covariate adjusted relative hazard of pyogenic liver abscess in relation to type 2 diabetes accompanied by selected clinical risk factors.
4. Discussion
With a large study cohort and 11-year follow-up, we noted that patients with T2DM were at a significantly increased hazard of PLA (HR = 2.88), especially in male and younger T2DM patients. A novel finding of our study was that we noted very high relative hazards of PLA in association with biliary tract diseases and liver cirrhosis, known risk factors for PLA, in patients with diabetes.
The reported annual incidence rates of PLA are low in many countries, being 2.3 per 100,000 person-years in Canada from 1999 to 2003 according to a population-based surveillance.[3] In a nationwide study spanning a 26-year period in Denmark, the annual incidence rate of PLA was 1.1 per 100,000 person-years from 1997 to 2002.[26] Another population-based study from United States reported that the annual incidence of hospitalization for PLA was 3.6 per 100,000 people between 1994 and 2005.[27] The differences in risk factors for PLA, including the prevalence of hepatobiliary disease such as cholangitis between American and Asian populations were ever mentioned to explain the higher incidence in Asian populations.[27] Compared with the figures reported in Western nations, the incidence of PLA noted in our study was much higher, which could be due to the spread of a pathogenic clone.[7] Several reports in Taiwan indicated that diabetes is the major predisposing factor of PLA and that K pneumoniae is the principal microbial agent causing PLA in this region.[8,14] Patients with T2DM are known to show defects in neutrophil chemotaxis and phagocytosis, which are thought to be the most important predisposing factors for K pneumoniae-pathogenic liver abscess. A previous Taiwanese study reported that diabetes was associated with a 9-fold increase of PLA incidence.[16]
A compatible report from a population-based case–control study in Denmark indicated that patients with DM had a 3.6-fold increased risk of experiencing PLA, (adjusted relative risk, 3.6; 95% CI = 2.9–4.5).[20] Although our findings were generally consistent with findings of the above population-based studies, we further noted significant interactions between diabetes and both sex and age, where, males and younger T2DM patients suffered greater increased risks of PLA. A number of previous studies noted a higher incidence of PLA in males.[3,5,14,18,26] The main etiology of PLA incidence is associated with hematogenous seeding of the liver, either through the portal system or the greater circulation, or local spread from infections within the peritoneal cavity.[28] Whether male patients with T2DM are vulnerable to the manifestation of this proposed etiology warrants further investigations.
One previous study argued that patients with PLA at an age of >50 years accounted for 76.8% of the total PLA patients and argued that the elderly people may have suffered from more comorbidities that predispose to the incidence of PLA.[29] Although elderly people had a higher incidence of PLA, younger patients with diabetes suffered from a greater increased hazard of PLA. Poorly controlled diabetes may induce tissue hyperglycemia and predilection for certain microorganisms, including E coli and Klebsiella species.[8] One previous study found that patients with uncontrolled glycemia tended to be younger, and may have a higher rate of cryptogenic liver abscess, gas-forming liver abscess, and metastatic infection than those with controlled glycemia. Additionally, young ages, newly diagnosed diabetes, cryptogenic liver abscess and metastatic infection were more common in the poor glycemic control group (HbA1c value >10%).[13] These findings might explain the significant effect-modification by age noted in our study. Similar findings were also noted in another study reporting that the relative risk of PLA tended to be 5 to 6 times higher in the younger age (<65 years) groups, as compared to those aged ≧65 years.[19] A lower relative hazard of PLA in patients with diabetes could be due to early diagnosis and treatment of T2DM that may have in turn decreased the risk of PLA in later years.
Biliary tract disease was previously reported as the most common cause of PLA, but its role in PLA incidence has become less important over the past years. There has been an increasing number of PLA patients who had no obvious underlying cause.[3,5,14] Such change is possibly due to an increasing use of ultrasound and computerized tomography, which results in earlier intervention and thus reduces the sequelae of biliary tract infections. One previous Taiwanese cohort study compared the risk of PLA between diabetes and non-diabetes and concluded that cryptogenic origin was the most common cause (87% vs 62%, P <.001) and biliary origin was much less frequent in the diabetes group (8% vs 26%, P <.001).[30] Liver cirrhosis is also a strong risk factor for PLA associated with a poor prognosis. The etiology is probably associated with impaired immune defenses and structural changes in the cirrhotic liver including reduced trans-hepatic blood flow, increased portal pressure, and often ascites.[2] The impaired macrophage function[31], less protective Kupffer cells, the impaired functions of neutrophilic leucocytes[32] and the complement system[33] may compromise immune defenses and increase intestinal permeability to bacteria. The contribution of biliary tract diseases and liver cirrhosis to the risk of PLA warrants attention especially on those T2DM patients with greater risks of PLA.
Several limitations should be noted in our study. First, exclusive reliance on the claims data might have resulted in potential misclassification bias in our study. The accuracy of single T2DM diagnosis in the NHI claims data in 2000 was reported to be 74.6%[34], but we used at least 2 diabetes-related diagnoses with the first and the last visits >30 days apart, which would greatly reduce the likelihood of disease misclassification. Despite that, the information of T2DM diagnosis in the NHI claims might still be subject to error. Although the T2DM diagnosis can be further confirmed by using the information of anti-glucose drugs, we were unable to do so mainly because our research project did not apply for drug data. Additionally, the control group might have been mixed up with new onset or undiagnosed diabetes cases. However, the above mentioned T2DM misclassification bias was likely to be non-differential, which would tend to underestimate rather than overestimate the true relative hazard. Second, using the linked administrative data, we were unable to make a comprehensive adjustment for risk factors of PLA incidence. Due to unavailability of laboratory and microbiologic data in NHI claims, the specific bacterial spectrum that causes PLA in Taiwan cannot be determined in our study. Nevertheless, the complex interactions between PLA, diabetes and the underlying risk factors are shown to worsen this condition warrants further investigations. Third, due to the claim data truncation, we were unable to obtain complete information of T2DM duration and adherence to treatment over the course of T2DM, which also limited interpretation of our study findings.
5. Conclusion
In conclusion, this large-scale cohort study confirmed the previously reported link between DM and risk of PLA onset. This study advanced the knowledge by highlighting a stronger association of interest noted for male and younger patients with T2DM, and that T2DM patients with underlying biliary tract diseases and liver cirrhosis are at even greater risks of PLA onset. These high-risk T2DM patients should be the objects of particular attention for the prevention of PLA onset. Nonetheless, generalizability of the findings from this study should be cautious due to possible dissimilarities in diabetes care at different settings.
Author contributions
Authors’ contributions: All authors participate sufficiently in the design, conduct, and data analysis through the production of this manuscript
Conceptualization: Ming-Chung Ko, Wei-Hung Lin, Santi Martini, Chang-Ta Chiu, Chung-Yi Li.
Data curation: Chung-Yi Li.
Formal analysis: Ming-Chung Ko, Ya-Hui Chang.
Funding acquisition: Chung-Yi Li.
Investigation: Ming-Chung Ko, Wei-Hung Lin, Santi Martini, Chang-Ta Chiu, Chung-Yi Li.
Methodology: Ming-Chung Ko, Wei-Hung Lin, Ya-Hui Chang, Chang-Ta Chiu, Chung-Yi Li.
Project administration: Chung-Yi Li.
Software: Ya-Hui Chang.
Writing – original draft: Ming-Chung Ko, Chang-Ta Chiu.
Writing – review & editing: Ming-Chung Ko, Wei-Hung Lin, Santi Martini, Ya-Hui Chang, Chang-Ta Chiu, Chung-Yi Li.
Footnotes
Abbreviations: DM = diabetes mellitus, ICD-9-CM = International Classification of Disease, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHIA = National Health Insurance Administration, NHRI = National Health Research Institute, PLA = pyogenic liver abscess, T2DM = type 2 diabetes mellitus.
C-TC and C-YL contributed equally to this work.
This study was partially supported by a grant from the Ministry of Science and Technology with National Scientific Council (MOST 106-2314-B-006-025), who however has no role in this study.
The authors have no conflicts of interest to disclose.
References
- [1].Mishra K, Basu S, Roychoudhury S, et al. Liver abscess in children: an overview. World J Pediatr 2010;6:210–6. [DOI] [PubMed] [Google Scholar]
- [2].Njoku VC, Howard TJ, Shen C, et al. Pyogenicliverabscess following pancreaticoduodenectomy: riskfactors, treatment, and long-term outcome. J GastrointestSurg 2014;18:922–8. [DOI] [PubMed] [Google Scholar]
- [3].Jun CH, Yoon JH, Wi JW, et al. Riskfactors and clinical outcomes for spontaneous rupture of pyogenicliverabscess. J Dig Dis 2015;16:31–6. [DOI] [PubMed] [Google Scholar]
- [4].Luo Y, Wang Y, Ye L, et al. Molecular epidemiology and virulence factors of pyogenic liver abscess causing Klebsiellapneumoniae in China. Clin Microbiol Infect 2014;20:O818–24. [DOI] [PubMed] [Google Scholar]
- [5].Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012;12:881–7. [DOI] [PubMed] [Google Scholar]
- [6].Wang WJ, Tao Z, Wu HL. Etiology and clinical manifestations of bacterial liver abscess: a study of 102 cases. Medicine (Baltimore) 2018;97:e12326.doi: 10.1097/MD.0000000000012326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lau YJ, Hu BS, Wu WL, et al. Identification of a major cluster of Klebsiella pneumoniae isolates from patients with liver abscess in Taiwan. J Clin Microbiol 2000;38:412–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998;26:1434–8. [DOI] [PubMed] [Google Scholar]
- [9].Fazili T, Sharngoe C, Endy T, et al. Klebsiella pneumoniae liver abscess: an emerging disease. Am J Med Sci 2016;351:297–304. [DOI] [PubMed] [Google Scholar]
- [10].Chu CS, Lin CC, Peng CY, et al. Does pyogenic liver abscess increase the risk of delayed-onset primary liver cancer? Evidence from a nationwide cohort study. Medicine (Baltimore) 2017;96:e7785.doi: 10.1097/MD.0000000000007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang CC, Yen CH, Ho MW, et al. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 2004;37:176–84. [PubMed] [Google Scholar]
- [12].Principi N, Berioli MG, Bianchini S, et al. Type 1 diabetes and viral infections: what is the relationship. J ClinVirol 2017;96:26–31. [DOI] [PubMed] [Google Scholar]
- [13].Lin YT, Wang FD, Wu PF, et al. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis 2013;13:56.doi: 10.1186/1471-2334-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu WL, Lai CC. Liverabscess in a diabetic patient. QJM 2012;105:1131–2. [DOI] [PubMed] [Google Scholar]
- [15].Takano Y, Hayashi M, Niiya F, et al. Life-threatening emphysematous liverabscess associated with poorly controlled diabetes mellitus: a case report. BMC Res Notes 2017;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Foo NP, Chen KT, Lin HJ, et al. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol 2010;105:328–35. [DOI] [PubMed] [Google Scholar]
- [17].Qian Y, Wong CC, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep 2016;6:38587.doi: 10.1038/srep38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004;2:1032–8. [DOI] [PubMed] [Google Scholar]
- [19].Thomsen RW, Jepsen P, Sorensen HT. Diabetes mellitus and pyogenic liver abscess: risk and prognosis. Clin Infect Dis 2007;44:1194–201. [DOI] [PubMed] [Google Scholar]
- [20].Tian LT, Yao K, Zhang XY, et al. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect 2012;18:E314–30. [DOI] [PubMed] [Google Scholar]
- [21].Chiang TL. Taiwan's 1995 health care reform. Health Policy 1997;39:225–39. [DOI] [PubMed] [Google Scholar]
- [22].Chen HF, Chen P, Li CY. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology 2010;52:155–63. [DOI] [PubMed] [Google Scholar]
- [23].Chen HF, Chang YH, Ko MC, et al. A large scale population-based cohort study on risk of ovarian neoplasm in patients with type 2 diabetes mellitus. Gynecol Oncol 2014;134:576–80. [DOI] [PubMed] [Google Scholar]
- [24].Chen HF, Ho CA, Li CY. Risk of heart failure in a population with type 2 diabetes versus a population without diabetes with and without coronary heart disease. Diabetes Obes Metab 2019;21:112–9. [DOI] [PubMed] [Google Scholar]
- [25].Tan HF, Tseng HF, Chang CK, et al. Accessibility assessment of the health care improvement program in rural Taiwan. J Rural Health 2005;21:372–7. [DOI] [PubMed] [Google Scholar]
- [26].Jepsen P, Vilstrup H, Schonheyder HC, et al. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977–2002. Aliment Pharmacol Ther 2005;21:1185–8. [DOI] [PubMed] [Google Scholar]
- [27].Meddings L, Myers RP, Hubbard J, et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol 2010;105:117–24. [DOI] [PubMed] [Google Scholar]
- [28].Doley J. Doley J. The liver in infections. Diseases of the liver and biliary system. Oxford: Blackwell Science; 1997. 497–500. [Google Scholar]
- [29].Chen YC, Lin CH, Chang SN, et al. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan. J Microbiol Immunol Infect 2016;49:646–53. [DOI] [PubMed] [Google Scholar]
- [30].Yu SC, Ho SS, Lau WY, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology 2004;39:932–8. [DOI] [PubMed] [Google Scholar]
- [31].Sabino KR, Petroianu A, Alberti LR. Influence of the acute alcoholism on the phagocytic function of the mononuclear phagocytic system. J Med Life 2011;4:421–3. [PMC free article] [PubMed] [Google Scholar]
- [32].Profita M, Sala A, Siena L, et al. Leukotriene B4 production in human mononuclear phagocytes is modulated by interleukin-4-induced 15-lipoxygenase. J PharmacolExpTher 2002;300:868–75. [DOI] [PubMed] [Google Scholar]
- [33].Zhang GL, Zhang T, Ye YN, et al. Complement factor 3 could be an independent risk Factor for mortality in patients with HBV related acute-on-chronic liver failure. Biomed Res Int 2016. 3524842.doi: 10.1155/2016/3524842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005;104:157–63. [PubMed] [Google Scholar]


