Supplemental Digital Content is available in the text
Keywords: BCG-CWS, cancer immunotherapy, clinical study, immune adjuvant, WT1
Abstract
The cell wall skeleton of Bacillus Calmette–Guérin (BCG-CWS) is a bioactive component that is a strong immune adjuvant for cancer immunotherapy. BCG-CWS activates the innate immune system through various pattern recognition receptors and is expected to elicit antigen-specific cellular immune responses when co-administered with tumor antigens. To determine the recommended dose (RD) of BCG-CWS based on its safety profile, we conducted a phase I dose-escalation study of BCG-CWS in combination with WT1 peptide for patients with advanced cancer.
The primary endpoint was the proportion of treatment-related adverse events (AEs) at each BCG-CWS dose. The secondary endpoints were immune responses and clinical effects. A BCG-CWS dose of 50, 100, or 200 μg/body was administered intradermally on days 0, 7, 21, and 42, followed by 2 mg of WT1 peptide on the next day. For the escalation of a dose level, 3 + 3 design was used.
Study subjects were 18 patients with advanced WT1-expressing cancers refractory to standard anti-cancer therapies (7 melanoma, 5 colorectal, 4 hepatobiliary, 1 ovarian, and 1 lung). Dose-limiting toxicity occurred in the form of local skin reactions in 2 patients at a dose of 200 μg although no serious treatment-related systemic AEs were observed. Neutrophils and monocytes transiently increased in response to BCG-CWS. Some patients demonstrated the induction of the CD4+ T cell subset and its differentiation from the naïve to memory phenotype, resulting in a tumor response.
The RD of BCG-CWS was determined to be 100 μg/body. This dose was well tolerated and showed promising clinical effects with the induction of an appropriate immune response.
1. Introduction
An immune adjuvant is a substance that accelerates or enhances antigen-specific immune responses by activating innate immunity when co-administered with an antigen given for a vaccination.[1,2,3] Most current immune adjuvants function as ligands for toll-like receptors (TLRs) and stimulate dendritic cells (DCs), leading to DC maturation and differentiation into antigen presenting cells (APCs).[3] The clinical use of immune adjuvants in cancer immunotherapy has been expected to overcome the immunosuppressive state in the tumor microenvironment, and to effectively induce or enhance pre-existing host anti-tumor immune responses to eradicate cancer cells.[4]
Bacillus Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis, has commonly been used worldwide as a prophylactic vaccine against tuberculosis.[5] BCG strongly enhances the non-specific immune response, thus augmenting specific protection, and has beneficial clinical effects. In the 1970s, Azuma et al isolated the bioactive component of BCG from its cell wall, and termed it the BCG cell wall skeleton (BCG-CWS).[6,51] BCG-CWS has a basic structure composed of mycolic acid, arabinogalactan, and peptidoglycan, all of which are ligands for TLR2 and TLR4.[7,8] Furthermore, BCG-CWS also contains trehalose dimycolate and mannose-capped lipoarabinomannan, which are recognized by C-type lectin receptors (CLRs) such as Mincle (macrophase inducible C-type lectin) and dectin-2.[9,10] The recognition of BCG-CWS by pattern recognition receptors such as TLRs and CLRs causes differentiation of DCs into master APCs and activates them to produce cytokines and proteins that can induce inflammation and an adaptive cellular immune response.[7,11,12,13] Pre-clinical studies of cancer vaccines using BCG-CWS (1 was a DC vaccine,[14] and the other was a peptide vaccine with a tumor-associated antigen (TAA) peptide[15]) showed that BCG-CWS functioned as an effective immune adjuvant that induced tumor-specific T cells to a sufficient degree to eradicate established tumors in mice. The clinical application of BCG-CWS in cancer immunotherapy was initiated for melanoma, lung cancer, and gastric cancer in the late 1970s.[16,17,18] Yamamura et al conducted a clinical study in patients advanced lung cancer, and found that overall survival (OS) in patients treated with BCG-CWS was prolonged compared to a historical control group, although the study used outdated methods and statistical analyses.[17] A recent case-control study analyzed BCG-CWS as maintenance therapy after surgery in patients with non–small cell lung cancer (NSCLC). The 5-year and 10-year survival rates in the BCG-CWS group were longer than those in the control group, although the difference in OS between the 2 groups was not statistically significant.[19] These results suggest that BCG-CWS may be expected to enhance the potency of cancer immunotherapies, including cancer vaccines and immune checkpoint inhibitors.
Many TAAs have been identified and used as therapeutic cancer vaccines.[20] One of the most promising TAAs is a Wilms’ tumor gene (WT1) product.[21] WT1 was originally isolated as a tumor suppressor gene responsible for Wilms’ tumor.[22] Numerous studies, however, demonstrated that WT1 was expressed at high levels in several kinds of cancers,[23,24,25] and that WT1 had oncogenic functions, including inhibition of differentiation, promotion of cell growth, cell death resistance, and tumor angiogenesis.[25,26,27,28,29] Further research demonstrated the beneficial clinical effects of WT1-targeted immunotherapy against several advanced cancers.[25,30,31,32,33,34,43,44,45,46,47,48]
No clinical studies thus far have administered BCG-CWS as an immune adjuvant together with any TAA-specific peptides or other cancer antigens. In addition, no definitive studies have evaluated the effects of BCG-CWS on the time course of innate or adaptive immune cells, including immunological phenotypes in CD4+ or CD8+ T cell subsets. The purpose of this study was to assess the safety of BCG-CWS in patients with advanced WT1-expressing solid cancers and to determine the recommended dose (RD) of BCG-CWS in combination with human leukocyte antigen (HLA)-A∗24:02–restricted WT1 peptide, based on identified dose-limiting toxicities (DLTs) and the estimated maximal tolerated dose (MTD). Another goal was to evaluate the effects of BCG-CWS on the immune system in cancer patients, especially adaptive cellular immunity, by assessing the immunological phenotypes of T cell subsets.
2. Methods
2.1. Study design
This study was designed as an open-label, dose-escalation phase I study to evaluate the safety and immunological effects of BCG-CWS in combination with HLA-A∗24:02–restricted 9-mer WT1 peptide in patients with advanced solid cancers who were refractory to standard anti-cancer therapies. The study was conducted at Osaka University Hospital. It was divided into a dose-escalating portion, with a maximum of 18 subjects, and an extended portion to confirm the RD of BCG-CWS, with a maximum of 5 subjects. The primary endpoint was the proportion of treatment-related adverse events (AEs) at each dose of BCG-CWS. The secondary endpoints included assessments of innate and adaptive immune-related cells, the WT1-specific immune response, and clinical response. The protocol was approved by the independent ethics committee of Osaka University Hospital and was conducted according to the ethical principles of the Declaration of Helsinki.
2.2. Patients
Patients with advanced WT1-expressing solid cancers who had failed standard anti-cancer therapies were eligible. Other major inclusion criteria were measurable disease; age between 16 and 79 years; HLA-A∗24:02; European Cooperative Oncology Group performance status of 0 to 1; adequate bone marrow, liver, and renal functions; and life expectancy greater than 3 months. Patients were excluded if they had a significant concomitant disease unrelated to the underlying malignancy, including co-existing malignancies, severe congestive heart failure, active coronary artery disease, uncompensated pulmonary disease, uncontrolled infectious disease, myeloproliferative disease, autoimmune disease, and severe mental disorders. All patients provided written informed consent.
2.3. Dose-limiting toxicity
DLT was defined as the occurrence of any of the followings between 8 and 10 weeks after the beginning of the study treatment:
-
1.
severe skin ulcer at a BCG-CWS injection site, with pus drainage that continued until the next vaccination;
-
2.
a fever of 39.0°C or higher within 48 hours after the administration of BCG-CWS; and
-
3.
any grade 3 or 4 treatment-related adverse event.
2.4. Dose escalation and determination of RD
Dose escalation was conducted using a modified Fibonacci method (3 + 3 design). At least 3 patients were enrolled at each dose level. If a DLT was observed in one of the initial 3 patients during the study treatment phase (Fig. 1A), 3 additional patients were entered at the same dose. Furthermore, if at least 2 of 6 patients developed a DLT, the associated dose was defined as the MTD. The RD of BCG-CWS was comprehensively determined, taking into consideration both the estimated MTD and the immune response.
Figure 1.
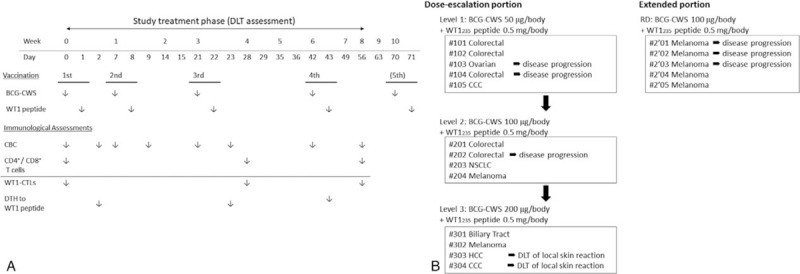
Study schedule and study profile. A, Schedule of the study treatment and immunological assessments during the study treatment phase. B, Study profile of the dose-escalation portion (left) and the extended portion (right) of the study.
2.5. Preparation of the BCG-CWS and WT1 peptide solution
We prepared the BCG-CWS as an oil-in-water emulsion product according to a previous report.[19,35] Briefly, 2 mg of BCG-CWS and 20 μl of Drakeol 6VR light mineral oil (Penreco, Karns City, PA) were gently mixed and homogenized for 1 minute. Then, the BCG-CWS/Drakeol solution was homogenized for 4 minutes with 1 ml of emulsion buffer (1% Tween 80 [polyoxyethylene sorbitan monooleate; Sigma, St Louis, MO] diluted by saline [Otsuka Pharmaceutical, Tokyo, Japan]). The BCG-CWS solution was then incubated at 60°C for 30 minutes. The final concentration of BCG-CWS in the oil-in-water emulsion was 1 mg/ml.
The sequence of HLA-A∗24:02–restricted modified 9mer WT1235 peptide (mp235) was CYTWNQMNL. Good manufacturing practice (GMP) –grade mp235 was synthesized by Peptide Institute (Osaka, Japan). For preparation of WT1 peptide solution, 3 mg of mp235 was dissolved in 100 μl of dimethyl sulfoxide (DMSO; Sigma) and then was diluted with 1400 μl of 5% glucose (Otsuka Pharmaceutical). The final concentration of WT1 peptide solution was 2 mg/ml. A volume of 250 μl peptide solution (0.5 mg of mp235) was administered to the patient.
2.6. Treatment
The treatment schedule is summarized in Figure 1A. BCG-CWS and WT1 peptide were inoculated 4 times during a period of 2 months (56 days) (study treatment phase). BCG-CWS (50, 100, or 200 μg/body) was administered on days 0, 7, 21 and 42. On the first day of each vaccination, BCG-CWS was administered intradermally in an upper arm, and then on the next day, WT1 peptide solution was administered in the same site, half intradermally and half subcutaneously. Inoculation was alternatively performed in the left and right upper arms (for example, the first and third vaccinations were administered in the left upper arm, and the second and forth given in the right upper arm). Patients were not allowed to receive any other cancer treatments including chemotherapeutic agents during the study treatment. Fever prophylaxis with either non-steroidal anti-inflammatory drugs or acetaminophen was also not allowed following vaccinations. However, the use of these drugs was not restricted when the indication was treatment of cancer pain. After the final safety assessment in the study treatment phase, all patients were permitted to receive the study treatment until the occurrence of disease progression, unacceptable AEs, or withdrawal of consent.
2.7. Study assessments
At baseline and at the time of every administration, we checked each patient's general conditions and vital signs, and carried out urine and blood tests, including blood cell counts and serum chemistry tests. AEs were assessed according the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Tumor response was defined by the investigator assessments according to the response evaluation criteria in solid tumors (RECIST). Radiological evaluation, such as computed tomography, was performed at baseline and at 1 and 2 months after the beginning of the study treatment.
2.8. Immunohistochemical analysis
Immunohistochemical analysis was performed to examine the expression of WT1 protein in malignant tumor cells using a procedure that has been previously described. Briefly, formalin-fixed tissue sections were cut from a paraffin block including the primary resected tumors or sometimes biopsy samples and stained with anti-WT1 mouse monoclonal antibody clone 6F-H2 (Dako cytomation, Carpinteria, CA). Visualization was performed by a standard avidin–biotin complex method using a Vectastain ABC elite kit (Vector Labs., Burlingame, CA). For malignant melanoma, a positive signal was detected by the alkaline phosphatase system using a ENVISION labeled polymer-AP kit (Dako cytomation).
2.9. Immunological assays
For the measurement of blood cell counts, peripheral blood (PB) was collected before and 24 to 48 hours after the administration of BCG-CWS (Fig. 1A). White blood cells (WBC) counts and cellularity in PB were measured with an automatic hemocytometer, and then absolute neutrophil, lymphocyte, and monocyte counts were calculated.
To assess the dynamic change of WT1-specific cytotoxic T lymphocytes (WT1-CTLs) and CD4+ or CD8+ T cells and their immunological phenotypes, peripheral mononuclear cells (PBMCs) were collected on days 0, 28, and 56 (Fig. 1A), and cryopreserved until use. WT1-CTLs, which were defined as WT1-tetramer+CD3+CD8+ T cells, were assessed by the WT1 peptide / HLA-A∗24:02 tetramer assay. The following tetramer and monoclonal antibodies were used: PE-conjugated WT1235 tetramer (MBL, Nagoya, Japan); anti–CD4-fluorescein isothiocyanate (FITC), anti–CD16-FITC, and anti–CD45RA-allophycocyanin (APC) (BioLegend, San Diego, CA); anti–CD19-FITC, and anti–CCR7-R-phycoerythrin (PE)-Cy7 (BD Pharmingen, San Diego, CA); anti–CD3-PerCP, anti–CD8-APC-Cy7, and anti–CD14-FITC (BD Biosciences, San Jose, CA); and anti–CD56-FITC (eBioscience, San Diego, CA). Data acquisition was performed on a fluorescence-activated cell sorter (FACS) Aria instrument (BD Biosciences), and data analysis was performed with FACS Diva software (BD Biosciences).
Delayed-type hypersensitivity (DTH) to WT1 peptide was examined as a WT1-specific immune response. Thirty micrograms of WT1 peptide diluted in saline were intradermally injected into the forearm on days 0, 21, and 42, and the maximal diameter of erythema was measured after 48 hours (Fig. 1A).
2.10. Statistical analysis
The chi-square test (χ 2 test) was used to calculate P values for associations between the frequency of each AE and the dose level of BCG-CWS. The Wilcoxon signed-rank test or the Friedman test was used to calculate P values for changes in immune cell counts and frequencies of T cell subsets and WT1-CTLs. For the assessment of adverse events and for the immunological assay, we judged P values of less than .05 and less than .01, respectively, to be significant. The statistical analyses were performed with StatView for Windows version 5.0 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient characteristics
A total of 18 patients with advanced solid cancers (13 in the dose-escalation portion, and 5 in the extended portion) were enrolled between July 2007 and March 2010. Patient characteristics at baseline are listed in Table 1. Eight patients were male, and 10 were females. The median age was 60.5 years (range: 36–79). In the dose-escalation portion of the study, the most common primary cancer types were colorectal cancer (n = 5, 38.5%), hepatobiliary cancers (n = 4, 30.8%), and melanoma (n = 2, 15.4%). In the extended portion, all were melanoma (n = 5). The main locations of metastasis were the lymph nodes (n = 14), lungs (n = 14), skin (n = 5), and liver (n = 4). The median time since the initial diagnosis of disease was 24.5 months (range 5–116) (median 34.0 [range 5–82] in the dose-escalation portion, and median 10.0 [range 6–116] in the extended portion). Except for 1 individual with intrahepatic cholangiocarcinoma (#105), all patients had received standard care, including a surgical resection of the primary tumor, and had undergone at least 1 prior treatment, including chemotherapy, for metastatic diseases before study enrollment. Two patients, 1 with colorectal cancer (#202) and the other with lung cancer (#203), had received radiation therapy for brain metastases.
Table 1.
Patient characteristics at baseline.

All patients received at least 1 vaccination. Ten of 13 patients (76.9%) in the dose-escalation portion and 2 of 5 patients (40.0%) in the extended portion completed the 10-week of treatment plan, consisting of 4 vaccinations followed by a final safety assessment (Fig. 1B). The remaining 6 patients in both groups, including 2 patients who had received 4 times of vaccinations, were withdrawn from the study due to rapid disease progression (Fig. 1B). Ten of 12 patients who had completed the 10-week of treatment plan continued to receive the study treatment until the occurrence of disease progression (Table 4).
Table 4.
Clinical effects.

3.2. Dose-limiting toxicities
Two patients at a BCG-CWS dose of 200 μg/body experienced DLTs, specifically severe skin ulcerations at the injection sites and pus drainage that persisted despite local skin treatment (Fig. 2A). There were no other systemic DLTs, including a sustained fever of 39.0°C or higher, even at a BCG-CWS dose of 200 μg/body. Thus, the MTD of BCG-CWS was determined to be 100 μg/body in this clinical setting.
Figure 2.
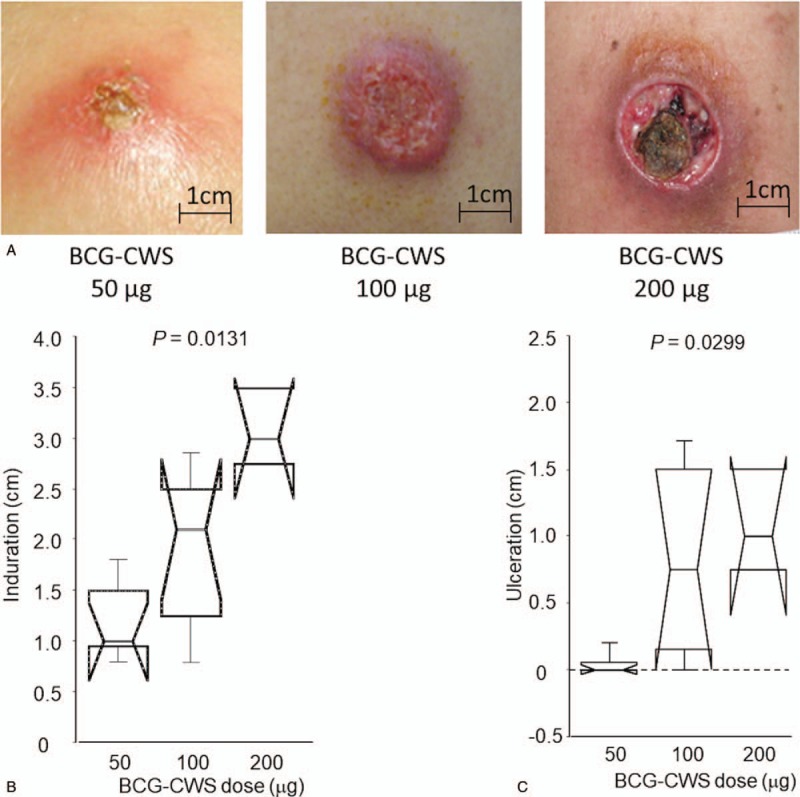
Local skin reactions at vaccine sites. A, Typical skin reactions according to BCG-CWS dose. B, Comparison of the size of induration with each BCG-CWS dose. C, Comparison of the size of ulceration with each BCG-CWS dose.
3.3. Safety and tolerability (1): systemic adverse events
The median number of vaccinations per patient was 5.5 times (range 1–31) (Table 4). All treatment-related AEs are summarized in Table 2. No patients discontinued the study treatment due to some treatment-related AEs. The most commonly reported (>15%) treatment-related AEs, excluding local skin toxicity at the vaccine sites, were lymphopenia, anemia, fatigue, pruritus, hypoalbuminemia, hyperkalemia, proteinuria, and hematuria. All treatment-related AEs were grade 1 or 2, and some were readily manageable with usual supportive care. The frequencies of AEs were not significantly associated with BCG-CWS dose levels. Although no patients developed vaccine-related fevers of 38.0°C or higher (≥grade 1), 6 patients (2, 3, and 1 receiving BCG-CWS dose of 50, 100, and 200 μg/body, respectively) exhibited transient increases in body temperature (<37.5°C) within 24 to 48 hours after vaccination.
Table 2.
Treatment-related adverse events.

In total, 5 ≥grade 3 AEs such as gastrointestinal obstruction, hypokalemia, motor paralysis, and hepatobiliary infection occurred in 4 patients (Supplementary Table S1). All of these events, however, were definitely related to a disease progression or cancer-associated complications.
3.4. Safety and tolerability (2): local skin reactions
Local skin reactions at vaccine sites, such as redness, induration, or ulcer occurred in all patients; however, 1 patient (#E01) could not be adequately evaluated because he discontinued the treatment after 1 vaccination. The severity of skin reaction was dependent on the dose of BCG-CWS (Fig. 2A). In particular, both the size of induration and the severity of ulceration were positively correlated with the dose of BCG-CWS (Fig. 2B and C). About 70% of patients (11/17) (20, 75, and 100% receiving BCG-CWS doses of 50, 100, and 200 μg/body, respectively) exhibited skin ulcers. In patients whose BCG-CWS dose was 200 μg/body, ulcerations after the second vaccination were severe, and pus drainage persisted for several days. In 2 patients (#303 and #304) the third or fourth vaccination had to be delayed, and their dose of BCG-CWS had to be reduced to 100 μg/body. Some patients complained of vaccine site symptoms such as pruritus and mild pain, which may also have been associated with the dose level of BCG-CWS (Supplementary Table S2).
3.5. Effects of BCG-CWS on innate immune cells
We examined WBC counts and their cellularity in PB before and 2 days after the administration of BCG-CWS. In the overall population, the median absolute neutrophil counts before the first, second, third, and fourth vaccinations were 4060, 3500, 3620, and 3740 /μl, respectively, and the median absolute monocyte counts before each vaccination were 499, 481, 455, and 411 /μl, respectively (Supplementary Table S3). There were no significant temporal changes in these counts. On the other hand, the median neutrophil counts 2 days after the first, second, and third administrations of BCG-CWS were 3475, 3720, and 4435 /μl, respectively, and the median monocyte counts at these points were 476, 519, and 491 /μl, respectively (Supplementary Table S3). Temporary increases in neutrophils and monocytes were seen after the administration of BCG-CWS, although these changes were not statistically significant (Fig. 3A and C).
Figure 3.
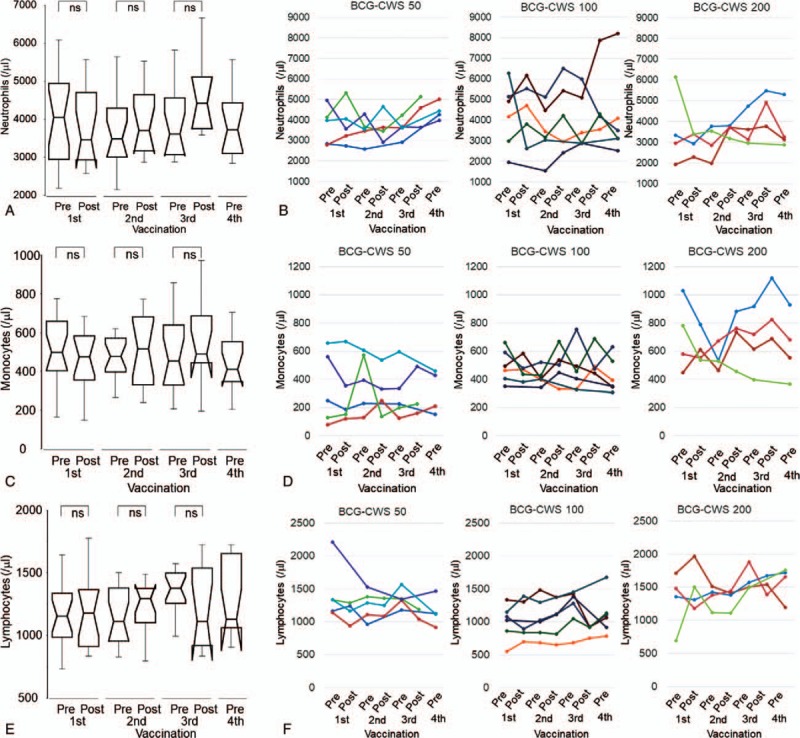
Dynamic changes in immune-related cells (1). A, B, Dynamic changes in absolute neutrophil counts in all patients and individual cases. C, D, Dynamic changes in absolute monocyte counts in all patients and individual cases. E, F, Dynamic changes in absolute lymphocyte counts in all patients and individual cases. In the line graphs, each color represents the same case in all panels. Peripheral blood samples were collected before (pre) and 24 to 48 hours after (post) the administration of BCG-CWS. Abbreviation: ns, not significant.
Compared to patients receiving 50 μg/body of BCG-CWS, those receiving 100 or 200 μg/body exhibited a greater increase in neutrophils or monocytes or both, especially following the second and the third vaccinations (Fig. 3B and D). The mean differences between each BCG-CWS dose group, however, were not significant (Supplementary Table S3).
3.6. Effects of BCG-CWS on adaptive immune cells and T cells subsets
In all patients, the median absolute lymphocyte counts before the first, second, third, and fourth vaccination were 1160, 1110, 1380, and 1130 /μl, respectively (Supplementary Table S3). The median lymphocyte counts 2 days after the first, second, and third administrations of BCG-CWS were 1180, 1300, and 1110 /μl, respectively (Supplementary Table S3). These pre- and post-vaccination changes in lymphocytes were not significant (Fig. 3E). The fluctuation of lymphocytes in each individual was relatively small (200 to 300/μl), and there was no significant difference between BCG-CWS dose groups (Fig. 3F).
Next, we evaluated the characteristics of lymphocytes, including both T cell subsets (CD4+ or CD8+) and their phenotypes, specifically naïve, central memory (CM), effector memory (EM), and effector, which were defined as CD45RA+ CCR7+, CD45RA+ CCR7-, CD45RA- CCR7-, and CD45RA+ CCR7-, respectively. We performed these analyses using blood samples collected from patients in the dose-escalation portion of the study. Ultimately, 11 patients could be assessed (3, 4, and 4 receiving BCG-CWS doses of 50, 100, and 200 μg/body, respectively). In all patients, the median frequencies of CD4+ T cells in whole lymphocytes (%CD4+ T cells) at baseline and at 1 and 2 months after vaccination were 40.1, 38.8, and 39.0%, respectively (Table 3). Immunological phenotype analyses showed a slight increase in the CM phenotype and a slight decrease in the naïve phenotype at 2 months after vaccinations (Table 3), but these differences were not significant. Two patients (#203 and #301) exhibited about a 10% increase in %CD4+ T cells after vaccination (Fig. 4A). Interestingly, in the analyses of immunological phenotypes, 3 patients (#102, #203, and #301), including 2 who exhibited an increase in %CD4+ T cells, demonstrated more than a 7% increase in the CM phenotype, and in 2 patients (#102 and #203) the naïve phenotype decreased proportionally to an increase in the CM phenotype (Fig. 4B).
Table 3.
CD4+ T cells, CD8+ T cells, and tumor-associated antigen-specific CTLs.

Figure 4.
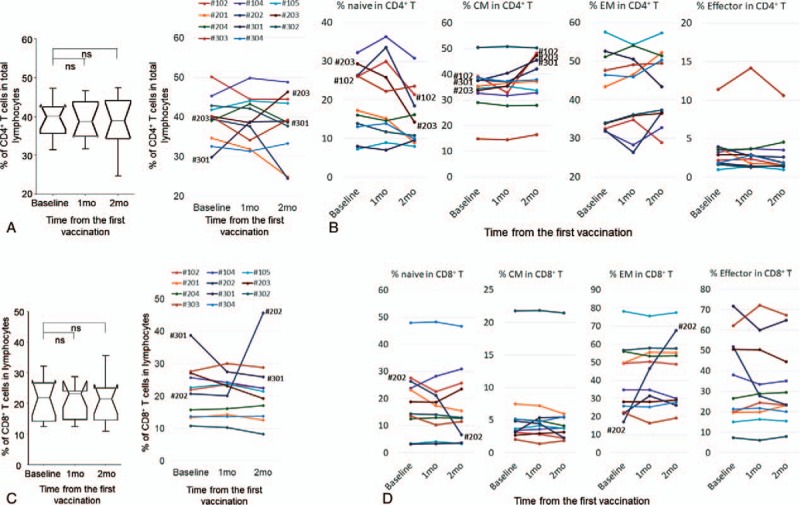
Dynamic changes in immune-related cells (2). A, Dynamic changes in percentages of CD4+ T cell in total lymphocytes in all patients (left) and individual cases (right). B, Frequencies of CD4+ T cell immunological phenotypes in individual cases. C, Dynamic changes in percentages of CD8+ T cells in total lymphocytes in all patients (left) and individual cases (right). D, Frequencies of CD8+ T cell immunological phenotypes in individual cases. In the line graphs, each color represents the same case in all panels. Peripheral blood mononuclear cells were collected before (baseline) and 1 or 2 months after the beginning of the study treatment. CM = central memory, EM = effector memory.
In all patients, the median frequencies of CD8+ T cells (%CD8+ T cells) at baseline and at 1 and 2 months after vaccination were 21.9, 23.2, and 21.5%, respectively (Table 3). Immunological phenotype analyses showed an increase in the EM phenotype and a slight decrease in the naïve phenotype at 2 months after vaccination, but these differences were not significant (Table 3). Of note, most patients exhibited minimal changes in %CD8+ T cells and of each immunological phenotype (Fig. 4C and D); large changes in both naïve and EM phenotypes in patient #202 significantly influenced the overall results.
3.7. Effect of BCG-CWS on tumor-associated antigen (TAA)-specific immunity
We used an HLA-A × 24:02–restricted WT1 peptide (mp235) to assess the induction of TAA-specific immunity by co-administration with BCG-CWS. We evaluated the WT1-specific immune response by a WT1-tetramer assay using PB samples collected from patients in the dose-escalation portion of the study (11 patients total: 3, 4, and 4 patients at BCG-CWS doses of 50, 100, and 200 μg/body, respectively). In the overall patient samples, the median percentages of WT1-CTLs in the entire CD8+ T cell population (%WT1-CTLs) at baseline and at 1 and 2 months after vaccination were 0.073, 0.078, and 0.085%, respectively (Table 3). The dose effect of BCG-CWS on the induction of WT1-CTLs was not clear (Supplementary Table S4). At an individual level, 2 of 11 patients (18.2%) (#202 and #302) exhibited a 3-fold or greater increase in %WT1-CTLs compared to baseline, while the remaining patients demonstrated no remarkable change (Figs. 5A and 3B). However, the induction of WT1-CTLs was not necessarily associated with DTH positivity to the WT1 peptide (Fig. 5B).
Figure 5.
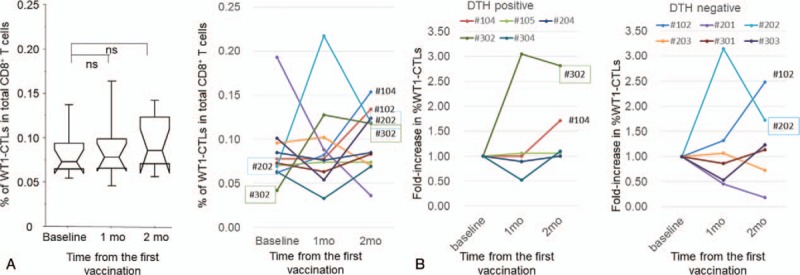
Tumor-associated antigen (TAA)-specific immune response. A, Dynamic changes in percentage of WT1-specific CTLs in total CD8+ T cells (%WT1-CTLs) in all patients (left) and individual cases (right). B, Fold-increase in %WT1-CTLs after the study treatment. Left and right graphs represent DTH-positive and DTH-negative patients, respectively. Peripheral blood mononuclear cells were collected before (baseline) and 1 or 2 months after the beginning of the study treatment. CTLs = cytotoxic T lymphocytes, DTH = delayed-type hypersensitivity, WT1 = Wilms’ tumor gene 1.
3.8. Clinical effects
The overall clinical responses are listed in Table 4. Nine of the 18 patients (50%) had stable disease at 2 months after vaccination although no patients reached complete response or partial response. Four patients (#105, #204, #304, and #E05) survived for more than 1 year from the date of enrollment. The association between BCG-CWS dose and clinical effect was not evident.
3.9. Case report
A 57-year-old female with relapsed NSCLC who was refractory to platinum-based chemotherapies and gefitinib received 100 μg/body of BCG-CWS in combination with WT1 peptide. Pleural invasions and lung metastases in the right upper lobe had gradually progressed from the final chemotherapy to the beginning of the study treatment, and pleural effusions also developed (Fig. 6A). While receiving the study treatment, the patient exhibited mild-to-moderate local skin reactions at vaccine sites but with no systemic treatment-related AEs. Interestingly, the disease involving the lung and pleura stabilized for about 3 months, and pleural effusion gradually decreased (Fig. 6A). Neutrophil and monocyte counts fluctuated with a temporary increase after the administration of BCG-CWS. Interestingly, %CD4+ T cells increased with CM-dominant differentiation. (Fig. 6C). WT1-specific immune responses, however, were not elicited (%WT1-CTLs at baseline and at 1 and 2 months after vaccination were 0.068%, 0.078%, and 0.061%, respectively).
Figure 6.
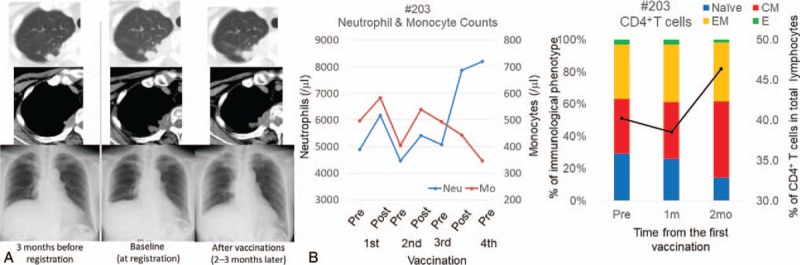
Clinical course and immunological monitoring in one case (#203). A, Chest computed tomography (CT) scan and chest X-ray radiograph (Chest-Xp) before and after the study treatment. Upper, intrapulmonary metastatic lesion; Middle, pleural disseminated lesions; Bottom, pleural effusion. B, Immunological assessment during the study treatment. (Left) Neutrophil and monocyte counts. Blue and red lines represent neutrophil and monocyte counts, respectively. (Right) CD4+ T cells. Black line represents %CD4+ T cells. Bar graph indicates immunological phenotypes of CD4+ T cells. Blue, red, orange, and green columns represent naïve, central memory (CM), effector memory (EM), and effector CD4+ T cells, respectively.
4. Discussion
We planned this phase 1 clinical study to determine the RD of BCG-CWS when co-administered with a TAA-specific peptide to patients with advanced solid cancer. In the dose-escalation portion of the study, local skin reactions occurred as a DLT in 2 patients receiving a BCG-CWS dose of 200 μg/body, while there were no DLTs in other groups. Importantly, there were no severe systemic treatment-related AEs at any BCG-CWS dose. Based on these results, we decided that the MTD of BCG-CWS was 100 μg/body. To determine the RD of BCG-CWS, we further assessed the dynamic changes of several immunological parameters in response to BCG-CWS. Overall, both innate and adaptive immune cells appeared to fluctuate more dynamically in response to a BCG-CWS dose of 100 μg/body than to that of 50 μg/body. There were no significant immunological advantages to 200 μg/body of BCG-CWS that would compensate for the disadvantages in terms of AEs, specifically more serious local skin reactions. Given these findings, we ultimately decided the RD of BCG-CWS as 100 μg/body in our clinical setting.
Immune adjuvants are expected to enhance the anti-tumor immune effects of other immunotherapies such as cancer vaccines, but at the same time there is concern that unacceptable immune-related AEs will develop.[36] The clinical application of BCG-CWS in cancer patients dates back to the 1970s.[16] A dose of 300 μg/body or more may cause liver injury (hepatitis), granulomatosis, or interstitial lung disease.[17] Even at a dose of 200 μg/body or less, BCG-CWS is known to cause severe skin ulcers at the injection site, which are sometimes difficult to manage with local treatments and may affect patients’ quality of life (QOL).[19] To determine the MTD while taking feasibility (including QOL) into account, we evaluated DLTs encompassing not only serious systemic AEs but also severe local skin reactions. Our final conclusion was that the MTD of BCG-CWS was 100 μg/body; while this was a lower dose than in previous reports, all systemic AEs related to BCG-CWS, including hematological, biochemical, and physiological findings, were mild, even at a BCG-CWS dose of 200 μg/body. Using a similar approach, a phase 1 dose-escalation study evaluated local skin toxicities of IMM-101, an immune adjuvant produced from Mycobacterium, in patients with melanoma.[37] After determining the RD based on consideration of skin toxicity, clinical studies of combination therapies with IMM-101 and chemotherapy or checkpoint inhibitors were conducted.[38,39] No decline in QOL due to skin toxicity secondary to IMM-101 was observed in these combinations. In the clinical development of immune adjuvants in cancer treatment, researchers should consider not only enhancing the immune response, thus leading to clinical effects, but also minimizing the deterioration of QOL due to immunological side effects.
Another purpose of this study was to assess the immune response induced by BCG-CWS. The immunological effects of BCG-CWS were not clear from the averaged results of the immunological parameters in the overall patient sample. However, a more granular analysis of individual patients revealed various immunological changes in response to BCG-CWS. First, innate immune cells such as neutrophils and monocytes increased over time. These effects seemed to be more pronounced at BCG-CWS dose levels of 100 μg and 200 μg than 50 μg. Second, the induction of the CD4+ T cell subset and its differentiation from the naïve to CM immunological phenotype occurred in some patients, but not all. Mycobacterium products exert multiple effects as immunomodulators because they contain various unique bioactive components that affect the host immune system through several types of pattern recognition receptors.[6,40] Consequently, BCG-CWS directly promotes the maturation of DCs and their differentiation into professional APCs, then indirectly activates the functional differentiation of antigen-specific CD4+ T cells and promotes antigen cross-presentation to antigen-specific CD8+ T cells.[7,11,12,41] In order to further understand the immunobioactivity of BCG-CWS, it is necessary to evaluate the effects of not only individual components of BCG-CWS, but also those of total components using conventional cytological and serological analyses as well as novel biological technologies, including bioinformatics.
All patients except for 1 (case #105) had advanced cancers that were refractory to all previously administered standard therapies. Nonetheless, these cancers stabilized in 9 of 18 patients after vaccinations, and 4 patients had an overall survival of more than 1 year. Most patients who experienced disease stabilization demonstrated an immune response. One patient (#203), in particular, exhibited disease stabilization and reduction of pleural effusion after induction and differentiation of CD4+ T cells. These clinical effects, which confirmed those of several previous reports,[17,18,19,42] suggested that non-specific immune reactions induced by BCG-CWS exerted anti-tumor effects against advanced cancer.
Based on our preclinical results in mice,[15] we expected that DCs would be initially activated by the administration of adjuvant, thus facilitating the effective uptake and presentation of antigens. Accordingly, we designed the vaccine schedule so that the adjuvant was administered first, and the peptide was administered the next day. Unfortunately, we did not identify an increase in TAA-specific WT1-CTLs although we observed a dynamic change in innate and non-specific adaptive immune responses. We also did not found the dose effect of BCG-CWS on the induction of WT1-specific immunity assessed by a WT1-tetramer assay and DTH to WT1 peptide. We, however, observed some WT1-specific immune responses in at least 1 case at all dose levels. Two patients exhibited 3-fold or higher increases in %WT1-CTLs after vaccination, but these elicitations did not always result in better clinical outcomes. There are at least 3 problems that we have to consider regarding future research. The first concerns the antigenicity of the WT1-peptide that we used as the TAA. We and others have reported induction of WT1-CTLs and clinical effects in numerous cancer immunotherapies targeting WT1,[30,31,32,33,34] suggesting that the WT1 peptide itself, namely mp235, is sufficiently antigenic. The second issue is the timing of antigen administration. A WT1 peptide as a TAA might have to be delivered at the same time that BCG-CWS stimulates innate cells, because activated DCs generally migrate immediately into regional lymph nodes.[2] Both an immune adjuvant and an antigenic peptide should be administered at the same time, as in a general prophylactic vaccine. The third problem, thought to be the most important in our current strategy, concerns drug delivery. Freund incomplete adjuvant has been widely used for the preparation of WT1 peptide–based vaccines in most previous clinical studies, including our own. This adjuvant forms a water-in-oil emulsion with an antigen solution, thus preventing WT1 peptide from being immediately transported to lymph nodes, and instead gradually releasing the antigen at local vaccine sites.[49] By contrast, in this study a peptide solution was intradermally administered. Therefore, intradermal spreading and degradation of the WT1 peptide at local sites, or immediate drainage of the WT1 peptide to lymph nodes could inhibit the effective uptake of antigens by activated DCs, resulting in poor induction of WT1-CTLs. Improvement of the antigen delivery system is necessary to exploit the powerful immunity-inducing ability of BCG-CWS in cancer vaccine therapy.[3,50]
This study had 2 main limitations. First, more detailed immunological analyses were not performed, for example, a T cell functional assay (cell-killing assay). Second, we used a small sample size that was insufficient to evaluate clinical and immunological assessments. In order to evaluate the adjuvant effect of BCG-CWS with respect to clinical and immune effects, it is necessary to conduct a verification phase 2 study.
In conclusion, the RD of BCG-CWS as an immune adjuvant co-administered with a TAA-specific peptide was 100 μg/body, taking into consideration the serious skin-related adverse events at vaccine sites. This dose of BCG-CWS was well tolerated and showed promising clinical effects in several patients with advanced cancer, including NSCLC and melanoma. BCG-CWS affected both innate and adaptive immunity. In particular, the induction and differentiation of CD4+ T cells through the direct activation of innate immunity occurred in patients with an advanced cancer. The multi-faceted activity of BCG-CWS in modulating immune systems is expected to overcome the immunosuppressive tumor microenvironment and to elicit a robust and sustained anti-cancer immune response. Further basic and clinical investigations are needed to establish effective treatment based on this approach.
Acknowledgments
The authors thank Tomoe Umeda (Osaka University) for her coordination of the clinical trial, and the members of the nursing team at Osaka University Hospital for their dedicated support and care of patients. We also thank all the patients who participated in this study, as well as their supportive families.
Author contributions
Conceptualization: Sumiyuki Nishida, Akihiro Tsuboi, Yusuke Oji, Yoshihiro Oka, Satoshi Morita, Haruo Sugiyama.
Data curation: Sumiyuki Nishida, Akihiro Tsuboi.
Formal analysis: Sumiyuki Nishida, Akihiro Tsuboi, Satoshi Morita.
Funding acquisition: Yoshihiro Oka, Haruo Sugiyama.
Investigation: Sumiyuki Nishida, Akihiro Tsuboi, Atsushi Tanemura, Toshinori Ito, Toshiaki Shirakata, Soyoko Morimoto, Fumihiro Fujiki, Naoki Hosen, Yusuke Oji, Ichiro Kawase, Yoshihiro Oka.
Methodology: Sumiyuki Nishida, Akihiro Tsuboi, Hiroko Nakajima, Soyoko Morimoto, Fumihiro Fujiki, Yusuke Oji, Yoshihiro Oka, Satoshi Morita.
Project administration: Sumiyuki Nishida, Akihiro Tsuboi.
Resources: Sumiyuki Nishida, Atsushi Tanemura, Toshinori Ito, Hiroko Nakajima, Toshiaki Shirakata, Ichiro Kawase, Ichiro Azuma, Haruo Sugiyama.
Supervision: Toshinori Ito, Atsushi Kumanogoh, Ichiro Kawase, Ichiro Azuma, Haruo Sugiyama.
Validation: Sumiyuki Nishida, Soyoko Morimoto, Fumihiro Fujiki, Yusuke Oji, Haruo Sugiyama.
Visualization: Sumiyuki Nishida, Yusuke Oji, Haruo Sugiyama.
Writing – original draft: Sumiyuki Nishida.
Writing – review & editing: Sumiyuki Nishida, Akihiro Tsuboi, Atsushi Tanemura, Atsushi Kumanogoh, Yoshihiro Oka, Ichiro Azuma, Satoshi Morita, Haruo Sugiyama.
Supplementary Material
Footnotes
Abbreviations: AE = adverse event, APC = allophycocyanin, APC = antigen presenting cell, BCG = Bacillus Calmette–Guérin, BCG-CWS = bacillus Calmette–Guérin cell wall skeleton, CLR = C-type lectin receptor, CM = central memory, CTCAE = Common Terminology Criteria for Adverse Events, CTL = cytotoxic T lymphocyte, DC = dendritic cell, DLT = dose-limiting toxicity, DMSO = dimethyl sulfoxide, DTH = delayed-type hypersensitivity, EM = effector memory, FACS = fluorescence-activated cell sorter, FITC = fluorescein isothiocyanate, GMP = good manufacturing practice, HLA = human leukocyte antigen, MTD = maximal tolerated dose, NSCLC = non-small cell lung cancer, OS = overall survival, PB = peripheral blood, PBMC = peripheral blood mononuclear cell, PE = R-phycoerythrin, RD = recommended dose, RECIST = response evaluation criteria in solid tumor, TAA = tumor-associated antigen, TLR = toll-like receptor, WBC = white blood cell, WT1 = Wilms tumor gene.
This study was supported by the Japanese Ministries of Education, Culture, Sports, Science and Technology, and of Health, Labor and Welfare.
All authors declared no potential conflicts of interest with regard to this work.
Supplemental Digital Content is available for this article.
References
- [1]. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013;19:1597–608. [DOI] [PubMed] [Google Scholar]
- [2]. Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci 2011;366:2748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol 2016;28:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016;16:219–33. [DOI] [PubMed] [Google Scholar]
- [5]. WHO. https://www.who.int/biologicals/areas/vaccines/bcg/Tuberculosis/en/. [Google Scholar]
- [6]. Azuma I, Kishimoto S, Yamamura Y, et al. Adjuvanticity of mycobacterial cell walls. Jap J Microbiol 1971;15:193–7. [DOI] [PubMed] [Google Scholar]
- [7]. Tsuji S, Matsumoto M, Takeuchi O, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of toll-like receptors. Infect Immun 2000;68:6883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Uehori J, Matsumoto M, Tsuji S, et al. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette-Guérin peptidoglycan. Infect Immun 2003;71:4238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Ishikawa E, Ishikawa T, Morita YS, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 2009;206:2879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Yonekawa A, Saijo S, Hoshino Y, et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 2014;41:402–13. [DOI] [PubMed] [Google Scholar]
- [11]. Matsumoto M, Seya T, Kikkawa S, et al. Interferon gamma-producing ability in blood lymphocytes of patients with lung cancer through activation of the innate immune system by BCG cell wall skeleton. Int Immunopharmacol 2001;1:1559–69. [DOI] [PubMed] [Google Scholar]
- [12]. Akazawa T, Masuda H, Saeki Y, et al. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res 2004;64:757–64. [DOI] [PubMed] [Google Scholar]
- [13]. Ishii K, Kurita-Taniguchi M, Aoki M, et al. Gene-inducing program of human dendritic cells in response to BCG cell-wall skeleton (CWS), which reflects adjuvancy required for tumor immunotherapy. Immunol Lett 2005;98:280–90. [DOI] [PubMed] [Google Scholar]
- [14]. Udagawa M, Kudo-Saito C, Hasegawa G, et al. Enhancement of immunologic tumor regression by intratumoral administration of dendritic cells in combination with cryoablative tumor pretreatment and Bacillus Calmette-Guerin cell wall skeleton stimulation. Clin Cancer Res 2006;12:7465–75. [DOI] [PubMed] [Google Scholar]
- [15]. Nakajima H, Kawasaki K, Oka Y, et al. WT1 peptide vaccination combined with BCG-CWS is more efficient for tumor eradication than WT1 peptide vaccination alone. Cancer Immunol Immunother 2004;53:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Yamamura Y, Sakatani M, Ogura T, et al. Adjuvant immunotherapy of lung cancer with BCG cell wall skeleton (BCG-CWS). Cancer 1979;43:1314–9. [DOI] [PubMed] [Google Scholar]
- [17]. Yasumoto K, Manabe H, Yanagawa E, et al. Nonspecific adjuvant immunotherapy of lung cancer with cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guérin. Cancer Res 1979;39:3262–7. [PubMed] [Google Scholar]
- [18]. Ochiai T, Sato H, Hayashi R, et al. Postoperative adjuvant immunotherapy of gastric cancer with BCG-cell wall skeleton. 3- to 6-year follow up of a randomized clinical trial. Cancer Immunol Immunother 1983;14:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Kodama K, Higashiyama M, Takami K, et al. Innate immune therapy with a Bacillus Calmette-Guérin cell wall skeleton after radical surgery for non-small cell lung cancer: a case-control study. Surg Today 2009;39:194–200. [DOI] [PubMed] [Google Scholar]
- [20]. Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2004;14:135–46. [DOI] [PubMed] [Google Scholar]
- [21]. Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15:5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Call KM, Glaser T, Ito CY, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 1990;60:509–20. [DOI] [PubMed] [Google Scholar]
- [23]. Inoue K, Sugiyama H, Ogawa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood 1994;84:3071–9. [PubMed] [Google Scholar]
- [24]. Nakatsuka S, Oji Y, Horiuchi T, et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol 2006;19:804–14. [DOI] [PubMed] [Google Scholar]
- [25]. Sugiyama H. WT1 (Wilms’ Tumor Gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol 2010;40:377–87. [DOI] [PubMed] [Google Scholar]
- [26]. Inoue K, Tamaki H, Ogawa H, et al. Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood 1998;91:2969–76. [PubMed] [Google Scholar]
- [27]. Nishida S, Hosen N, Shirakata T, et al. AML1-ETO rapidly induces acute myeloblastic leukemia in cooperation with the Wilms tumor gene. WT1 Blood 2006;107:3303–12. [DOI] [PubMed] [Google Scholar]
- [28]. Ito K, Oji Y, Tatsumi N, et al. Antiapoptotic function of 17AA(+)WT1 (Wilms’ tumor gene) isoforms on the intrinsic apoptosis pathway. Oncogene 2006;25:4217–29. [DOI] [PubMed] [Google Scholar]
- [29]. Wagner KD, Cherfils-Vicini J, Hosen N, et al. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun 2014;5:5852. [DOI] [PubMed] [Google Scholar]
- [30]. Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA 2004;101:13885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Miyatake T, Ueda Y, Morimoto A, et al. WT1 peptide immunotherapy for gynecologic malignancies resistant to conventional therapies: a phase II trial. J Cancer Res Clin Oncol 2013;139:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Hashimoto N, Tsuboi A, Kagawa N, et al. Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: safety and impact on immunological response. Cancer Immunol Immunother 2015;64:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Nishida S, Ishikawa T, Egawa S, et al. Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: a phase II randomized study. Cancer Immunol Res 2018;6:320–31. [DOI] [PubMed] [Google Scholar]
- [34]. Oka Y, Tsuboi A, Nakata J, et al. Wilms’ Tumor Gene 1 (WT1) peptide vaccine therapy for hematological malignancies: from CTL epitope identification to recent progress in clinical studies including a cure-oriented strategy. Oncol Res Treat 2017;40:682–90. [DOI] [PubMed] [Google Scholar]
- [35]. Hayashi A, Doi O, Azuma I, et al. Immuno-friendly use of BCG-cell-wall skeleton remarkably improves the survival rate of various cancer patients. Proc Japan Acad 1998;74:50–5. [Google Scholar]
- [36]. Seya T, Shime H, Takeda Y, et al. Adjuvant for vaccine immunotherapy of cancer--focusing on Toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity. Cancer Sci 2015;106:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Stebbing J, Dalgleish A, Gifford-Moore A, et al. An intra-patient placebo-controlled phase I trial to evaluate the safety and tolerability of intradermal IMM-101 in melanoma. Ann Oncol 2012;23:1314–9. [DOI] [PubMed] [Google Scholar]
- [38]. Dalgleish AG, Stebbing J, Adamson DJ, et al. Randomised, open-label, phase II study of gemcitabine with and without IMM-101 for advanced pancreatic cancer. Br J Cancer 2016;115:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Dalgleish AG, Mudan S, Fusi A. Enhanced effect of checkpoint inhibitors when given after or together with IMM-101: significant responses in four advanced melanoma patients with no additional major toxicity. J Transl Med 2018;16:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Maruyama C. Treatment of malignant tumors by an extract from tubercle bacilli (tuberculosis vaccine). Nihon Ika Dagaku Zasshi 1971;38:267–76. [PubMed] [Google Scholar]
- [41]. Nishioka M, Tanemura A, Nishida S, et al. Vaccination with WT-1 (Wilms’ tumor gene-1) peptide and BCG-CWS in melanoma. Eur J Dermatol 2012;22:258–9. [DOI] [PubMed] [Google Scholar]
- [42]. Hayashi A, Nishida Y, Yoshii S, et al. Immunotherapy of ovarian cancer with cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guérin: effect of lymphadenectomy. Cancer Sci 2009;100:1991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Keilholz U, Letsch A, Busse A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 2009;113:6541–8. [DOI] [PubMed] [Google Scholar]
- [44]. Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 2010;107:13824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Anguille S, Van de Velde AL, Smits EL, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017;130:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Ueda Y, Ogura M, Miyakoshi S, et al. Phase 1/2 study of the WT1 peptide cancer vaccine WT4869 in patients with myelodysplastic syndrome. Cancer Sci 2017;108:2445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Zauderer MG, Tsao AS, Dao T, et al. A randomized phase ii trial of adjuvant galinpepimut-S, WT-1 analogue peptide vaccine, after multimodality therapy for patients with malignant pleural mesothelioma. Clin Cancer Res 2017;23:7483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Maslak PG, Dao T, Bernal Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv 2018;2:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol 2007;5:505–17. [DOI] [PubMed] [Google Scholar]
- [50]. Nordly P, Madsen HB, Nielsen HM, et al. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin Drug Deliv 2009;6:657–72. [DOI] [PubMed] [Google Scholar]
- [51]. Azuma I, Ribi EE, Meyer TJ, et al. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst 1974;52:95–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


