Abstract
Background:
We performed this meta-analysis to assess the efficacy and safety of Jianpi Liqi therapy (JLT), a traditional Chinese medicine therapy, in treating functional dyspepsia (FD).
Methods:
We systematically searched 13 databases from their inception to 15th, May 2019. Eligible studies were randomized controlled trials (RCTs) that compared JLT medicine with conventional pharmacotherapy (CP) in treating patients with FD. Cochrane Collaboration tool, Review Manager 5.3 and STATA 11.0, GRADE profiler 3.6 were used for evaluating risk of bias, analyzing, and assessing quality of evidence respectively.
Results:
After exclusions, 15 RCTs including a total of 1451 participants were included for analysis. We found evidence that JLT had better efficacy than CP (domperidone, omeprazole, esomeprazole, mosapride, lansoprazole, compound digestive enzymes, lactasin tablets) for FD (OR 0.34; 95% CI 0.26, 0.45; P < .00001). Moreover, JLT had more improvement on symptoms including abdominal pain, abdominal distention, early satiety, belching, poor appetite, and fatigue compared with CP. In addition, serious adverse events were not observed in treatment courses.
Conclusion:
This meta-analysis suggested that JLT appears to have better efficacy in treating FD compared with CP. It may be an effective and safe therapy option for patients with FD. Though, more large-sample and strictly designed RCTs are needed to confirm our findings.
PROSPERO registration number: CRD42019133241.
Keywords: Chinese traditional medicine, functional dyspepsia, meta-analysis
1. Introduction
Functional dyspepsia (FD) is a chronic, recurrent, and non-organic disease, which presents with typical gastroduodenal symptoms of epigastric pain or burning, early satiety, postprandial fullness, or a combination of them.[1,2,3] FD places high healthcare cost and financial burden on families and society.[4,5,6,7] It also significantly reduces the quality of life and productivity of individuals suffering from it. Furthermore, the global prevalence of FD ranged from 5% to 11%.[8] However, causes of FD remain to be established and current pharmacological treatments for FD cannot satisfy clinical needs.[9,10,11,12,13,14,15,16]
JLT (Invigorating spleen and regulating qi therapy, named Jianpi Liqi in Chinese pinyin) is a widely used therapeutic method in traditional Chinese medicine (TCM). JLT includes various herbal formulas which have the same aims for invigorating spleen and regulating qi. TCM, as an alternative treatment for FD, has been reported to be effective frequently.[17,18,19,20,21,22,23,24] But evidences of JLT medicine in treating FD were still insufficient. Therefore, to provide solid evidence for its efficacy, we performed this meta-analysis of randomized controlled clinical trials. In this study, our primary objective was to determine whether use of JLT in patients with FD results in better efficacy compared with CP. Our secondary goal was to identify whether use of JLT leads to greater alleviations on individual symptoms of FD.
2. Methods
2.1. Search strategy
Literature searches was conducted using the following databases: PubMed, Embase, Cochrane Library, Web of Science Core Collection, KCI-Korean Journal Database, MEDLINE, Russian Science Citation Index and SciELO Citation Index, Springer Link, China National Knowledge Infrastructure, Chinese Scientific Journals Database, Wan-fang database, and Chinese Biomedicine Database from their inception to 15th May 2019. RCTs comparing JLT medicine alone with conventional medical treatment were potentially eligible for inclusion. Our search has no language limitation. Key words used for search were “functional dyspepsia”, “epigastric pain syndrome”, “postprandial discomfort syndrome”, “FD”, “traditional Chinese medicine”, “Jianpi”, “Liqi”, “herb formula”, “randomized controlled trial”, “controlled clinical trial” and “clinical trials”. These searched words were used separately and collectively. Moreover, manual searches in cited references were performed to prevent missing relevant articles.
2.2. Selection criteria
Studies were chosen for this meta-analysis when they met the following criteria:
-
1.
Randomized controlled trial;
-
2.
Patients were diagnosed with FD by ROME III or IV criteria;
-
3.
The experiment group used JLT medicine alone;
-
4.
The control group used CP.
-
5.
The Jadad score was at least 1.
While major exclusions were:
-
1.
Not clinical trial;
-
2.
Duplicated publication;
-
3.
Patients accompanied by other digestive diseases;
-
4.
Review article, case report or selective reporting.
2.3. Data extraction and quality assessment
Two reviewers independently extracted data from the selected studies. The information consisted of:
-
1.
general information including name of first author, publication year, sex, sample size, age of subjects, intervention, and duration of treatment;
-
2.
methodological information including details of randomization, blinding, allocation concealment, and description of withdrawals;
-
3.
outcome measures, follow-up periods, and adverse events.
Evaluation of methodological quality was also conducted independently by 2 reviewers using the Cochrane Collaboration's risk of bias tool[25] and Jadad scale.[26] Disagreements were resolved by discussion or by consulting a third reviewer.
3. Data analysis
Data analysis was performed by using Review Manager 5.3, STATA 11.0 and GRADE profiler 3.6. Efficacy of JLT compared with CP in treating FD was estimated by odds ratio (OR) and 95% confidence interval (95%CI) for each study. The TCM symptoms score, as continuous data, was estimated by standardized mean difference (SMD) and 95%CI. Heterogeneity was statistically assessed by χ 2 test and I 2 test, and it was presented as significant when I 2 was over 50% or P < .1.[27] A random effect model was applied to calculate the pooled statistics when there was significant heterogeneity, or else the fixed effect model was used.[27] Sensitivity analysis was performed to investigate potential study which would obviously influence results. Begg test was used for evaluating publication bias. In addition, GRADE profiler 3.6 was used to assess the quality of outcomes.
4. Result
4.1. Description of studies
The search results and the number of studies reviewed, excluded, and included were presented in a flow diagram in Figure 1. The eligible 15 RCTs included 1451participants (727 in experiment group and 724 in control group).[28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] All included studies were single-center trials and were published in Chinese. Interventions between experiment groups and control groups were all JLT versus CP. Eleven studies used TCM decoction in experiment groups,[28,29,30,31,32,33,34,35,39,40,42] 3 used TCM granule[36,37,38] and the remain used TCM oral liquid.[41] More characteristics of the included studies were described in Table 1. The constituents of herbal formulae were listed in Table 2 and the frequencies of usage were summarized in Table 3. Atractylodes macrocephala Koidz (Bai Zhu), Radix Glycyrrhizae preparata (Gan Cao), Poria cocos (Schw.)Wolf (Fu Ling), and Amomum villosum Lour (Sha Ren) were the most frequently used among 51 kinds of herbs used in experiment group.
Figure 1.

Flow chart of study selection process.
Table 1.
Characteristics of included studies.

Table 2.
The ingredients of each formula.

Table 3.
Frequencies of usage and distribution in TCM.

4.2. Methodological quality of included studies
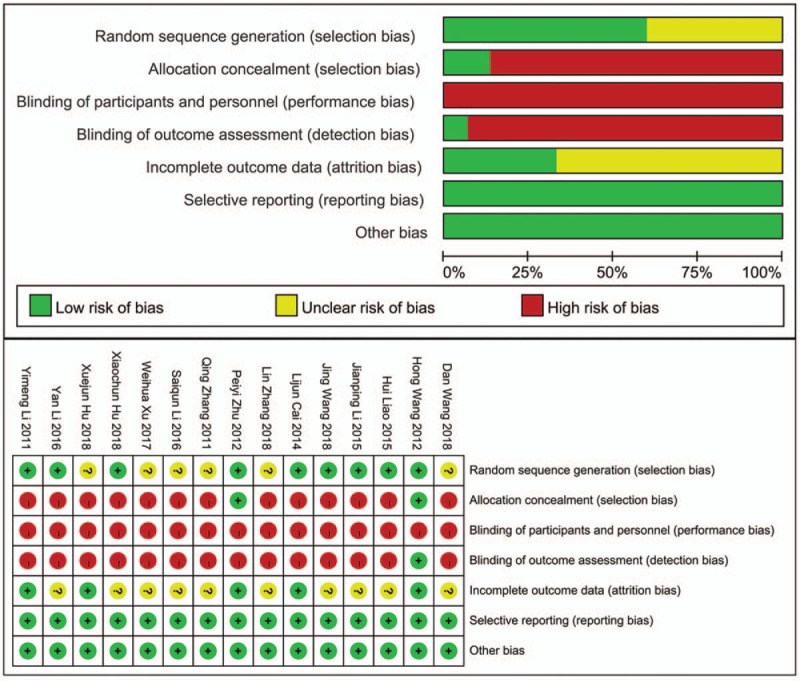
All included studies reported that baselines were comparable among groups. Jadad scores of the included RCTs ranged from 1 to 3 points. Studies got 3 points in Jadad scores were considered as high quality,[28,30,31,32] and those got 1 or 2 points were considered as low quality.[29,33,34,35,36,37,38,39,40,41,42] There are respectively 13,[28,29,32,33,34,35,36,37,38,39,40,41,42] 15,[28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] and 14[28,29,30,32,33,34,35,36,37,38,39,40,41,42] trials which did not mention allocation concealment, blinding of participants and personnel, blinding of outcome assessment. Therefore, these studies were considered having high risk of selection bias, performance bias and detection bias respectively. A description of methodological quality of the selected trials were summarized in Table 4. And the risk of bias assessment of selected studies was shown in Figure 2.
Table 4.
Evaluation of methodological quality of included studies.
Figure 2.
Risk of bias summary and graph.
4.3. Primary outcomes: clinical efficacy rate
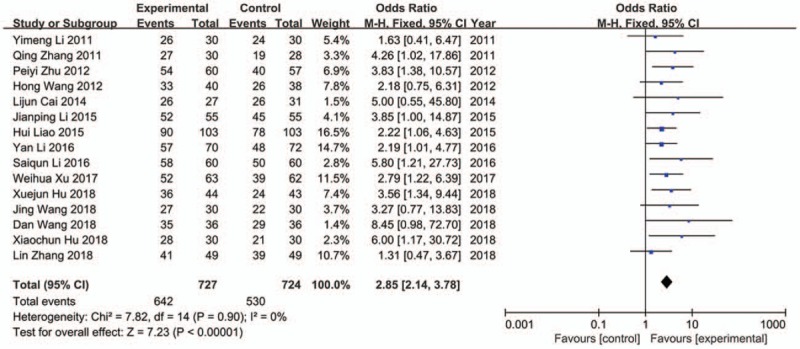
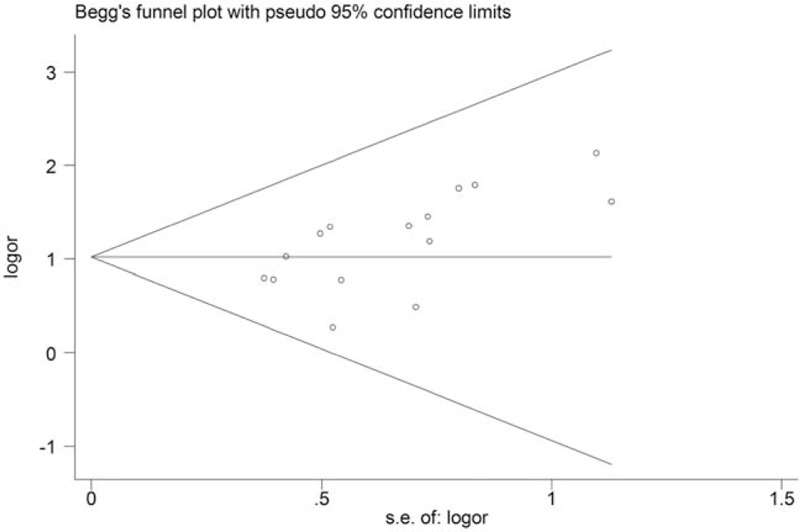
Fifteen comparisons from all included studies were pooled for the primary outcome of clinical efficacy rate, which were calculated according to the standards of the Guiding Principles for the Clinical Research of New TCM.[43] There were 727 patients in experiment groups received JLT, while 724 patients received CP in control groups. Under 90% significance level, heterogeneity analysis indicated that there was no statistical heterogeneity among these studies (Chi2 = 7.82, P = .90, I 2 = 0%) (Fig. 3). Therefore, fixed effect model was chosen to perform the trial and the result showed that JLT had significantly better clinical efficacy than CP on treating FD (OR 2.85; 95%CI 2.14, 3.78; P < .00001) (Fig. 3). Besides, potential publication bias was identified by funnel plot analysis (Begg test P = .018) (Fig. 4).
Figure 3.
Forest plot of effective rate (fixed effect model).
Figure 4.
Funnel plot of effective rate.
4.4. Second outcomes: Improvement of TCM symptoms scores
4.4.1. Abdominal pain scores
Among all included trials, 9 trials reported the improvement of abdominal pain,[28,29,30,31,34,35,37,38,41] but 4 of them used different scoring criteria.[28,31,35,41] Therefore, only 5 studies were used for analysis.[29,30,34,37,38] The result showed that JLT medicine had better efficacy in relieving abdominal pain (SMD −0.45; 95%CI −0.61, −0.29; P < .00001) with no statistical heterogeneity (Chi2 = 3.78, P = 0.44, I 2 = 0%) (Fig. 5).
Figure 5.
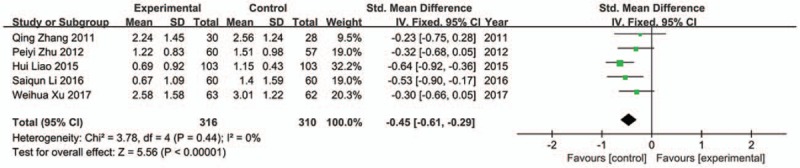
Forest plot of abdominal pain (fixed effect model).
4.4.2. Abdominal distention scores
Although 11 studies reported abdominal distention,[28,29,30,31,34,35,37,38,39,41,42] 6 trials used different scoring criteria.[28,30,31,35,41,42] Thus, only 5 trials were included in analysis.[29,34,37,38,39] The fixed effect model was used as there was no apparently statistical heterogeneity (Chi2 = 2.63, P = .62, I 2 = 0%). The result indicated that JLT medicine had greater abdominal distention alleviation compared to control groups (SMD −0.34; 95%CI −0.50, −0.18; P < .0001) (Fig. 6).
Figure 6.
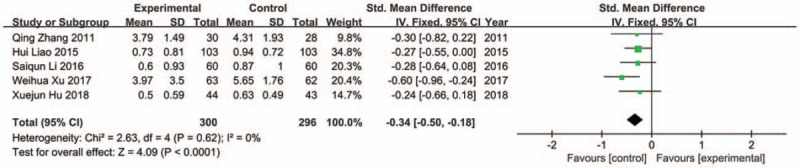
Forest plot of abdominal distention (fixed effect model).
4.4.3. Early satiety scores
In the included studies, 4 studies reported early satiety.[30,34,38,41] One trial was excluded from analysis because of significant heterogeneity.[41] No apparently heterogeneity was found among the other 3 trials (Chi2 = 0.27, P = .88, I 2 = 0%). The result showed that JTL groups was superior to control groups in relieving early satiety (SMD −0.37; 95%CI −0.56, −0.19; P < .0001) (Fig. 7).
Figure 7.
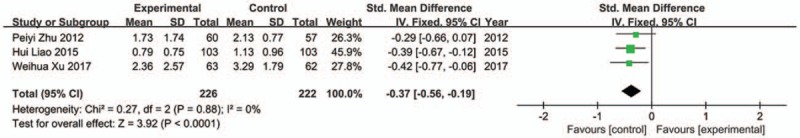
Forest plot of early satiety (fixed effect model).
4.4.4. Belching scores
Three trials were included in analysis[31,37,39] and fixed model was used because of no apparently statistical heterogeneity (Chi2 = 1.71, P = .42, I 2 = 0%). The result indicated that JLT groups had more significant improvement on belching than control groups (SMD −0.50; 95%CI −0.74, −0.27; P < .0001) (Fig. 8).
Figure 8.
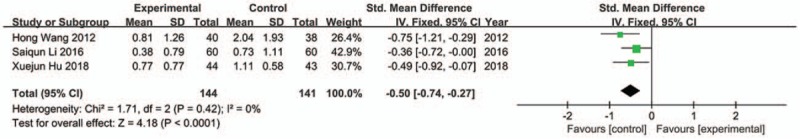
Forest plot of belching (fixed effect model).
4.4.5. Poor appetite scores
Fixed model was conducted as no apparently heterogeneity was found among the 3 trials[29,31,37] which were included in analysis (Chi2 = 0.90, P = .64, I 2 = 0%). The result showed that JLT had better efficacy in alleviating poor appetite (SMD −0.52; 95%CI −0.77, −0.27; P < .0001) (Fig. 9).
Figure 9.
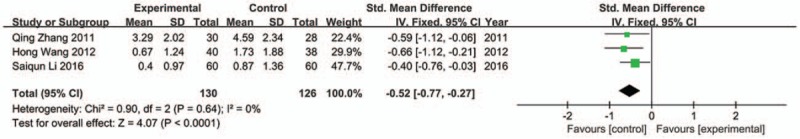
Forest plot of poor appetite (fixed effect model).
4.4.6. Fatigue scores
Three trials were included in analysis,[29,37,39] and there was no significant heterogeneity among them (Chi2 = 1.53, P = .47, I 2 = 0%). The result showed greater improvement in fatigue for JLT groups compared with control groups (SMD −0.76; 95%CI −1.01, −0.51; P < .00001) (Fig. 10).
Figure 10.
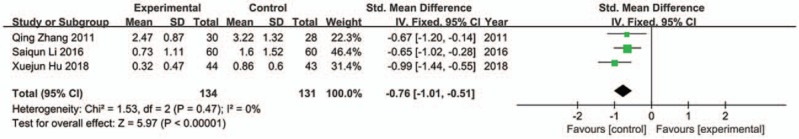
Forest plot of fatigue (fixed effect model).
4.5. GRADE evidence of quality
In order to assess the quality of evidences and reliability of this meta-analysis, we performed an evaluation by using GRADE profiler software. The results showed that the evidence quality was “low”. Detailed information of assessment and basis of classification were showed in Figure 11 and Table 5.
Figure 11.
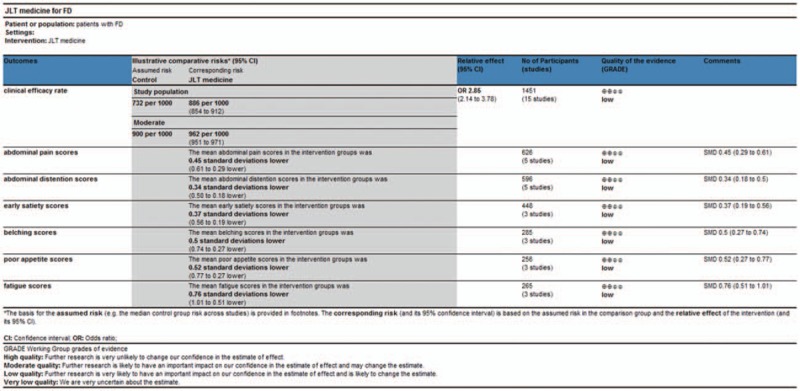
GRADE quality grading evaluation.
Table 5.
GRADE quality grading evaluation.
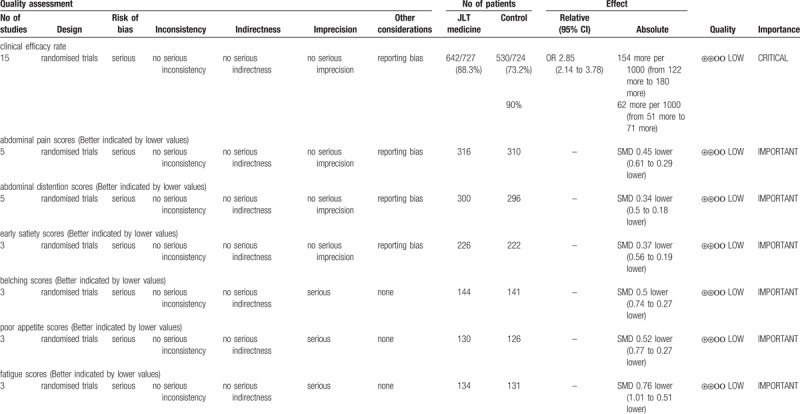
4.6. Adverse events
Ten studies reported adverse events.[28,29,31,32,35,36,37,38,39,40] Among them, 8 trials mentioned no adverse event.[29,31,32,35,36,37,39,40] One study reported that 2 patients in experiment group had loose stool for 2 days, 3 patients in control group appeared mild diarrhea for 3 days[28]. These discomforts disappeared without any intervention. Another study reported that 2 patients in experiment group experienced mild diarrhea and 1 patient appeared rash.[38] All discomforts disappeared after drug withdrawal and no further measure was needed.
5. Discussion
In the meta-analysis of RCTs comparing JLT with CP in participants with FD, use of JLT resulted in better efficacy and greater alleviations on individual symptoms (including abdominal pain, abdominal distention, early satiety, belching, poor appetite, and fatigue). However, because of the usage of different scoring criteria or measures in the second outcome, not all studies reporting these symptoms were included in analyses. That could lead to small sample size and bias in the second outcome.
Current study has not fully understood the pathogenesis of FD. It is generally believed that various factors can lead to FD, including gastric motility and compliance, altered gut microbiome, psychological distress (particularly anxiety), visceral hypersensitivity and infection (specially Helicobacter pylori).[1,44,45,46,47] Present treatments for FD are mostly based on individual symptoms and experience, including H. pylori eradication therapy, acid-suppression therapy, prokinetic agents, antidepressants, and psychological therapy.[9,10,11,12,13,14,15] On the other hand, FD belongs to the category of Wei Tong (stomachache) or Wei Pi (stomach distention and fullness) in TCM, and the basic pathogenesis is widely considered as Pi deficiency and Qi stagnation.[2,48] The representative formula of JLT is Xiangsha Liujunzi Tang. JLT medicine has been reported to have better clinical efficacy than CP (such as domperidone, mosapride, lansoprazole) in treating patients with FD.[19,49,50,51] Moreover, numerous studies have found modern pharmacology evidences for JLT medicine's efficacy in treating FD. According to clinical trials, JLT medicine could raise the level of Ghrelin to ameliorate gastric empty rate and consequently relieve symptoms of FD.[52,53] Meanwhile, the study of Pan[54] indicated that JLT medicine can also raise the level of acetylcholin esterase (AChE) to ameliorate gastric emptying rate. Besides, animal experiment proved that JLT medicine can enhance rats’ gastric emptying by increasing the content of motilin, gastrin, ghrelin, 5-hydroxytryptamine, decreasing the content of calcitonin gene related peptide and up-regulating the expressions of 5-HT4R (5-hydroxytryptamine 4 receptor) mRNA and 5-HT4R protein.[55] There was also experiment showed that JLT medicine can raise the levels of ghrelin, cholecystokinin, and vasoactive intestinal polypeptide in rats to alleviate the symptoms of FD.[56] Study of Xiaona Wang showed that JLT medicine can increase the expression level of CaMKII (Ca[2+]/calmodulin-dependent kinase II) to promote gastric motility in rats.[57] Experiment of Yuhong Ge proved that Sijun Zi Decoction can lower visceral hypersensibility by decreasing the expression level of phospholipase C-γ and transient receptor potential vanilloid 1 mRNA in rats.[58]
We also summarized the frequency of each single herb used in included trails. The most frequently used herbs were Atractylodes macrocephala Koidz (Bai Zhu), Radix Glycyrrhizae preparata (Gan Cao), Poria cocos(Schw.)Wolf (Fu Ling), and Amomum villosum Lour (Sha Ren). There were plenty of ingredients in Bai Zhu, including sesquiterpenoids, phenylpropanoids, polyacetylenes, coumarins, triterpenoids, flavonoids, and flavonoid glycosides, steroids, benzoquinones, and polysaccharides. Bai Zhu was also proved to have varied pharmacological effects, including anti-tumor, anti-inflammatory, anti-aging activity, immunomodulatory, and improving gastrointestinal function.[59,60,61,62,63,64,65] Gan Cao was found to mainly contain flavonoid and triterpenoid, and have the effects of anti-inflammatory, analgesia as well as reducing intestinal motility, according to studies.[66,67,68,69] Experiments revealed that triterpenoid and pachymaran were the main components of Fu Ling and anti-tumor effect, hepatoprotective effect, immunization as well as anti-inflammatory effect were pharmacological actions of Fu Ling.[70,71,72,73] Sha Ren mainly contains volatile oil and polysaccharide. It has effects of protecting gastric mucosa, anti-inflammatory, facilitating gastric emptying as well as intestine peristalsis, according to researches.[74,75,76,77,78]
Several potential limitations of this study should be noted. First, allocation concealment and blinding were not conducted adequately in most of the included studies (only 2 studies reported detailed description of allocation concealment and only 1 reported blind method). It led to high risk of biases of these trials and consequently resulted in low quality evidence of this meta-analysis. Second, meta-analysis of recurrence rate was not performed, due to the insufficiency of follow-up period in most included trials (only 4 studies reported 1-month or 6-months follow-up period).
In this study the limited evidence available suggests that JLT was superior to CP on treating FD patients. However, the reported effectiveness of JTL for FD can be consider as encouraging but not convincing, the low-quality evidence is insufficient to recommend the use of JTL. But it is sufficient to support the necessity of further study. This study indicated that the assessment of recurrence rate should be performed in further study to evaluate the long-term effect of JLT. The problem that how to perform adequate allocation concealment and blinding should be emphatically solved in future RCTs for TCM versus CP.
6. Conclusions
In summary, this meta-analysis could provide a degree of evidence for the efficacy and safety of JLT medicine in treating patients with FD. However, further standardized, rigorously designed, and large-scale RCTs are required to provide more convincing and solid evidence.
Author contributions
Conceptualization: Jin-Tong Ye, Ling Hu.
Data curation: Yun-kai Dai, Dan-yan Li.
Formal analysis: Jin-Tong Ye, Yun-zhan Zhang, Meng-Xin Huang.
Funding acquisition: Ling Hu.
Investigation: Ling Hu.
Methodology: Jin-Tong Ye, Meng-Xin Huang, Wei-Jing Chen.
Project administration: Ru-Liu Li, Ling Hu.
Resources: Ru-Liu Li, Dan-yan Li.
Software: Jin-tong Ye, Yun-kai Dai.
Supervision: Ru-Liu Li, Ling Hu.
Validation: Ru-Liu Li, Ling Hu.
Visualization: Jin-tong Ye, Yun-zhan Zhang, Dan-yan Li, Meng-Xin Huang.
Writing – original draft: Jin-tong Ye.
Writing – review & editing: Jin-tong Ye, Yun-zhan Zhang, Dan-yan Li, Yun-kai Dai.
Ling Hu orcid: 0000-0003-3104-8050.
Footnotes
Abbreviations: JLT = Jianpi Liqi therapy, FD = functional dyspepsia, RCTs = randomized controlled trials, CP = conventional pharmacotherapy, TCM = traditional Chinese medicine, OR = odds ratio, CI = confidence interval, SMD = standardized mean difference, AChE = acetylcholin esterase, 5-HT4R = 5-hydroxytryptamine 4 receptor, CaMKII = Ca2+/calmodulin-dependent kinase II, ITT = Intention to treat.
J-TY and Y-KD contributed equally to this work.
This study was supported by National Natural Science Foundation of China, No.81774238, 81373563, 30772689; Project of Guangdong Province “South China collaborative innovation center of traditional Chinese medicine - Spleen and stomach disease research team” (2016) No. 2016KYTD07; Construction of high-level university of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine (2016) No.64; Special fund for “construction of first-class discipline” of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine (2017) No.70. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1]. Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med 2015;373:1853–63. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- [2]. Zhang SS, Zhao LQ. TCM guidelines on clinical diagnosis and treatment of functional dyspepsia (Chinese). China J Tradition Chin Med Pharmacy 2017;2017:2595–8. [Google Scholar]
- [3]. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterol 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- [4]. Sander GB, Mazzoleni LE, Francesconi CF, et al. Influence of organic and functional dyspepsia on work productivity: the HEROES-DIP study. Value Health 2011;14 5 Suppl 1:S126–9. doi: 10.1016/j.jval.2011.05.021. [DOI] [PubMed] [Google Scholar]
- [5]. Halder SL, Locke GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case-control study. Aliment Pharmacol Ther 2004;19:233–42. [DOI] [PubMed] [Google Scholar]
- [6]. Lacy BE, Weiser KT, Kennedy AT, et al. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther 2013;38:170–7. doi: 10.1111/apt.12355. [DOI] [PubMed] [Google Scholar]
- [7]. Brook RA, Kleinman NL, Choung RS, et al. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol 2010;8:498–503. doi: 10.1016/j.cgh.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [8]. Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut 2015;64:1049–57. doi: 10.1136/gutjnl -2014- 307843. [DOI] [PubMed] [Google Scholar]
- [9]. Talley NJ. Functional dyspepsia: new insights into pathogenesis and therapy. Korean J Intern Med 2016;31:444–56. doi: 10.3904/kjim.2016.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Lacy BE, Talley NJ, Locke GR, et al. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther 2012;36:3–15. doi: 10.1111/j 1365 -2036 2012 05128 x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013;10:187–94. doi: 10.1038/nrgastro.2013.11. [DOI] [PubMed] [Google Scholar]
- [12]. Wysowski DK, Corken A, Gallo-Torres H, et al. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol 2001;96:1698–703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- [13]. Moayyedi P, Soo S, Deeks J, et al. Eradication of helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev 2000;D2096 doi: 10.1002/14651858.CD002096. [DOI] [PubMed] [Google Scholar]
- [14]. den Hollander WJ, Kuipers EJ. Current pharmacotherapy options for gastritis. Expert Opin Pharmacother 2012;13:2625–36. doi: 10.1517/14656566.2012.747510. [DOI] [PubMed] [Google Scholar]
- [15]. Lahner E, Bellentani S, Bastiani RD, et al. A survey of pharmacological and nonpharmacological treatment of functional gastrointestinal disorders. United European Gastroenterol J 2013;1:385–93. doi: 10.1177/2050640613499567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Cote GA, Howden CW. Potential adverse effects of proton pump inhibitors. Curr Gastroenterol Rep 2008;10:208–14. [DOI] [PubMed] [Google Scholar]
- [17]. Zhao L, Zhang S, Wang Z, et al. Efficacy of modified ban xia xie xin decoction on functional dyspepsia of cold and heat in complexity syndrome: a randomized controlled trial. Evid Based Complement Alternat Med 2013;2013:812143 doi: 10.1155/2013/812143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Du HG, Ming L, Chen SJ, et al. Xiaoyao pill for treatment of functional dyspepsia in perimenopausal women with depression. World J Gastroenterol 2014;20:16739–44. doi: 10.3748/wjg.v20.i44.16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Arai M, Matsumura T, Tsuchiya N, et al. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology 2012;59:62–6. doi: 10.5754/hge11246. [DOI] [PubMed] [Google Scholar]
- [20]. Zhang SS, Zhao LQ, Wang HB, et al. Efficacy of Gastrosis No.1 compound on functional dyspepsia of spleen and stomach deficiency-cold syndrome: a multi-center, double-blind, placebo-controlled clinical trial. Chin J Integr Med 2013;19:498–504. doi: 10.1007/s11655-013-1503-x. [DOI] [PubMed] [Google Scholar]
- [21]. Suzuki H, Matsuzaki J, Fukushima Y, et al. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia--a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil 2014;26:950–61. doi: 10.1111/nmo.12348. [DOI] [PubMed] [Google Scholar]
- [22]. Oikawa T, Ito G, Hoshino T, et al. (Banxia-houpo-tang), a Kampo Medicine that treats functional dyspepsia. Evid Based Complement Alternat Med 2009;6:375–8. doi: 10.1093/ecam/nem101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Tominaga K, Arakawa T. Kampo medicines for gastrointestinal tract disorders: a review of basic science and clinical evidence and their future application. J Gastroenterol 2013;48:452–62. doi: 10.1007/s00535-013-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Chu M, Wu I, Ho R, et al. Chinese herbal medicine for functional dyspepsia: systematic review of systematic reviews. Therap Adv Gastroenterol 2018;11: 322992859. doi: 10.1177/1756284818785573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
- [26]. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [27]. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Li Y-M, Jin H-L, Li A, et al. Clinical observation of “Jianpi Liqi Decoction” in treating functional dyspepsia of spleen deficiency and qi stagnation (Chinese). Shanghai J Tradition Chin Med 2011;27–30. [Google Scholar]
- [29]. Zhang Q, Liang C. Clinical observation of using spleen invigorating and qi promoting method to treat functional dyspepsia (Chinese). J Sichuan Tradition Chin Med 2011;29:67–9. [Google Scholar]
- [30]. Zhu Pei-yi, Zhang L, Wang Hong-bing. Study on functional dyspepsia with epigastric pain syndrome treated by Jianpi Liqi decoction (Chinese). Chin J Basic Med Tradition Chin Med 2012;18:874–7. [Google Scholar]
- [31]. Wang H, Wang Hong-bing, Deng Jin-mei, et al. Study on functional dyspepsia with syndrome of spleen deficiency and Qi stagnation treated by invigorating spleen and regulating Qi method and its effect on visceral sensitivity (Chinese). China J Tradition Chin Med Pharmacy 2012;1321–4. [Google Scholar]
- [32]. Cai Li-jun, Fan Yi-hong, Lv Bin, et al. Effect observation of xiangsha six mild-drug decoction in treatment of spleen and stomach deficiency type of functional dyspepsia (Chinese). Chin Arch Tradition Chin Med 2014;1974–6. [Google Scholar]
- [33]. Hu Xiao-chun, Li Xue-jun. Clinical observation of jianpi xiaopi decoction in treating functional dyspepsia with qi defciency syndrome (Chinese). Clin J Tradition Chin Med 2018;817–20. doi: 10.13359/j.cnki.gzxbtcm.2015.05.008. [Google Scholar]
- [34]. Liao H, Wang Xiao-jun, Zou Zhi-hong. Clinical observation of using janpi tiaozhong xiaopi decoction to treat functional dyspepsia (Chinese). J Pract Tradition Chin Med 2015;898–9. [Google Scholar]
- [35]. Li Jian-ping, Cai Cui-zhu, Liu De-xi. Clinical observation of using spleen invigorating and qi promoting method to treat functional dyspepsia (Chinese). J Guangzhou Univ Tradition Chin Med 2015;817–20. [Google Scholar]
- [36]. Li Yan, Liu Hua-yi, Wang Xiu-juan, et al. Clinical observation of effect of an wei I Granules on functional dyspepsia (Chinese). Shanxi J Tradition Chin Med 2016;32:16–8. [Google Scholar]
- [37]. Li Sai-qun, Yang Zheng-yu, Jiang H, et al. Efficacy of Weiguangping granules in treatment of functional dyspepsia: a clinical analysis of 60 cases (Chinese). Hunan J Tradition Chin Med 2016;4–6. [Google Scholar]
- [38]. Xu Wei-hua, Wang W, Li Ni-jiao, et al. Effects of modifed Xiangsha Liujunzi Decoction combined with Zhizhu Pills in the treatment of functional dyspepsia with spleen defciency and qi stagnation and its influence on radio nuclide gastric emptying (Chinese). China J Tradition Chin Med Pharmacy 2017;1025–8. [Google Scholar]
- [39]. Hu Xue-jun, He Gui-hua, Wu Zi-an, et al. Clinical effect of Jianpi Liqi Recipe for treating postprandial distress syndrome of functional dyspepsia with spleen deficiency and qi stagnation type in 45 cases and its effect on gastrointestinal hormone (Chinese). China Pharmaceut 2018;29–32. [Google Scholar]
- [40]. Wang Jin. Clinical observation of Zhishi Xiaopi pills combined with Huangqi Jianzhong decoction in treating for functional dyspepsia with anxiety (Chinese). Acta Chinese Med 2018;1542–7. [Google Scholar]
- [41]. Wang Dan. Clinical evaluation of Liqixiaozhang oral liquid in treating functional dyspepsia of spleen deficiency syndrome (Chinese). J Yanan Univ (Med Sci) 2018S;60–3. [Google Scholar]
- [42]. Zhang L, Zhang W, Feng Shi-min, et al. Clinical study on Tiaozhong Jianpi decoction in treatment of spleen deficiency and Qi stagnation syndrome of functional dyspepsia (Chinese). Liaoning J Tradition Chin Med 2018;1435–7. [Google Scholar]
- [43]. Zheng XY. Guiding Principles for Clinical Research on New Drugs of Traditional Chinese Medicine (Chinese). 2005;Beijing: Chinese Medical Science and Technology Press, 124–151. [Google Scholar]
- [44]. Dal K, Deveci OS, Kucukazman M, et al. Decreased parasympathetic activity in patients with functional dyspepsia. Eur J Gastroenterol Hepatol 2014;26:748–52. doi: 10.1097/MEG.0000000000000111. [DOI] [PubMed] [Google Scholar]
- [45]. Overland MK. Dyspepsia. Med Clin North Am 2014;98:549–64. doi: 10.1016/j.mcna.2014.01.007. [DOI] [PubMed] [Google Scholar]
- [46]. Burri E, Barba E, Huaman JW, et al. Mechanisms of postprandial abdominal bloating and distension in functional dyspepsia. Gut 2014;63:395–400. doi: 10.1136/gutjnl -2013- 304574. [DOI] [PubMed] [Google Scholar]
- [47]. Futagami S, Shimpuku M, Yin Y, et al. Pathophysiology of functional dyspepsia. J Nippon Med Sch 2011;78:280–5. [DOI] [PubMed] [Google Scholar]
- [48]. Cheng QS. Research progress on TCM in treatment of functional dyspepsia (Chinese). Chin Arch Tradition Chin Med 2015;33:70–2. [Google Scholar]
- [49]. Zhao L, Gan AP. Clinical and psychological assessment on Xinwei decoction for treating functional dyspepsia accompanied with depression and anxiety. Am J Chin Med 2005;33:249–57. doi: 10.1142/S0192415X05002801. [DOI] [PubMed] [Google Scholar]
- [50]. Wang C, Zhu M, Xia W, et al. Meta-analysis of traditional Chinese medicine in treating functional dyspepsia of liver-stomach disharmony syndrome. J Tradit Chin Med 2012;32:515–22. [DOI] [PubMed] [Google Scholar]
- [51]. Ling W, Li Y, Jiang W, et al. Common mechanism of pathogenesis in gastrointestinal diseases implied by consistent efficacy of single chinese medicine formula: a PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1111 doi: 10.1097/MD.0000000000001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Tatsuta M, Iishi H. Effect of treatment with liu-jun-zi-tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther 1993;7:459–62. [DOI] [PubMed] [Google Scholar]
- [53]. Matsumura T, Arai M, Yonemitsu Y, et al. The traditional Japanese medicine Rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol 2010;45:300–7. doi: 10.1007/s00535-009-0166-z. [DOI] [PubMed] [Google Scholar]
- [54]. Pan QH, Wei WB. Study on Jianpi Hewei Xiaopi herbs in treating functional dyspepsia with spleen and stomach weakness syndrome and its’ influence on acetylcholin esterase, motilin, somatostatin (Chinese). Modern J Integr Tradition Chin West Med 2017;26:2121–3. doi: 10.3969/j.issn.1008-8849.2017.19.022. [Google Scholar]
- [55]. Li XL. Study on Renzhu Jianpi Liqi fomular in treating rats with functional dyspepsia and its influence on gastric emptying function, Ghrelin, 5-HT and CGRP (Chinese). Chin J Integr Tradition West Med Digest 2014;355–9. doi: 10.3969/j.issn.1671-038X.2014.07.02. [Google Scholar]
- [56]. Liu J, Li F, Tang XD, et al. XiangshaLiujunzi decoction alleviates the symptoms of functional dyspepsia by regulating brain-gut axis and production of neuropeptides. BMC Complement Altern Med 2015;15:387 doi: 10.1186/s12906-015-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Wang XN, Wang BB, Zhou T, et al. The study on the effects of Jianpi Liqi Decoction on rats with functional dyspepsia and its mechanism (Chinese). Chin J Integr Tradition West Med Digest 2012;297–300. [Google Scholar]
- [58]. Ge YH. Study on Treatment Regularity of Professor Huang Suiping and Experiments on Treating Functional Dyspepsia of Spleen Deficiency with the Decoction of Si Junzi (Chinese). [dissertation]. Guangzhou University of Chinese Medicine; 2017. [Google Scholar]
- [59]. Xu D, Li B, Cao N, et al. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget 2017;8:70394–405. doi: 10.18632/oncotarget.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Ji GQ, Chen RQ, Zheng JX. Macrophage activation by polysaccharides from Atractylodes macrocephala Koidz through the nuclear factor-kappaB pathway. Pharm Biol 2015;53:512–7. doi: 10.3109/13880209.2014.929152. [DOI] [PubMed] [Google Scholar]
- [61]. Song HP, Li RL, Chen X, et al. Atractylodes macrocephala Koidz promotes intestinal epithelial restitution via the polyamine--voltage-gated K+ channel pathway. J Ethnopharmacol 2014;152:163–72. doi: 10.1016/j.jep.2013.12.049. [DOI] [PubMed] [Google Scholar]
- [62]. Wang R, Zhou G, Wang M, et al. The metabolism of polysaccharide from atractylodes macrocephala koidz and its effect on intestinal microflora. Evid Based Complement Alternat Med 2014;2014:926381 doi: 10.1155/2014/926381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Zhu B, Zhang QL, Hua JW, et al. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: a review. J Ethnopharmacol 2018;226:143–67. doi: 10.1016/j.jep.2018.08.023. [DOI] [PubMed] [Google Scholar]
- [64]. Liu Z, Sun Y, Zhang J, et al. Immunopotentiation of polysaccharides of Atractylodes macrocephala Koidz-loaded nanostructured lipid carriers as an adjuvant. Int J Biol Macromol 2018;120 (Pt A):768–74. doi: 10.1016/j.ijbiomac.2018.08.108. [DOI] [PubMed] [Google Scholar]
- [65]. Li W, Guo S, Xu D, et al. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) relieves immunosuppression in cyclophosphamide-treated geese by maintaining a humoral and cellular immune balance. Molecules 2018;23: doi: 10.3390/molecules23040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Zhang Q, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A 2009;1216:1954–69. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]
- [67]. Baltina LA. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr Med Chem 2003;10:155–71. [DOI] [PubMed] [Google Scholar]
- [68]. Yang R, Yuan BC, Ma YS, et al. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol 2017;55:5–18. doi: 10.1080/13880209.2016.1225775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Chen G, Zhu L, Liu Y, et al. Isoliquiritigenin, a flavonoid from licorice, plays a dual role in regulating gastrointestinal motility in vitro and in vivo. Phytother Res 2009;23:498–506. doi: 10.1002/ptr.2660. [DOI] [PubMed] [Google Scholar]
- [70]. Sun Y. Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives. Int J Biol Macromol 2014;68:131–4. doi: 10.1016/j.ijbiomac.2014.04.010. [DOI] [PubMed] [Google Scholar]
- [71]. Rios JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med 2011;77:681–91. doi: 10.1055/s-0030-1270823. [DOI] [PubMed] [Google Scholar]
- [72]. Wang YZ, Zhang J, Zhao YL, et al. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: a review. J Ethnopharmacol 2013;147:265–76. doi: 10.1016/j.jep.2013.03.027. [DOI] [PubMed] [Google Scholar]
- [73]. Okui Y, Morita M, Iizuka A, et al. Effects of Hoelen on the efferent activity of the gastric vagus nerve in the rat. Jpn J Pharmacol 1996;72:71–3. [DOI] [PubMed] [Google Scholar]
- [74]. Xue X, Yang D, Wang D, et al. Solidification of floating organic drop liquid-phase microextraction cell fishing with gas chromatography-mass spectrometry for screening bioactive components from Amomum villosum Lour. Biomed Chromatogr 2015;29:626–32. doi: 10.1002/bmc.3324. [DOI] [PubMed] [Google Scholar]
- [75]. Jafri MA, Farah, Javed K, et al. Evaluation of the gastric antiulcerogenic effect of large cardamom (fruits of Amomum subulatum Roxb). J Ethnopharmacol 2001;75:89–94. [DOI] [PubMed] [Google Scholar]
- [76]. Zhang D, Li S, Xiong Q, et al. Extraction, characterization and biological activities of polysaccharides from Amomum villosum. Carbohydr Polym 2013;95:114–22. doi: 10.1016/j.carbpol.2013.03.015. [DOI] [PubMed] [Google Scholar]
- [77]. Chen Z, Ni W, Yang C, et al. Therapeutic effect of amomum villosum on inflammatory bowel disease in rats. Front Pharmacol 2018;9: 639. doi: 10.3389/fphar.2018.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Lu S, Zhang T, Gu W, et al. Volatile oil of amomum villosum inhibits nonalcoholic fatty liver disease via the gut-liver axis. Biomed Res Int 2018;2018: 3589874. doi: 10.1155/2018/3589874. [DOI] [PMC free article] [PubMed] [Google Scholar]


