Abstract
Background:
The effects of early continuous renal replacement therapy (CRRT) on mortality in patients with septic acute kidney injury (AKI) remain controversial. A systematic review and meta-analysis was performed to investigate the impact of timing of CRRT on clinical outcomes in patients with septic AKI.
Methods:
The PubMed, Cochrane, and Embase databases were searched from inception to the 31st of March 2019, to identify trials that assessed the timing of initiation of CRRT in patients with septic AKI.
Results:
Five trials including 900 patients were included. The results of this meta-analysis showed that there was no significant difference between 28-day mortality (odds ratio = 0.76;95% CI, 0.58–1.00; P = .05) and 90-day mortality(odds ratio = 0.79;95% CI, 0.59–1.06; P = .12)of early and late initiation of CRRT group. In addition, compared with late initiation strategy, early initiation showed no significant advantage in length of stay in ICU (Mean difference = −0.9;95% CI, −2.37 to 0.57; P = .23) and length of stay in hospital (Mean difference = −1.43;95% CI, −5.28 to 2.41; P = .47).
Conclusion:
Our meta-analysis revealed that early initiation of CRRT could not reduce mortality in patients with septic AKI. The study also showed no significant difference in ICU length of stay or hospital length of stay between early and late CRRT group. To achieve optimal timing of CRRT for septic AKI, large multicenter randomized trials with better design are still needed.
Keywords: acute kidney injury, continuous renal replacement therapy, meta-analysis, sepsis, septic shock, timing
1. Introduction
Acute kidney injury(AKI) is a frequent complication in patients hospitalized in the intensive care unit (ICU) for septic shock[1,2,3] and is associated with high mortality.[3,4,5,6,7] The kidney is one of the first organs to fail, as occurs in 51% of patients with septic shock.[8] The mortality of septic AKI remains substantial, despite the effort expended in recent decades. Septic AKI was associated with more severe hemodynamic instability, greater requirement of mechanical ventilation, longer hospital stay and higher hospital mortality when compared with non-septic AKI.[9]
Although continuous renal replacement therapy (CRRT) is a well-known treatment for AKI and the technical advances for CRRT treatment have been performed,[10,11,12,13] the mortality rate among these patients still remains extremely high. On average, 20% of hospitalized patients develop AKI; of those, 1% to 2% develop AKI requiring RRT. Among patients with AKI, 40% to 60% die and 40% to 50% survive. Depending on the degree of preexisting chronic kidney disease (CKD), 70% to 90% of the survivors recover function, whereas10% to 30% remain on dialysis at hospital discharge. Importantly, up to 80% of those who recover may develop CKD in the future.[14] CRRT has an advantage in patients with hemodynamic instability, which is frequently encountered in sepsis. Potential advantages of accelerated initiation of CRRT include early control of electrolyte and acid-base derangements and uremia, avoidance of volume overload and clearance of inflammatory mediators.[15] Although the optimal timing of CRRT initiation remains controversial, recent studies comparing early and late initiation have suggested that early initiation may have a beneficial impact on survival of critically ill patients with AKI.[16,17,18] However, in managing septic AKI, whether to provide renal-replacement therapy and, if so, when to initiate it are unclear.
Recent meta-analysis mainly focuses on the impact of CRRT timing on AKI in critically ill patients. There are many reasons for AKI in critically ill patients, such as hypovolemic shock, glomerulonephritis, ureteral calculi, etc, but there are few studies on the impact of CRRT timing on AKI caused by sepsis.
In this study, we conducted a meta-analysis, which extracted results from recently published trials to investigate whether early initiation of CRRT in critically ill patients with septic AKI improves patient survival.
2. Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[19] Ethical approval was not necessary for this study because it was a review of the published literature.
2.1. Search strategy
We searched the PubMed, Cochrane, and Embase databases from inception to the 31st of March 2019 using the following search terms: acute renal failure, AKI, CRRT, sepsis, septic shock, septic AKI, septic-associated AKI, dialysis, hemodialysis, hemofiltration, time to treatment, time factors, timing, early, earlier, time, late, initiation, start, and randomized controlled trials. The search was slightly adjusted according to the requirements of the different databases. The authors’ personal files and reference lists of relevant review articles were also reviewed. The flow chart of the search strategies is summarized in (Fig. 1).
Figure 1.
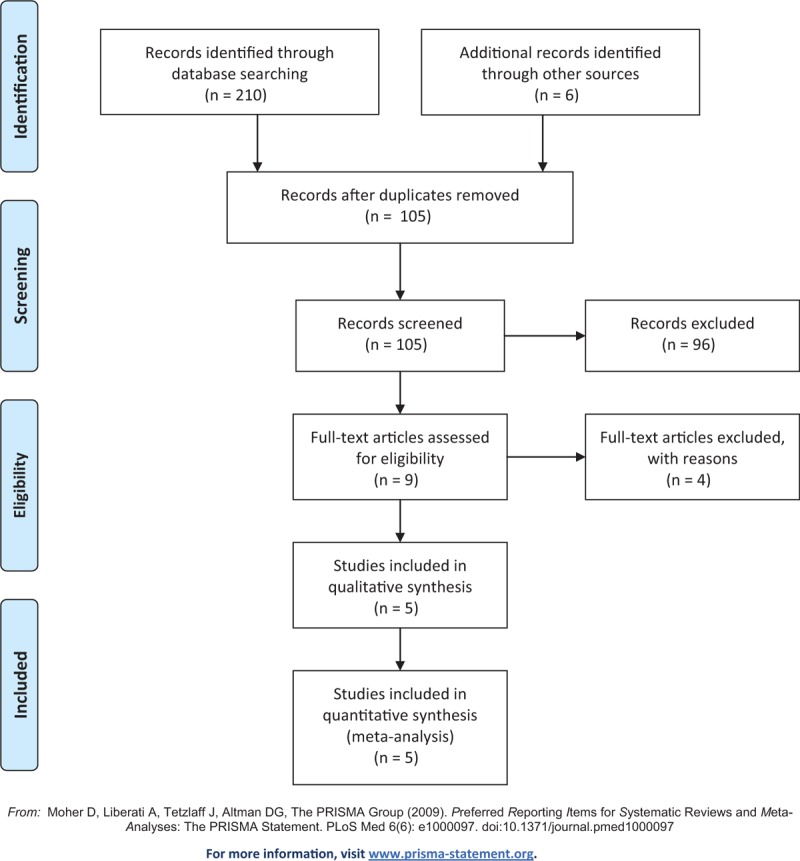
Flow chart of literature selection.
2.2. Types of outcome measures
The primary outcome was 28-day mortality in early and late initiation of CRRT. The 90-day mortality, ICU, and hospital length of stay were secondary outcomes. Weighted means were calculated based on the number of patients in each study.
2.3. Study selection
The inclusion criteria were as follows:
-
(1)
randomized controlled trials;
-
(2)
adult sepsis or septic shock population;
-
(3)
AKI development following sepsis or septic shock without other causes;
-
(4)
clearly comparing early vs late CRRT initiation with effect on mortality and clinically relevant secondary outcomes.
We excluded studies without clear comparisons of the outcomes.
2.4. Quality assessment
Two reviewers (YL and HL) independently performed quality assessment using the Newcastle-Ottawa scale (NOS),[20] which allocates a maximum of 9 points according to the quality of the selection, comparability, and outcomes of the study populations. Study quality was defined as poor (0–3), fair (4–6), or good (7–9).
2.5. Statistical analysis
Statistical analyses were performed using Review Manager Version 5.3 (RevMan, The Cochrane Collaboration, Oxford, United Kingdom). Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated for dichotomous variables. A random-effects model was used to pool studies with significant heterogeneity, as determined by the chi-squared test (P < .10) and inconsistency index (I 2 ≥ 50%).[21] The selected continuous variables were represented by the median (interquartile range). We calculated their mean and standard deviation according to the sample size with an on-line calculator (http://www.comp.hkbu.edu.hk/∼xwan/median2mean.html), and then performed meta-analysis. A P value <.05 was set as the threshold of statistical significance.
3. Result
3.1. Study characteristics
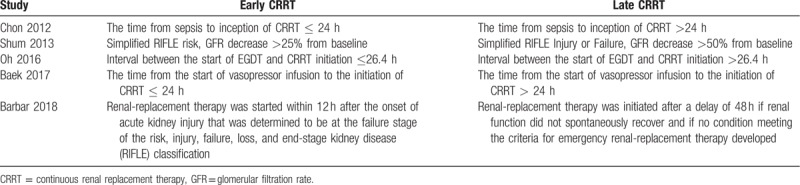
The search strategy identified 216 studies, and the data were from 5 studies comprising 900 patients (Table 1).[22,23,24,25,26] The characteristics of the included studies are shown in Table 1. A total of 5 eligible studies were published between 2012 and 2018. Among these studies, 3 studies were conducted in Korea, 1 study was conducted in China, 1 study was conducted in France. Of these studies, one of the studies was multi-center study,[26] and 4 were single-center studies,[22,23,24,25] The definition of early and late initiation of CRRT for each specific study is outlined in Table 2.
Table 1.
The basic characteristics of studies included in meta-analysis.
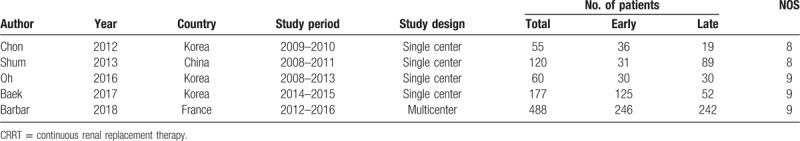
Table 2.
Definitions of early and late CRRT in studies included in the meta-analysis.
3.2. Primary outcome
A total of 5 studies including 900 patients were included, and the 28-day mortality in patients with septic AKI was about 43.0% (184/468 in the early group and 203/432 in the late group). There was no significant difference between 28-day mortality of early and late strategy for the initiation of CRRT using the random effect model (odds ratio [OR] = 0.76;95% CI, 0.58–1.00; P = .05), with wild heterogeneity (Chi2 = 16.88, I 2 = 76%) (Fig. 2). A funnel plot was used to assess the publication bias (Fig. 3).
Figure 2.
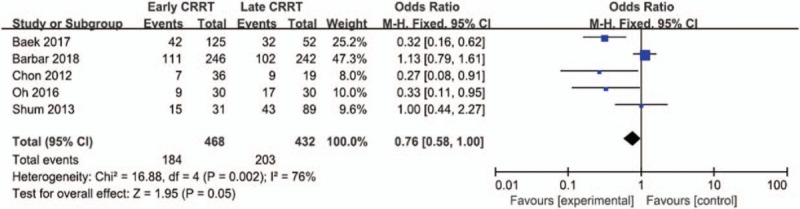
Forest plot for 28-day mortality.
Figure 3.

Funnel plot of 28-day mortality.
3.3. Secondary outcomes
3.3.1. Effect of early CRRT on 90-day mortality
Three of included studies were analyzed to assess effect of early CRRT on the 90-day mortality. There was no statistically significant difference in the 90-day mortality between 2 groups (odds ratio [OR] = 0.79;95% CI, 0.59–1.06; P = .12), with wild heterogeneity (Chi2 = 14.32, I 2 = 86%) (Fig. 4).
Figure 4.

Forest plot for 90-day mortality.
3.3.2. Effect of early CRRT on length of ICU stay
Four of included studies were analyzed to assess effect of early CRRT on the ICU length of stay. There was no statistically significant difference in the ICU length of stay between 2 groups (Mean difference = −0.9;95% CI, −2.37 to 0.57; P = .23), with wild heterogeneity (Chi2 = 2.39, I 2 = 0%) (Fig. 5).
Figure 5.
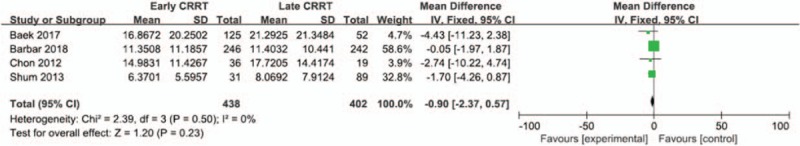
Forest plot for ICU length of stay.
3.3.3. Effect of early CRRT on length of hospital stay
Three of included studies were analyzed to assess effect of early CRRT on the hospital length of stay. There was no statistically significant difference in the hospital length of stay between 2 groups (Mean difference = −1.43;95% CI, −5.28 to 2.41; P = .47), with wild heterogeneity (Chi2 = 0.47, I 2 = 0%) (Fig. 6).
Figure 6.
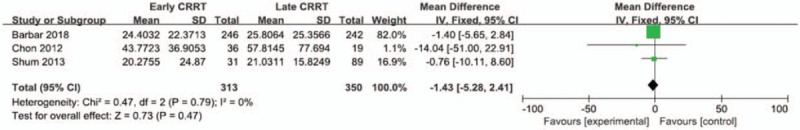
Forest plot for hospital length of stay.
4. Discussion
This systematic review and meta-analysis of five unique studies compared early vs late initiation of CRRT in septic AKI patients and the results were contradictory. Three studies supported that early CRRT could improve 28-day mortality.[22,24,25] Two studies showed that early and late CRRT had no effect on 28-day mortality.[23,26] Our meta-analysis suggests that earlier initiation is not associated with improved survival. No significant differences were found in length of stay in ICU and that in hospital.
The potential advantage of earlier initiation of dialysis in AKI is that it may improve acid-base, electrolyte, and fluid balance, thereby preventing more severe complications of AKI and perhaps also enhancing removal of toxins.[27] Removal of nonspecific proinflammatory or anti-inflammatory mediators by convection and adsorption has been demonstrated during CRRT.[28] Early CRRT can stabilize the internal environment while facilitating the timely removal of inflammatory mediators, and reduce the impact on the kidney. Excessive inflammation can be controlled as early as possible, which can reduce tissue damage caused by uncontrolled inflammation. Furthermore, liquid management can be easily targeted through the fluid regulation function of CRRT. However, in our meta-analysis we did not observe lower mortality with early initiation than with late initiation. A possible explanation is that fluid removal with CRRT cannot be performed easily or safely in patients with hemodynamic instability in the early phases of septic shock, so starting such therapy earlier would not improve fluid balance.
Our meta-analysis of secondary outcomes did not demonstrate a statistical reduction in ICU or hospital length of stay, this is consistent with a recent multicenter randomized controlled trial.[26] Therefore, the timing of CRRT initiation is still a matter of debate. The determination to start CRRT in critically ill patients with AKI depends on numerous factors and thus may be a complex process.[29]
Our meta-analysis has several characteristics:
-
(1)
Previous studies focused on the timing of CRRT in critically ill patients with AKI, but did not limit the etiology of AKI. The study included only septic AKI and clearly defined etiology of AKI. Because CRRT itself has the function of clearing inflammatory mediators, this study anticipates that early CRRT in septic AKI can improve the prognosis, but the results are different from expectations.
-
(2)
The research period included in this study was less than 10 years.
-
(3)
The results of the included studies are contradictory, so the meta-analysis of the controversial results increases the significance of this study.
This meta-analysis was associated with several limitations. First, the number of included studies was small. In the future, large, multicenter prospective observational studies are required to make sure the impact of CRRT timing on septic AKI. Second, there was substantial heterogeneity among the included studies. Therefore, our findings should be interpreted with caution. Third, publication bias was not assessed due to the small sample size.
5. Conclusion
Our meta-analysis revealed that early initiation of CRRT showed no survival benefits in patients with septic AKI. The analysis of secondary outcomes showed no significant difference in ICU length of stay or hospital length of stay between early and late CRRT group. To achieve optimal timing of CRRT for septic AKI, large multicenter randomized trials with better design are still needed.
Author contributions
Data curation: Hongxiang Li.
Resources: Hongxiang Li.
Software: Yuting Li.
Supervision: Dong Zhang.
Visualization: Dong Zhang.
Writing – original draft: Yuting Li.
Footnotes
Abbreviations: AKI = acute kidney injury, CIs = confidence intervals, CKD = chronic kidney disease, CRRT = continuous renal replacement therapy, ICU = intensive care unit, NOS = Newcastle-Ottawa scale, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RRs = risk ratios.
This work was supported by the Project of Natural Science Foundation of Jilin Province (No. 20160101142JC).
References
- [1]. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- [2]. Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015;385:2616–43. [DOI] [PubMed] [Google Scholar]
- [3]. Quenot JP, Binquet C, Kara F, et al. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care 2013;17:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicenter evaluation. Crit Care 2008;12:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. The RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009;361:1627–38. [DOI] [PubMed] [Google Scholar]
- [6]. The VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294:813–8. [DOI] [PubMed] [Google Scholar]
- [8]. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 2004;351:159–69. [DOI] [PubMed] [Google Scholar]
- [9]. Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2007;2:431–9. [DOI] [PubMed] [Google Scholar]
- [10]. Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant 2009;24:512–8. [DOI] [PubMed] [Google Scholar]
- [11]. Uchino S, Bellomo R, Morimatsu H, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. Kidney) investigators. Intensive Care Med 2007;33:1563–70. [DOI] [PubMed] [Google Scholar]
- [12]. Allegretti AS, Steele DJ, David-Kasdan JA, et al. Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: a cohort study. Crit Care 2013;17:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Oh HJ, Park JT, Kim JK, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant 2012;27:589–94. [DOI] [PubMed] [Google Scholar]
- [14]. Cerda J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol 2015;10:1859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Joannidis M, Forni LG. Clinical review: timing of renal replacement therapy. Crit Care 2011;15:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Seabra VF, Balk EM, Liangos O, et al. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis 2008;52:272–84. [DOI] [PubMed] [Google Scholar]
- [17]. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 2011;15:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Lee J, Cho JH, Chung BH, et al. Classical indications are useful for initiating continuous renal replacement therapy in critically ill patients. Tohoku J Exp Med 2014;233:233–41. [DOI] [PubMed] [Google Scholar]
- [19]. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- [20]. Wells GA, Shea BJ, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric 2014;18:727–34. [Google Scholar]
- [21]. Biggerstaff BJ, Jackson D. The exact distribution of Cochran's heterogeneity statistic in one-way random effects meta-analysis. Stat Med 2008;27:6093–110. [DOI] [PubMed] [Google Scholar]
- [22]. Chon GR, Chang JW, Huh JW, et al. A comparison of the time from sepsis to inception of continuous renal replacement therapy versus RIFLE criteria in patients with septic acute kidney injury. Shock 2012;38:30–6. [DOI] [PubMed] [Google Scholar]
- [23]. Shum HP, Chan KC, Kwan MC, et al. Timing for initiation of continuous renal replacement therapy in patients with septic shock and acute kidney injury. Ther Apher Dial 2013;17:305–10. [DOI] [PubMed] [Google Scholar]
- [24]. Oh HJ, Kim MH, Ahn JY, et al. Can early initiation of continuous renal replacement therapy improve patient survival with septic acute kidney injury when enrolled in early goal-directed therapy? J Crit Care 2016;35:51–6. [DOI] [PubMed] [Google Scholar]
- [25]. Baek SD, Yu H, Shin S, et al. Early continuous renal replacement therapy in septic acute kidney injury could be defined by its initiation within 24 hours of vasopressor infusion. J Crit Care 2017;39:108–14. [DOI] [PubMed] [Google Scholar]
- [26]. Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018;379:1431–42. [DOI] [PubMed] [Google Scholar]
- [27]. Wald R, Bagshaw SM. The timing of renal replacement therapy initiation in acute kidney injury: is earlier truly better? Crit Care Med 2014;42:1933–4. [DOI] [PubMed] [Google Scholar]
- [28]. Davenport A. Can modification of renal replacement therapy improve the outcome of patients with systemic inflammatory response syndrome? Blood Purif 2006;24:317–8. [DOI] [PubMed] [Google Scholar]
- [29]. Matson J, Zydney A, Honore PM. Blood filtration: new opportunities and the implications of systems biology. Crit Care Resusc 2004;6:209–17. [PubMed] [Google Scholar]


