Supplemental Digital Content is available in the text
Keywords: cognition, obesity, older adults
Abstract
The relationship between body weight changes in late life and cognitive function is controversial.
We investigated whether weight gain or loss in late life affected cognitive function in community-dwelling older adults over a 3-year period.
Our study used data from the Survey of Living Conditions and Welfare Needs of Korean Older Persons and included 3859 subjects (aged ≥65 years) with normal cognition at baseline. At baseline and the 3-year follow-up, body weight and height were measured, and cognitive function was assessed using the mini-mental state examination. Based on their body mass index (BMI) at baseline and follow-up, we divided the subjects into 4 groups: weight gain (baseline BMI <23 kg/m2 and follow-up BMI ≥23 kg/m2); weight loss (baseline BMI ≥23 kg/m2 and follow-up BMI <23 kg/m2); stable overweight/obese (BMI ≥23 kg/m2 at both visits); and stable non-overweight/obese (BMI <23 kg/m2 at both visits). Incidence rates (IRs) of cognitive impairment per 100 persons and IR ratios (IRRs) were calculated for each group and adjusted for confounding variables.
At the 3-year follow-up, 610 cases of cognitive impairment (15.8%) were identified. The stable overweight/obese group had the lowest IR (14.0, 95% confidence interval [CI] 12.45–15.71) and was therefore used as the reference group when calculating IRRs for cognitive impairment. When men and women were evaluated separately, IRs between groups were significantly different only for women. The stable non-overweight/obese group (IRR 1.65, 95% CI 1.22–2.22) and the weight gain group (IRR 1.93, 95% CI 1.24–3.01) had higher IRs than those in the stable overweight/obese group. As a gain or loss of adiposity, the IR of the weight gain group (IRR 1.17, 95% CI 0.74–1.84) was not different from that of the stable non-overweight/obese group. Also, the IR of weight loss group (IRR 1.09, 95% CI 0.71–1.67) was not significantly different from that of the stable overweight/obese group.
We suggest that overweight or obese older women at baseline had cognitive benefits. However, additional gain or loss of adiposity in late life did not affect the risk of cognitive impairment.
1. Introduction
Dementia is a major health problem in aging populations, and its prevalence increases rapidly with age, with 20% to 25% of people aged ≥85 years being affected worldwide.[1] Several studies have investigated the association between changes in body weight in late life and cognitive function. The population-based prospective Mayo Clinic Study of Aging found that a marked decrease in body mass index (BMI) per decade triggered mild cognitive impairment.[2] An elderly African-American cohort study reported that subjects with incident dementia or mild cognitive impairment had a greater decline in BMI than those with normal cognitive function.[3] A cohort study of African-Americans with hypertension correlated decreases in BMI in late life with lower cognitive Z-scores but found no association between increases in BMI and cognitive Z-scores.[4] The Women's Health Initiative Study of Cognitive Aging reported similar findings; an association was observed between weight loss and cognitive impairment in older women but not between weight gain or stable weight and cognition.[5] On the other hand, the Longitudinal Assessment of Women Study in Australia associated both weight loss and gain (compared with stable weight) with poor visual delayed index scores in middle-aged and older women.[6]
In contrast to previous studies that reported that weight loss in late life adversely affected cognitive function, intentional weight loss through diet was associated with cognitive improvement in obese elderly patients with mild cognitive impairment.[7] Based on existing research findings, the effect of weight gain or loss in late life on cognitive function remains unclear.
Because body weight can change with age and is modifiable,[8] determining how such changes affect cognitive function is important for developing appropriate weight management strategies for older adults. Furthermore, most studies examining the relationship between changes in body weight and cognitive function were performed in Western countries, with no studies on Asian populations who have lower BMI thresholds for overweight (23 kg/m2) and obesity (25 kg/m2) than Western populations.[9] Although a Korean longitudinal study showed that middle-aged and elderly subjects with baseline obesity had a lower risk of cognitive decline,[10] it did not consider changes in body weight.
In this regard, we investigated whether body weight at baseline and weight gain or loss considering the BMI threshold for overweight or obesity in late life affected cognitive function in community-dwelling Korean older adults over a 3-year period.
2. Methods
2.1. Study population
For our study, we used the data obtained in the Survey of Living Conditions and Welfare Needs of Korean Older Persons, which was conducted by the Korea Institute for Health and Social Affairs. The quality of the survey data was approved by Statistics Korea, a service of the Korean Government.[11] The survey, which began in 2008, is performed at 3-year intervals on a representative sample of non-institutionalized elderly Korean subjects. A stratified, 2-stage, cluster-sampling design was used. Face-to-face interviews were conducted by trained interviewers. The survey collected sociodemographic and health-related information, including functional disabilities, history of chronic diseases, cognitive function, geriatric depression scale (GDS) score, health behaviors, and body weight and height.
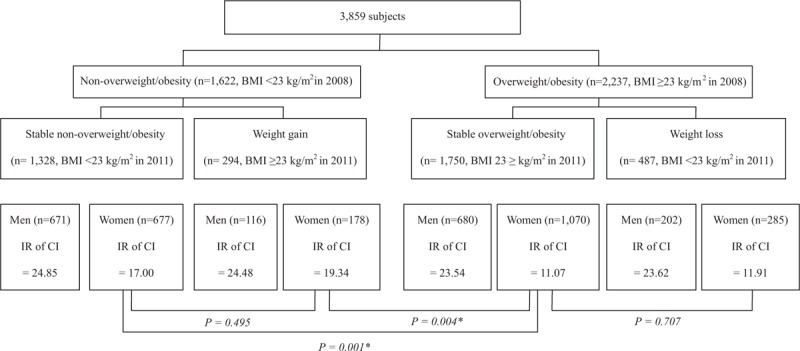
The first-wave survey in 2008 included 15,146 subjects, among whom 3984 were lost to follow-up and 1159 were admitted to a hospital or nursing home (and hence were no longer eligible) or died. We excluded subjects who were aged <65 years (n = 1078); had dementia or cognitive impairment (n = 1417); were frail, disabled, or had cancer (n = 2160); or were missing body weight, height, or cognitive function data (n = 544). The remaining 4804 subjects were included in the second-wave survey in 2011; those with missing anthropometric or cognitive function data were excluded from our study (n = 945). Finally, 3859 subjects (all ≥65 years of age) constituted our study population (Fig. 1). All subjects provided informed consent before participation in the study, either by themselves or (in cases of decision-impaired adults) a legally authorized representative. The current study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital (approval number, 2018-04-028).
Figure 1.

Flowchart showing the process of selecting the study population.
2.2. Measurements
At baseline and the 3-year follow-up, anthropometric information was collected from subjects wearing light clothes and no shoes. Height was measured to the nearest one-tenth of a centimeter; weight was measured to the nearest one-tenth of a kilogram in the upright position. If subjects refused or could not be measured, self-reported data were used; the validity of the self-reported data was assessed by checking the health records of community health centers or hospitals.[11] Body weight in kilograms was divided by height in square meter to determine BMI.
In accordance with the guidelines of the World Health Organization for Asian Pacific populations, BMI of 23 kg/m2 was the threshold for overweight in Asian populations.[10] Unlike previous studies, which did not consider BMI cut-off points for changes in body weight,[2,4–6] we used BMI ≥23 kg/m2 as the threshold at which increased health risks required medical intervention. Based on changes in BMI over a 3-year period, the subjects were divided into 4 groups: weight gain (n = 294, baseline BMI <23 kg/m2, follow-up BMI ≥23 kg/m2); weight loss (n = 487, baseline BMI ≥23 kg/m2, follow-up BMI <23 kg/m2); stable overweight/obese (n = 1750, BMI ≥23 kg/m2 at both visits); and stable non-overweight/obese (n = 1328, BMI <23 kg/m2 at both visits).
At baseline and follow-up visits, cognitive function was evaluated using the Korean version of the mini-mental status examination (MMSE-KC). Trained interviewers administered the MMSE-KC in an isolated area where the subjects would not be disturbed for about 10 minutes. The MMSE-KC uses the Korean language and has been standardized; the scores range from 0 to 30. Cognitive impairment was defined as an MMSE-KC score <1.5 standard deviations from the age-, sex-, and education-adjusted norm for elderly Koreans.[12]
The demographic variables recorded were age, sex, educational level, and household income. Smoking status was classified as never-smoker, ex-smoker, or current smoker. Alcohol drinking status was classified as non-drinker, moderate drinking, and high-risk drinking. High-risk alcohol consumption was defined as drinking alcohol ≥2 d/wk and ≥7 (men) or ≥5 (women) standard-sized drinks/drinking day.[13] Physical activity was classified as adequate if >150 min/wk of exercise was reported.[14] Disease history (hypertension, diabetes mellitus, dyslipidemia, and depression) was considered as a covariate.
The short version of the GDS contains 15 questions, which we translated into Korean. GDS scores range from 0 to 15, with scores ≥8 indicating depression.[15] Nutritional status was assessed using 7 questions from the Nutrition Screening Initiative DETERMINE checklist. The total score ranged from 0 to 7, with higher scores indicating poorer nutritional status. Physical performance was assessed by asking patients whether they could run around a playground (400 m); walk around a playground (400 m); climb 10 stairs without rest; bend their body, squat, and sit with bent knees; and stretch their arms to place something above their heads. The possible answers were “can do it easily” (4 points), “can do it but slightly difficult” (3 points), “can do it but very difficult” (2 points), and “cannot do it” (1 point). The scores were summed and graphed using a 100-point scale.
2.3. Statistical analysis
For general characteristics, we calculated means with standard deviation or frequencies (percentages). The assumption of normality of the data was evaluated by the Shapiro–Wilk test, and a P-value > .05 indicated that the observed distribution of a variable was not significantly different from the normal distribution. MMSE-KC was log-transformed because it did not fit a normal distribution. We used the analysis of variance test to compare the continuous variables and the chi-squared test to compare the categorical variables among the 4 BMI groups. The incidence rate (IR) of cognitive impairment per 100 persons over a 3-year period was calculated and adjusted for demographic factors such as age, sex, household income, and marital status in Model 1. Health behaviors (smoking, alcohol consumption, and exercise), nutritional status, physical performance, and GDS score were added as adjusted variables in Model 2. Comorbidities and the number of medications were added as adjusted variables in Model 3. Finally, the baseline MMSE-KC score in 2008 was added as an adjusted variable in Model 4.
To assess the risk of cognitive impairment, IR ratios (IRRs) were estimated by dividing the IR of a particular group by that of the reference group (the stable overweight/obese group) via generalized linear models. Because there were sex differences among the BMI groups, we performed an additional analysis after stratifying for sex. All tests were 2-sided, and the significance level was set at P < .05. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
3. Results
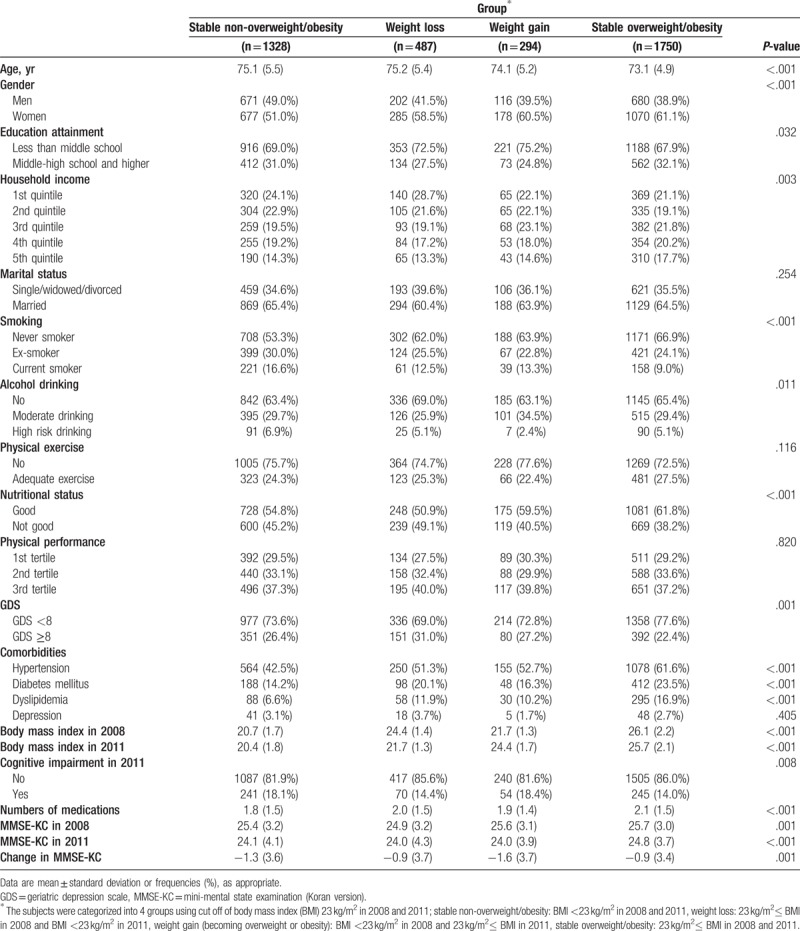
The general characteristics of the 4 BMI groups are shown in Table 1. The weight loss group had the highest mean age (75.2 ± 5.4 years), and the stable non-overweight/obese group had the highest percentage of men (49.0%). The stable overweight/obese group had the highest education level and household income and the lowest percentage of current smokers, poor nutritional status, and higher GDS score (≥8) indicating depression. The stable non-overweight/obese had the highest percentage of high-risk drinking, and the weight gain group had the highest percentage of moderate drinking. Marital status, physical exercise, physical performance, and history of depression did not differ significantly among the 4 groups. The prevalence of chronic diseases, such as hypertension, diabetes mellitus, and dyslipidemia, was highest in the stable overweight/obese group.
Table 1.
Characteristics of the study subjects according to the body mass index groups.
At the 3-year follow-up, 610 subjects (15.8%) had cognitive impairments. Cognitive impairment was the highest in the weight gain group (18.4%) and the lowest in the stable overweight/obese group (14.0%). The stable overweight/obese group had the highest MMSE-KC score both at baseline and the 3-year follow-up (25.7 ± 3.0 and 24.8 ± 3.7, respectively), whereas the weight loss group had the lowest scores at both visits (24.9 ± 3.2 and 24.0 ± 4.3, respectively). The MMSE-KC score over 3 years declined the most in the weight gain group (−1.6 ± 3.7, P-value = .001).
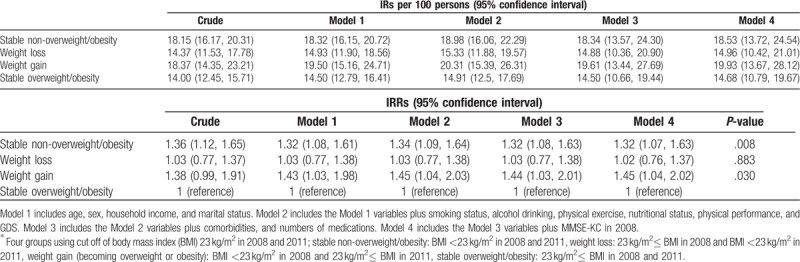
The IRs and IRRs of cognitive impairment in the 4 BMI groups over the 3-year period are presented in Table 2. The stable overweight/obese group had the lowest IR (14.0; 95% confidence interval [CI] 12.45–15.71) and was therefore used as the reference group when calculating IRRs. The fully adjusted IRs were significantly higher in the stable non-overweight/obese group (IRR 1.32, 95% CI 1.07–1.63) and weight gain group (IRR 1.45, 95% CI 1.04–2.02) than in the reference group (Model 4). However, the IR of the weight loss group was not significantly different from that of the reference group (IRR 1.02, 95% CI 0.76–1.37).
Table 2.
Cognitive impairment incidence rates (IRs) and incidence rate ratios (IRRs) of in the body mass index groups∗.
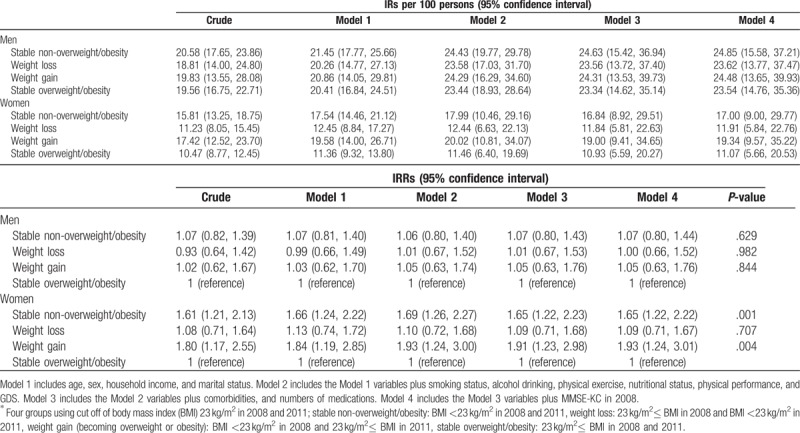
Given the sex differences among the 4 BMI groups (Table 1), we performed a sex-stratified analysis (Table 3). Among women, the crude IR of cognitive impairment was the lowest in the stable overweight/obese group (10.47, 95% CI 8.77–12.45) and the highest in the weight gain group (17.42, 95% CI 12.52–23.70). Using the stable overweight/obese group as the reference, fully adjusted IRRs for cognitive impairment in the weight gain and stable non-overweight/obese groups were 1.93 (95% CI 1.24–3.01) and 1.65 (95% CI 1.22–2.22), respectively (Model 4). As a loss of adiposity, the IR of weight loss group (IRR 1.09, 95% CI 0.71–1.67) was not significantly different from that of the stable overweight/obese group. Also, as a gain of adiposity, the IR of weight gain group (IRR 1.17, 95% CI 0.74–1.84) was not different from that of the stable non-overweight/obese group. In men, there was no significant difference in the fully adjusted IRR in the weight gain group (1.05, 95% CI 0.63–1.76), stable non-overweight/obese group (1.07, 95% CI 0.80–1.44), and weight loss group (1.00, 95% CI 0.66–1.52) using the stable overweight/obese group as the reference. As a gain of adiposity, the IR of weight gain group (IRR 0.98, 95% CI 0.59–1.63) was not different from that of the stable non-overweight/obese group.
Table 3.
Cognitive impairment incidence rates (IRs) and incidence rate ratios (IRRs) of in the body mass index groups∗ according to sex.
In Figure 2, we present the comparisons between the adjusted IRs of cognitive impairment in the BMI groups according to sex (Fig. 2).
Figure 2.
Comparisons between adjusted IRs of cognitive impairment in the body mass index groups according to sex. IRs = incidence rates.
4. Discussion
In this nationally representative prospective study, we found that there were sex differences in the relationship between obesity and cognitive function. Older women with baseline adiposity had a lower risk of cognitive impairment than those who were not overweight/obese. However, additional gain or loss of adiposity considering the BMI threshold in late life did not affect the risk of cognitive impairment in women. In older men, no relationship was found between cognitive function and baseline body weight or changes in body weight.
Previous studies have described the obesity paradox, which proposes that late-life obesity reduces the risk of dementia. However, the relationship between obesity and dementia is somewhat controversial. On one hand, a large retrospective cohort study in the UK found that underweight individuals (BMI <20 kg/m2) had a 34% higher risk of dementia than did normal weight individuals (20–25 kg/m2), whereas very obese individuals (>40 kg/m2) had a 29% lower risk.[16] On the other hand, the TREVISO LONGEVA study equated a higher baseline BMI with cognitive decline in an elderly population.[17] Moreover, in an unadjusted prospective analysis in the US, middle-aged and older women who were overweight (BMI = 25–30 kg/m2) or obese (≥30 kg/m2) had a significantly higher risk of cognitive impairment than did their normal weight counterparts (18.5–25 kg/m2); however, the significance was lost after multivariate adjustment.[18]
Only a few studies have examined the effects of weight on cognitive function in Asian populations, which have lower BMI thresholds for overweight (≥23 kg/m2) and obesity (≥25 kg/m2).[19] A Chinese cross-sectional study found no association between overweight or obesity, as defined by BMI, although subjects with central obesity were at risk for cognitive impairment.[20] A Korean longitudinal study showed a reduced risk of cognitive decline in middle- and older-aged adults with baseline obesity (BMI ≥25 kg/m2).[10] In addition, our previous cross-sectional study associated a large fat mass, as measured via dual-energy X-ray absorptiometry, with a relatively low risk of cognitive impairment in older women.[21] Unlike previous Asian studies, which focused on the effects of baseline adiposity on future cognitive function, our current study considered changes in BMI in late life.
In this study, we found that there were sex differences in the relationship between obesity and cognitive function. The differential effects between regional fats, such as subcutaneous fat and visceral fat, on cognitive function might explain our results. Kim et al reported that brain cortical thickness correlated positively with the percentage of body fat and negatively with the waist-hip ratio.[22] In the AGES-Reykjavik study, subcutaneous fat accumulation was associated with a decreased risk for dementia, whereas visceral fat accumulation was not associated with dementia.[23]
Sex differences in fat distribution might lead to a sex-specific association between obesity and cognitive function. Compared to men, women have an increased amount of peripheral fat depots (subcutaneous fat), whereas intra-abdominal fat depots (visceral fat) are preferentially increased in men.[24] There are possible mechanisms by which obesity could have positive effects on cognitive function in women. First, subcutaneous fat produces leptin, which may improve learning and memory capacity, increase grey matter volume in the hippocampus, and lower the risk of dementia.[25,26] Second, the peripheral fat distribution in women is associated with lower metabolic risk.[24] In contrast, visceral fat causes insulin resistance and increased risk of cerebrovascular disease and diabetes mellitus, which are potential risk factors for vascular dementia[27] and brain atrophy.[28] Third, in women, obesity may improve cognitive function by increasing the amount of adipose tissue and consequently estrogen levels. Estrogens protect against dementia by reducing amyloid β-peptide production and promoting peptide clearance via microglial phagocytosis and degradation.[29] They also stimulate neurogenesis in various brain regions, such as the dentate gyrus of the hippocampus.[30]
However, in elderly men, obesity may accelerate cognitive decline,[31] in part by reducing the level of testosterone, which protects against amyloid β toxicity and oxidative stress.[32,33]
In this study, despite the positive effect of adiposity at baseline on cognitive function in women, a gain of adiposity considering the BMI threshold in late life was not associated with the risk of cognitive impairment. Our results suggest that there was a differential effect of obesity on cognitive function with aging in women. Aging is accompanied by a loss of lean mass and a shift to central fat accumulation.[34] Weight gain in the elderly mainly reflects fat gain, particularly visceral fat rather than subcutaneous fat. The effect of visceral adiposity on brain function might be different with aging and remains controversial. In healthy middle-aged adults, visceral fat was associated with lower brain volume atrophy.[35] Yoon et al reported that visceral adiposity correlated with poor cognitive function in Korean individuals aged <70 years but not in those aged ≥70 years.[36] Other study reported that there was a negative correlation between visceral adipose tissue and cerebellar function in young to mid-age adults (20–45 years of age), but not in older adults (65–70 years of age).[37] In the AGES-Reykjavik study, visceral fat accumulation was not associated with dementia in older adults (mean age 76 years).[23] The relationship between visceral adiposity and cognitive function seems to be prominent in relatively younger aged adults.
However, recent longitudinal prospective studies have revealed that metabolic syndrome, which is characterized by abdominal obesity, increases the risk of cognitive decline in older adults. Insulin resistance or hyperglycemia in mid-life predicts cognitive decline, and brain insulin resistance could be a triggering factor in the development of Alzheimer disease.[38,39] In very older adults, metabolic syndrome in late life, which was closely associated with insulin resistance, could accelerate cognitive decline.[40,41] To clarify the effect of weight gain in late life on cognitive function, further large prospective studies are needed.
This study had 2 limitations. First, although it included many health-related variables, it lacked laboratory data and blood pressure measurements owing to their unavailability. Second, body weights and heights were self-reported by subjects who refused or could not be measured, as is commonly done in community surveys. However, to improve the validity of self-reported values, the health records of community health centers and hospitals were examined.[11]
Despite these limitations, our study had several strengths. First, we used a large nationally representative database of community-dwelling older adults. Hence, our findings are applicable to older Korean adults in general. Second, to our knowledge, this is the first Asian-based longitudinal study that investigated the effect of changes in body weight in late life on cognitive function. Third, we selected relatively healthy older adults without functional limitations to minimize the effects of confounding factors on body weight and cognitive function. We also adjusted for a wide range of confounding factors, including health behaviors, nutritional status, physical performance, comorbidities, and the number of medications.
In conclusion, we found that older women with adiposity before late life had a lower risk of cognitive impairment than those who were not overweight/obese. However, gain or loss of adiposity in late life did not affect the risk of cognitive impairment. It suggests that there were differential effects of obesity on a cognitive function related to life course in women. Further prospective studies are needed to establish the optimal age-related body weights for preventing cognitive decline.
Author contributions
Conceptualization: Hye-Mi Noh, Yeo Jin Kim, Hong JI Song.
Data analysis: Jin-Hyung Jung, Junhee Han.
Data curation: Hye-Mi Noh, Jin-Hyung Jung, Yong Kyun Roh, Hong JI Song.
Formal analysis: Junhee Han.
Funding acquisition: Yong Kyun Roh, Hong JI Song.
Methodology: Hye-Mi Noh, Jin-Hyung Jung, Yong Kyun Roh, Hong JI Song.
Writing – original draft: Hye-Mi Noh, Hong JI Song.
Writing – reviewing and editing: Hye-Mi Noh, Hong Ji Song, Yong Kyun Roh, Junhee Han.
Hong JI Song orcid: 0000-0002-3563-9504.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, GDS = geriatric depression scale, IR = incidence rate, IRR = incidence rate ratio, MMSE-KC = mini-mental state examination-Korean version.
YKR and HJS contributed equally to this work.
This study was supported by a grant from the Hallym University Medical Center Research Fund (HURF-2015-33).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Supplementary Figures:.
References
- [1].Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alhurani RE, Vassilaki M, Aakre JA, et al. Decline in weight and incident mild cognitive impairment: Mayo clinic study of aging. JAMA Neurol 2016;73:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gao S, Nguyen JT, Hendrie HC, et al. Accelerated weight loss and incident dementia in an elderly African-American cohort. J Am Geriatr Soc 2011;59:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].West NA, Lirette ST, Cannon VA, et al. Adiposity, change in adiposity, and cognitive decline in mid- and late life. J Am Geriatr Soc 2017;65:1282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Driscoll I, Espeland MA, Wassertheil-Smoller S, et al. Weight change and cognitive function: findings from the women's health initiative study of cognitive aging. Obesity 2011;19:1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lo AHY, Pachana NA, Byrne GJ, et al. Relationship between changes in body weight and cognitive function in middle-aged and older women. Int J Geriatr Psychiatry 2012;27:863–72. [DOI] [PubMed] [Google Scholar]
- [7].Horie NC, Serrao VT, Simon SS, et al. Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab 2016;101:1104–12. [DOI] [PubMed] [Google Scholar]
- [8].Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the health, aging and body composition study. Am J Clin Nutr 2005;82:872–8. [DOI] [PubMed] [Google Scholar]
- [9].Low S, Chin MC, Ma S, et al. Rationale for redefining obesity in Asians. Ann Acad Med Singapore 2009;38:66–9. [PubMed] [Google Scholar]
- [10].Rosenfeld CS, Kim S, Kim Y, et al. Body mass index and decline of cognitive function. Plos One 2016;11:e0148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Statistics Korea. The Report of Statistics Quality in the Survey of Living Conditions and Welfare Needs of Korean Older Persons 2015;22–31. [Google Scholar]
- [12].Park JI, Park TW, Yang JC, et al. Factors associated with depression among elderly Koreans: the role of chronic illness, subjective health status, and cognitive impairment. Psychogeriatrics 2016;16:62–9. [DOI] [PubMed] [Google Scholar]
- [13].Lee HK, Lee BH. The epidemiology of alcohol use disorders. J Korean Diabetes 2012;13:69–75. [Google Scholar]
- [14].Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1081–93. [DOI] [PubMed] [Google Scholar]
- [15].Bae JN, Cho MJ. Development of the Korean version of the geriatric depression scale and its short form among elderly psychiatric patients. J Psychosom Res 2004;57:297–305. [DOI] [PubMed] [Google Scholar]
- [16].Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol 2015;3:431–6. [DOI] [PubMed] [Google Scholar]
- [17].Gallucci M, Mazzuco S, Ongaro F, et al. Body mass index, lifestyles, physical performance and cognitive decline: the “Treviso Longeva (TRELONG)” study. J Nutr Health Aging 2013;17:378–84. [DOI] [PubMed] [Google Scholar]
- [18].Xiang X, An R. Body weight status and onset of cognitive impairment among U.S. middle-aged and older adults. Arch Gerontol Geriatr 2015;60:394–400. [DOI] [PubMed] [Google Scholar]
- [19].Deurenberg-Yap M, Yian TB, Kai CS, et al. Manifestation of cardiovascular risk factors at low levels of body mass index and waist-to-hip ratio in Singaporean Chinese. Asia Pac J Clin Nutr 1999;8:177–83. [DOI] [PubMed] [Google Scholar]
- [20].Cui GH, Guo HD, Xu RF, et al. The association of weight status with cognitive impairment in the elderly population of a Shanghai suburb. Asia Pac J Clin Nutr 2013;22:74–82. [DOI] [PubMed] [Google Scholar]
- [21].Noh HM, Oh S, Song HJ, et al. Relationships between cognitive function and body composition among community-dwelling older adults: a cross-sectional study. BMC Geriatr 2017;17:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim HJ, Kim C, Jeon S, et al. Association of body fat percentage and waist-hip ratio with brain cortical thickness: a study among 1777 cognitively normal subjects. Alzheimer Dis Assoc Disord 2015;29:279–86. [DOI] [PubMed] [Google Scholar]
- [23].Spauwen PJJ, Murphy RA, Jónsson PV, et al. Associations of fat and muscle tissue with cognitive status in older adults: the AGES-Reykjavik study. Age Ageing 2017;46:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karastergiou K, Smith SR, Greenberg AS, et al. Sex differences in human adipose tissues – the biology of pear shape. Biol Sex Differ 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gustafson DR. Adiposity and cognitive decline: underlying mechanisms. J Alzheimers Dis 2012;30Suppl 2:S97–112. [DOI] [PubMed] [Google Scholar]
- [26].McGuire MJ, Ishii M. Leptin dysfunction and Alzheimer's disease: evidence from cellular, animal, and human studies. Cell Mol Neurobiol 2016;36:203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Niedowicz DM, Reeves VL, Platt TL, et al. Obesity and diabetes cause cognitive dysfunction in the absence of accelerated beta-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol Commun 2014;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ho AJ, Raji CA, Becker JT, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging 2010;31:1326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Singh M, Setalo G, Jr, Guan X, et al. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci 1999;19:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Galea LA, Wainwright SR, Roes MM, et al. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol 2013;25:1039–61. [DOI] [PubMed] [Google Scholar]
- [31].Cao J, Chen TM, Hao WJ, et al. Correlation between sex hormone levels and obesity in the elderly male. Aging Male 2012;15:85–9. [DOI] [PubMed] [Google Scholar]
- [32].Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res 2001;919:160–5. [DOI] [PubMed] [Google Scholar]
- [33].Ahlbom E, Grandison L, Bonfoco E, et al. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci 1999;11:1285–91. [DOI] [PubMed] [Google Scholar]
- [34].Vlassopoulos A, Combet E, Lean ME. Changing distributions of body size and adiposity with age. Int J Obesity 2014;38:857–64. [DOI] [PubMed] [Google Scholar]
- [35].Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol 2010;68:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yoon DH, Choi SH, Yu JH, et al. The relationship between visceral adiposity and cognitive performance in older adults. Age Ageing 2012;41:456–61. [DOI] [PubMed] [Google Scholar]
- [37].Raschpichler M, Straatman K, Schroeter ML, et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open 2013;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ekblad LL, Rinne JO, Puukka P, et al. Insulin resistance predicts cognitive decline: an 11-year follow-up of a nationally representative adult population sample. Diabetes Care 2017;40:751–8. [DOI] [PubMed] [Google Scholar]
- [39].Tortelli R, Lozupone M, Guerra V, et al. Midlife metabolic profile and the risk of late-life cognitive decline. J Alzheimers Dis 2017;59:121–30. [DOI] [PubMed] [Google Scholar]
- [40].Viscogliosi G, Chiriac IM, Andreozzi P, et al. The metabolic syndrome predicts longitudinal changes in clock drawing test performance in older nondemented hypertensive individuals. Am J Geriatr Psychiatry 2016;24:359–63. [DOI] [PubMed] [Google Scholar]
- [41].Viscogliosi G, Donfrancesco C, Palmieri L, et al. The metabolic syndrome and 10-year cognitive and functional decline in very old men. A population-based study. Arch Gerontol Geriatr 2017;70:62–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


