Abstract
Our study aimed to evaluate the value of neutrophil to lymphocyte ratio (NLR) and gamma-glutamyl transpeptidase to platelet ratio (GPR) in patients with hepatocellular carcinoma (HCC).
A total of 565 patients with pathological diagnosis of HCC were retrospectively analyzed and 414 patients diagnosed with cirrhosis were treated as a control group. All clinical materials were collected from the First Affiliated Hospital of Guangxi Medical University.
The preintervention NLR, GPR, and α-fetoprotein (AFP) were significantly higher in HCC patients than in the controls (PNLR < .000, PGPR < .000, PAFP < .000). The NLR and GPR were correlated with the Barcelona clinic liver cancer (BCLC) stages, Child-Pugh grades, and tumor size, but not with Edmondson–Steiner grades. Combined use of NLR or GPR with AFP produced larger area under the curve (AUC) (AUCNLR+AFP = 0.916; AUCNLR+AFP = 0.953) than NLR (P < .000), GPR (P < .000), or AFP (P < .000) used alone.
The preintervention hematologic parameters (NLR and GPR) studied herein were associated with the BCLC stages of HCC. Combined use of NLR or GPR with AFP may improve early detection and diagnosis of HCC.
Keywords: Barcelona clinic liver cancer (BCLC) stages, Edmondson–Steiner grades, gamma-glutamyl transpeptidase to platelet ratio, hepatocellular carcinoma, neutrophil to lymphocyte ratio
1. Introduction
Hepatocellular carcinoma (HCC) is the third most common malignancy responsible for mortality after gastric and esophageal cancers.[1] HCC is difficult to detect at an early stage and is characterized by a long incubation period, rapid development, high malignancy, and high mortality.[2] The incidence rate of HCC is particularly high in developing countries due to environmental variables, dietary habits, and inadequate medical care.[3]
Tumor progression is closely related to inflammatory factors.[4] The process of hepatitis, cirrhosis, and HCC staging is linked to a network of inflammatory signaling pathways and changes in the tumor microenvironment.[5,6] Previous studies have demonstrated the clinical importance of inflammation-based hematological markers, like the C-reactive protein,[7] and the aspartate aminotransferase to platelet count ratio index.[8] Recently, hematologic markers of inflammation, such as the neutrophil to lymphocyte ratio (NLR) and gamma-glutamyl transpeptidase to platelet ratio (GPR), have attracted increasing attention due to their clinical application in various diseases (e.g., cervical squamous cell carcinoma, colorectal cancer, liver fibrosis, and cirrhosis).[9–11] However, their clinical importance in HCC staging remains unexplored.
Inflammatory factors have been closely associated with the stages of tumor.[12] Previous studies have evaluated the predictive value of NLR and GPR in HCC.[13,14] Limited numbers of studies have examined the relationship between hematologic markers and tumor clinicopathological features.[9,15] An analysis of hematologic parameters and clinicopathological features would provide information for early diagnosis of diseases. Our study aimed to explore the preintervention values of the NLR and GPR in HCC.
2. Materials and methods
2.1. Study population and design
A total of 565 patients with pathologically diagnosed HCC were enrolled in this study. All preintervention hematologic parameters, pathological grades, and clinical features of HCC patients who attended the First Affiliated Hospital of Guangxi Medical University between 2012 and 2017 were collected from the hospital's electronic medical records. Patients in HCC group need to meet the following 5 criteria to be included: Barcelona clinic liver cancer (BCLC) stages A, B, or C; Edmondson–Steiner grades I, II, or III; Child-Pugh grades A, B, or C; pathologically diagnosed as HCC; surgical resection treatment. Patients will be excluded if they meet one of the following: past history of HCC; blood system diseases; immune-related diseases; organic disease outside liver; presence of other types of cancers. The control group comprised 441 age- and sex-matched individuals diagnosed with cirrhosis. This study was approved by the Ethics Committee of First Affiliated Hospital of Guangxi Medical University. Informed consent was obtained from all the participants.
2.2. Data collection
All laboratory data were collected about a week before the patient underwent the first treatment. Data on the participant's age and sex were obtained. The values of platelet (PLT), neutrophil (NEU), and lymphocyte (LYM) counts; and the levels of α-fetoprotein (AFP) and gamma-glutamyl transpeptidase (GGT) were recorded. NLR and GPR were calculated using the following formulas:
NLR = neutrophil count/lymphocyte count
GPR = gamma-glutamyl transpeptidase level/platelet count
2.3. Statistical analysis
SPSS software version 17.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 5 software (http://www.graphpad.com/scientific-software/prism/) were used for data analysis. The distribution of variables was based on Kolmogorov–Smirnov test. Summary data were represented as the median, with interquartile ranges. A receiver operating characteristic (ROC) curve analysis was used to assess the sensitivity and specificity of preintervention NLR and GPR. Appropriate cut-off values for the above hematological parameters were obtained through ROC analysis. The Mann–Whitney U test was conducted to compare the laboratory parameters of the HCC patients and controls, and a chi-square test or Kruskal–Wallis test was performed to compare categorical variables. P value <.05 (two-tailed) was considered to be statistically significant.
3. Results
3.1. Comparison of hematologic features of the HCC patients with controls
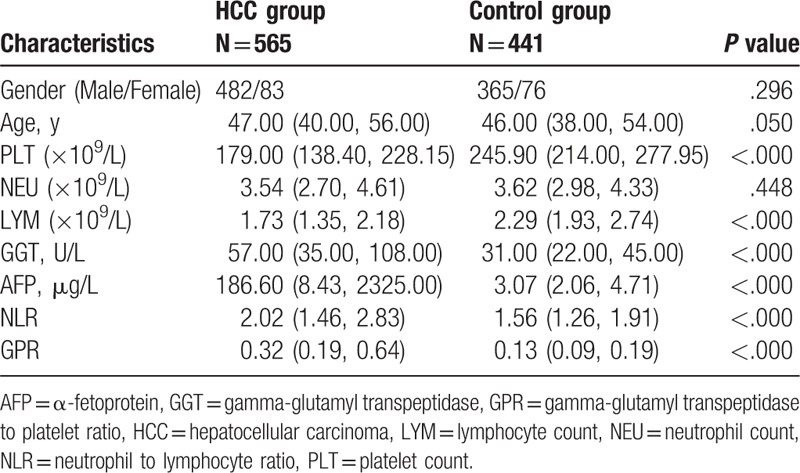
As shown in Table 1, the median serum AFP and GGT levels in HCC patients were significantly higher than those in controls (all P < .001). HCC patients had significantly lower platelet and lymphocyte counts compared with controls. Moreover, the median NLR and GPR values were higher in the HCC group as than in the control group (all P < .001), however, there was no significant difference in neutrophil counts between HCC patients and the controls (P = .447).
Table 1.
Comparison of hematologic characteristics between HCC group and control group.
3.2. Correlation between hematologic parameters and clinicopathological features of HCC patients
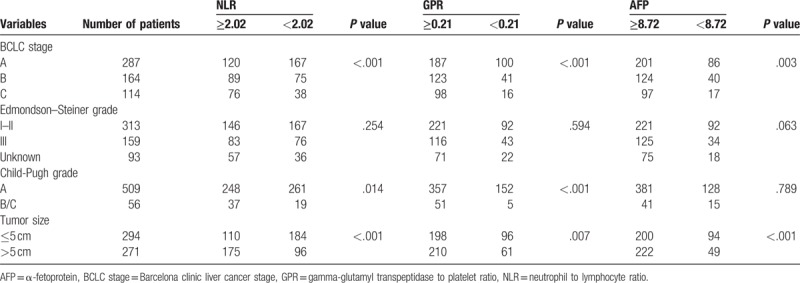
As shown in Table 2, the columns of the table were stratified by the cut-off values for the HCC patients, and the rows of the table were stratified according to different clinicopathological features. The correlation of clinicopathological features with NLR, GPR, and AFP values was analyzed using the chi-square test. The NLR (P < .001), GPR (P < .001), and AFP levels (P = .003) were correlated with BCLC stages. However, the Edmondson–Steiner grades were not correlated with any of the hematologic parameters (NLR and GPR) or AFP level (all P > .05). NLR > 2.02 (P = .014) and GPR > 0.21 (P < .001) were positively correlated with Child-Pugh grades. However, the level of AFP was not significantly related to the Child-Pugh grades. Tumor size was correlated with all the hematologic parameters (NLR, P < .001; GPR, P = .007; and AFP level, P < .001).
Table 2.
Relationship between hematological characteristics and clinicopathological features.
3.3. Comparisons of different hematologic parameters according to the HCC staging
The significance of the HCC staging with preintervention NLR, GPR, and AFP values was determined by the Mann–Whitney U test using the median with interquartile range to indicate the level of each hematologic ratio at different BCLC stages (Fig. 1) or Edmondson–Steiner grades (Fig. 2). Sustained rises in the NLR (all P < .05) and GPR (all P < .05) levels were significantly correlated with the BCLC stages, while AFP (P = .066) was not statistically significant in comparing BCLC stage B and BCLC stage C. Thus, NLR and GPR may be comparable to or even exceed AFP in differentiating between BCLC stage B and BCLC stage C. NLR and GPR cannot distinguish Edmondson–Steiner grades, while AFP (all P < .05) made a significant difference in distinguishing gradually increasing Edmondson–Steiner grades.
Figure 1.
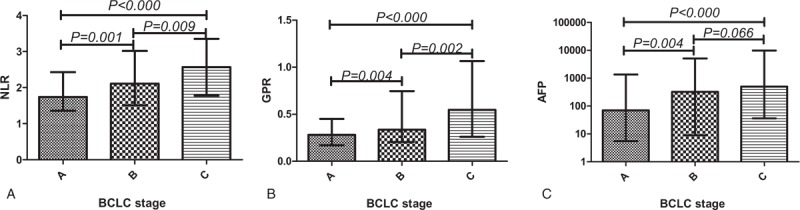
Comparisons of different hematologic parameters in HCC patients with different BCLC stages. BCLC = Barcelona clinic liver cancer, HCC = hepatocellular carcinoma.
Figure 2.
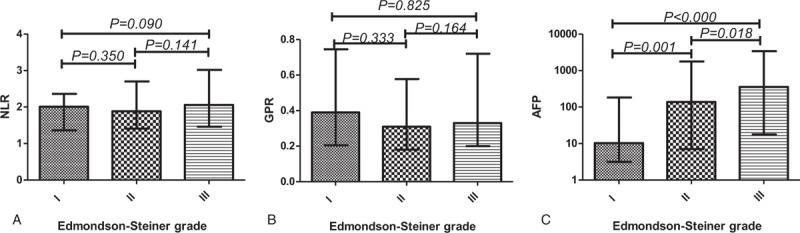
Comparisons of different hematologic parameters in HCC patients with different Edmondson–Steiner grades. HCC = hepatocellular carcinoma.
3.4. Diagnostic efficacy of NLR, GPR, and AFP alone or in combination in differentiating patients with HCC from controls
ROC analysis method was used for evaluating the diagnostic ability of preintervention NLR, GPR, and AFP values alone or in combination to differentiate HCC patients from controls. The results are presented in the Table 3 and Fig. 3. AFP had high sensitivity and specificity (74.69% and 98.41%, respectively) in differentiating HCC group from control group. Combined use of NLR and AFP produced larger AUC (0.916, 0.897–0.932) than NLR (P < .000) or AFP (P < .000) used alone. The sensitivity of differentiating HCC group from control group increased when GPR was used in combination with AFP. Moreover, the combination of GPR and AFP produced performance larger AUC (0.953, 0.938–0.966) compared with GPR (P < .000) or AFP (P < .000) used alone.
Table 3.
Diagnostic efficacy of NLR, GPR, and AFP alone or in combination in differentiating patients with HCC from controls.

Figure 3.
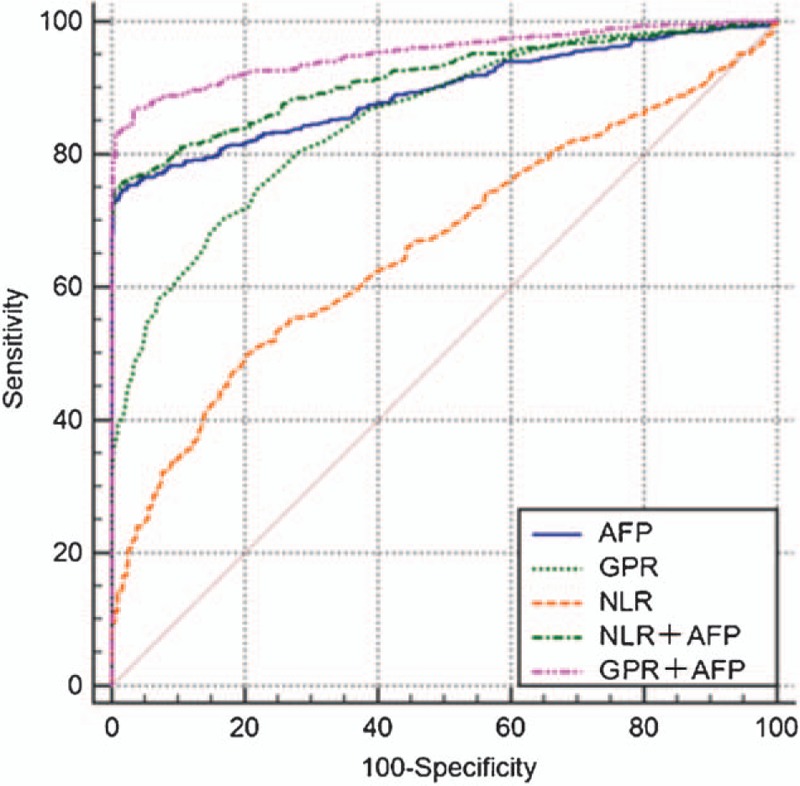
ROC curves of NLR, GPR, and AFP alone or in combination in differentiating patients with HCC from controls. AFP = α-fetoprotein, GPR = gamma-glutamyl transpeptidase to platelet ratio, HCC = hepatocellular carcinoma, NLR = neutrophil to lymphocyte ratio, ROC = receiver operating characteristic.
4. Discussion
Inflammation plays an important role in the development of HCC.[16] In the current study, we mainly assessed the diagnostic value of inflammatory markers (NLR and GPR) in HCC. The results suggested that NLR and GPR in the HCC group were significantly higher than those in the control group, which was consistent with previously reported findings.[14,17]
Edmondson–Steiner grades, Child-Pugh grades, and tumor size were closely related to HCC.[18–20] Songlin et al[21] revealed the association between AFP and clinicopathological features of HCC, including Child-Pugh grades and tumor size, which was consistent with our findings. In other studies, the relationship between hematologic parameters (NLR and GPR) and clinical characteristics (Child-Pugh grades and tumor size) has been demonstrated.[22,23] The NLR has traditionally been regarded as a marker of inflammation (systemic and subclinical).[9,24] Previous studies have reported a significant difference in the NLR of patients with severe preeclampsia and systemic lupus erythematosus versus that of controls.[25,26] Studies have also demonstrated that the NLR was significantly associated with the pathological grading of laryngeal carcinomas.[27] Thus, the relationship between NLR and the clinicopathological features of HCC appears to be different from other cancers or diseases. However, none of the studies reveal the relationship between NLR, GPR, and Edmondson–Steiner grades in HCC. Our current findings indicated that NLR and GPR have no correlation with Edmondson–Steiner grades.
The mechanism by which the hematologic ratios in this study can be used to monitor the stages of HCC is unclear. Du et al[28] followed 230 patients with cirrhosis and found that preoperative NLR elevation was associated with an increased risk of progression to HCC in patients with cirrhosis. Jin et al[29] evaluated the prognostic value of NLR in 556 patients with HCC in BCLC stage A, and found that increased NLR was a poor prognostic indicator. Park et al[14] evaluated the predictive value of GPR in patients with HCC and divided the included patients into low-risk and high-risk groups according to the GPR cut-off value. The results indicated that the relative risk of HCC development in the high-risk GPR group was significantly higher than in the low-risk GPR group. In the present study, an increase in the NLR and GPR was associated with the BCLC stages of HCC, which was consistent with previous studies.[30]
AFP is a well-known marker for the diagnosis of HCC.[31,32] The results of this study are consistent with those of Bertino et al,[33] who suggested that AFP had important predictive value in HCC and that the AUC was very large.Interestingly, in this study, the value of NLR and GPR was comparable to or higher than the commonly used marker AFP in differentiating between BCLC stage B and BCLC stage C. In clinical laboratory applications, accurate detection of AFP requires expensive and special equipment, and its detection speed and application are limited. In contrast, hematologic parameters have a number of advantages, including simple operation, low cost, rapid detection, and wide application. Hematologic ratios, such as the NLR and GPR, may become an alternative or auxiliary diagnostic indicator to AFP in the clinical values of HCC, especially in developing countries with limited resources where HCC is most prevalent.
AFP is a glycoprotein, which is closely related to the occurrence and development of various tumors (e.g., testicular cancer and dedifferentiated endometrioid adenocarcinoma).[34,35] In our study, the comparison of HCC group and control group revealed that AFP used alone gave the best diagnostic efficacy compared with NLR or GPR used alone, which was consistent with the results reported by previous studies.[36] In addition, our study found that the combined use of NLR or GPR with AFP was larger in AUC than that of NLR, GPR, and AFP used alone. Hence, we speculate that the combined use of NLR or PLR with AFP may improve the early diagnosis of HCC.
There were some limitations to our study. Firstly, this was a retrospective analysis. As such, it was prone to a variety of biases (e.g., selection bias and recall bias). Secondly, we were not able to directly analyze the relationship between factors and diseases. Finally, the sample size was limited and was not sufficient to explain the value of the NLR and GPR in predicting HCC progression. These problems could be resolved by increasing the sample size, setting strict inclusion criteria, and conducting rigorous prospective studies. However, our study is the first to assess the relationship between hematological parameters (NLR and GPR) and HCC staging (BCLC stages and Edmondson–Steiner grades), and establish that NLR and GPR had no correlation with Edmondson–Steiner grades. Our study is also the first to explore the diagnostic value of combined use of NLR, GPR, and AFP in HCC.
In summary, the hematologic ratios (NLR and GPR) studied herein might be associated with the BCLC staging of HCC. Combined use of NLR or GPR with AFP may improve early detection and diagnosis of HCC in order to enable early treatment of the disease.
Author contributions
Xue Qin and Shan Li drafted the overall design of this paper as the co-corresponding authors. Zuojian Hu and Huaping Chen wrote the article as the co-first authors. Siyuan Chen and Zhili Huang collected the laboratory data. Shanzi Qin and Jianing Zhong analyzed the data.
Data curation: Siyuan Chen, Shanzi Qin.
Formal analysis: Jianing Zhong.
Investigation: Shan Li.
Software: Zhili Huang.
Writing – original draft: Zuojian Hu.
Writing – review & editing: Xue Qin, Huaping Chen.
Footnotes
Abbreviations: AFP = α-fetoprotein, BCLC = Barcelona clinic liver cancer, GGT = gamma-glutamyl transpeptidase, GPR = gamma-glutamyl transpeptidase to platelet ratio, HCC = hepatocellular carcinoma, LYM = lymphocyte count, NEU = neutrophil count, NLR = neutrophil to lymphocyte ratio, PLT = platelet.
Zuojian Hu and Huaping Chen wrote the article as the co-first authors.
This work is supported by National Natural Science Foundation of China (No. 81460431), and the Key Laboratory of Early Prevention and Treatment of Regional High-incidence Tumors, Ministry of Education (GKE2017-ZZ03).
The authors report no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [4].Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [5].Francescone R, Hou V, Grivennikov SI. Microbiome, inflammation, and cancer. Cancer J 2014;20:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol 2014;816:401–35. [DOI] [PubMed] [Google Scholar]
- [7].Rekik S, Guyot E, Bhais M, et al. The CRP level and STATE score predict survival in cirrhotic patients with hepatocellular carcinoma treated by transarterial embolization. Dig Liver Dis 2016;48:1088–92. [DOI] [PubMed] [Google Scholar]
- [8].Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer 2016;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang L, Jia J, Lin L, et al. Predictive value of hematological markers of systemic inflammation for managing cervical cancer. Oncotarget 2017;8:44824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shibutani M, Maeda K, Nagahara H, et al. The pretreatment albumin to globulin ratio predicts chemotherapeutic outcomes in patients with unresectable metastatic colorectal cancer. BMC Cancer 2015;15:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2016;65:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tarocchi M, Polvani S, Marroncini G, et al. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol 2014;20:11630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta-analysis. Int J Surg 2018;55:73–80. [DOI] [PubMed] [Google Scholar]
- [14].Park YE, Kim BK, Park JY, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol 2017;32:1221–9. [DOI] [PubMed] [Google Scholar]
- [15].Liao W, Zhang J, Zhu Q, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol 2014;7:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Margetts J, Ogle LF, Chan SL, et al. Neutrophils: driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer 2018;118:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Okamura Y, Ashida R, Ito T, et al. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg 2015;39:1501–9. [DOI] [PubMed] [Google Scholar]
- [18].Tian F, Wu JX, Rong WQ, et al. Retrospective evaluation of discrepancies between radiological and pathological size of hepatocellular carcinoma masses. Asian Pac J Cancer Prev 2014;15:9487–94. [DOI] [PubMed] [Google Scholar]
- [19].Ogasawara S, Chiba T, Ooka Y, et al. Sorafenib treatment in Child-Pugh A and B patients with advanced hepatocellular carcinoma: safety, efficacy and prognostic factors. Invest New Drugs 2015;33:729–39. [DOI] [PubMed] [Google Scholar]
- [20].Zhou L, Rui JA, Zhou WX, et al. Edmondson-Steiner grade: a crucial predictor of recurrence and survival in hepatocellular carcinoma without microvascular invasio. Pathol Res Pract 2017;213:824–30. [DOI] [PubMed] [Google Scholar]
- [21].Songlin A, Weiqi R, Liming W, et al. [Analysis of clinicopathological features and prognosis between alpha-fetoprotein negative and positive hepatocellular carcinoma patients after R0 radical hepatectomy]. Zhonghua Zhong Liu Za Zhi 2015;37:308–11. [PubMed] [Google Scholar]
- [22].Gao F, Li X, Geng M, et al. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Medicine (Baltimore) 2015;94:e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pang Q, Bi JB, Wang ZX, et al. Simple models based on gamma-glutamyl transpeptidase and platelets for predicting survival in hepatitis B-associated hepatocellular carcinoma. Onco Targets Ther 2016;9:2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ozer S, Yilmaz R, Sönmezgöz E, et al. Simple markers for subclinical inflammation in patients with Familial Mediterranean Fever. Med Sci Monit 2015;21:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yucel B, Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy Hypertens 2017;7:29–32. [DOI] [PubMed] [Google Scholar]
- [26].Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol 2016;26:372–6. [DOI] [PubMed] [Google Scholar]
- [27].Kara M, Uysal S, Altinişik U, et al. The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol 2017;274:535–42. [DOI] [PubMed] [Google Scholar]
- [28].Du Z, Dong J, Bi J, et al. Predictive value of the preoperative neutrophil-to-lymphocyte ratio for the development of hepatocellular carcinoma in HBV-associated cirrhotic patients after splenectomy. PLoS One 2018;13:e0195336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jin C, Li C, Peng W, et al. Changes of platelet times neutrophil to lymphocyte ratio predict BCLC stage A hepatocellular carcinoma survival. Medicine (Baltimore) 2017;96:e7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sukato DC, Tohme S, Chalhoub D, et al. The prognostic role of neutrophil-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol 2015;26:816 e1–24 e1. [DOI] [PubMed] [Google Scholar]
- [31].Li L, Chen J, Xu W, et al. Clinical characteristics of hepatocellular carcinoma patients with normal serum alpha-fetoprotein level: a study of 112 consecutive cases. Asia Pac J Clin Oncol 2017;Oct 26. doi: 10.1111/ajco.12816. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [32].Gomez-Rodriguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012;104:298–304. [DOI] [PubMed] [Google Scholar]
- [33].Bertino G, Ardiri A, Malaguarnera M, et al. Hepatocellualar carcinoma serum markers. Semin Oncol 2012;39:410–33. [DOI] [PubMed] [Google Scholar]
- [34].Shimada S, Kinoshita H, Yoshida T, et al. [Late relapse of testicular cancer at the pelvis with elevated AFP levels: a case report]. Hinyokika Kiyo 2018;64:131–4. [DOI] [PubMed] [Google Scholar]
- [35].Cai H, Zhou R, Liang W, et al. Dedifferentiated endometrioid adenocarcinoma with trophoblastic components and elevated serum alfa-fetoprotein: a case report and literature review. Medicine (Baltimore) 2018;97:e0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen H, Zhang Y, Li S, et al. Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res 2018;10:1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


