Supplemental Digital Content is available in the text
Keywords: diabetes mellitus, hypertension, laparoscopic sleeve gastrectomy, laparoscopy adjustable gastric banding, obesity
Abstract
Background:
Laparoscopic adjustable gastric banding (LAGB) and laparoscopic sleeve gastrectomy (LSG) are common weight loss procedures. Our meta-analysis compared these procedures for the treatment of morbid obesity and related diseases.
Methods:
We systematically searched the PubMed, Embase, and the Cochrane Library through January 2018. The percentage of excess weight loss (%EWL), improvement or remission of type 2 diabetes mellitus (T2DM) and hypertension were analyzed and compared.
Results:
Thirty-three studies with 4109 patients were included. Greater decreases in excess weight were found in patients who received LSG at 6 months (weighted mean difference (WMD) −9.29, 95% confidence interval (CI): −15.19 to −3.40, P = .002), 12 months (WMD −16.67 95% CI: −24.30 to −9.05, P < .0001), 24 months (WMD −19.63, 95% CI: −29.00 to −10.26, P < .0001), and 36 months (WMD −19.28, 95% CI: −27.09 to −11.47, P < .0001) than in patients who received LAGB. However, there were no significant differences in the 3-month outcomes between the 2 groups (WMD −1.61, 95% CI: −9.96 to 6.73, P = .70). T2DM patients after LSG experience more significant improvement or remission of diabetes (odds ratio (OR): 0.22, 95% CI: 0.06–0.87, P = .03). The 2 groups did not significantly differ regarding improvement or remission of hypertension (OR 0.80, 95% CI: 0.46–1.38, P = .42).
Conclusion:
LSG is a more effective procedure than LAGB for morbidly obese patients, contributing to a higher %EWL and greater improvement in T2DM.
1. Introduction
World Health Organization data indicate that there were 422 million diabetic patients in 2014, and 10% of them were obese. Bariatric surgery is the most effective available therapy for obese patients with type 2 diabetes mellitus (T2DM); in fact, diabetes remission often occurs before significant weight loss, and the effects are superior to those of conventional therapy in randomized controlled trials (RCTs).[1–4]
Conventionally, 4 bariatric procedures are used for morbidly obese patients: laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG), laparoscopic Roux-en-Y gastric bypass (LRYGB), and biliopancreatic diversion (BPD).[5,6] LAGB initially accounted for most procedures and exerts a weight loss effect through a restrictive mechanism.[3] The band is placed 1 to 2 cm below the gastroesophageal junction and secured in place with a monofilament suture of the cardia and fundus below the band to the pouch above the band.[7] This operation is the least complex common procedure to perform and has the lowest early postoperative morbidity. However, the prevalence of this procedure has markedly declined in the past 10 years.[8] LSG is a bariatric procedure involving resection of most of the stomach along the greater curvature, leaving only a narrow tube between the gastroesophageal junction and the pylorus.[9] LSG has received increasing attention because of the relatively low rate of complications and the degree of percentage of excess weight loss (%EWL) and glucose reductions.[10] Given the increasing popularity of LSG over LAGB as a restrictive procedure, we performed a systematic review and meta-analysis of the available published literature to compare outcomes of the 2 approaches.
2. Methods
2.1. Literature search strategy
We searched PubMed, Embase, and the Cochrane Library for relevant articles (through January 2018). The following medical subject heading (MeSH) terms and their combinations were searched [in the Title/Abstract]: “body weight,” “weight loss,” “weight gain,” “weight change,” “body fat,” “adipose tissue,” “sleeve gastrectomy,” “gastric banding,” and “bariatric surgery.” The search strategy also used several text terms to identify relevant information. Reference lists from relevant primary studies and review articles were also examined to find additional publications.
2.2. Study inclusion and exclusion criteria
The following inclusion criteria were applied: RCTs and nonrandomized studies that compared LAGB with LSG regardless of publication date, and studies reporting outcomes of %EWL and/or diabetes mellitus and hypertension. Remission of T2DM is defined as fasting plasma glucose levels less than 125 mg/dL with A1c < 6.5% maintained for at least 1 year.[11] Hypertension remission was defined as normal blood pressure (BP) levels without antihypertensive therapy at 1 year (systolic BP < 140 mm Hg and diastolic BP < 90 mm Hg) and hypertension improvement was considered when a decrease in dosage or number of antihypertensive medications was required or when a decrease in systolic or diastolic BP levels was observed with the same medication.[12] The major exclusion criteria were duplicate publications; studies using animal models; studies that did not report usable data; case reports, letters, or articles that were not full texts; and commentaries, reviews and non-English publications.
2.3. Data extraction and risk-of-bias assessment
Two investigators independently extracted and evaluated all eligible studies. The authors, publication year, number of patients, mean age, %male subjects, and mean body mass index (BMI) were recorded for each study. We captured the following outcome variables: %EWL and (or) improvement or remission of T2DM and (or) hypertension. %EWL was stratified according to different follow-up time points (3, 6, 12, 24, and 36 months). In addition, major obesity-related diseases, including T2DM and hypertension, were also pooled and compared. Discrepancies were resolved by discussion between the 2 authors. If the 2 authors could not reach a consensus, the third author was consulted, and a final decision was made by voting. Two authors independently assessed the risk of bias using the approach recommended by the Cochrane Handbook for Systematic Reviews of Interventions.[13] Efforts were made to obtain exact numerical data from authors via email if the data were not available in the article.
2.4. Statistical analysis
Analysis of outcomes was performed with STATA/SE version 12.0 and Review Manager Version 5.0. Continuous variables were pooled using the weighted mean difference (WMD) with 95% confidence intervals (CIs), while odds ratios (ORs) with 95% CIs were applied to perform the statistical analysis for dichotomous variables. A χ2 test was performed to assess the heterogeneity of the included studies.[14] If P > .1 and/or I2 < 50%, the fixed effect model was used for data analysis; otherwise, the random-effect model was adopted. Publication bias of studies was estimated by Begg funnel plot with Egger test. If publication bias was present, we further evaluated the number of missing studies by the Duval and Tweedie trim and fill procedure and recalculated the pooled risk estimates with the addition of those missing studies. The statistical tests were 2-sided, and P < .05 was considered statistically significant. If data on continuous outcomes were reported as medians and ranges, we estimated the mean and standard deviation according to the Hozo method.[15]
2.5. Ethical review
This is a meta-analysis article, does not involve ethical review, and ethical approval is not necessary after inquiring the ethical review committee in our hospital.
3. Results
A flow chart of the literature search strategies is presented in Fig. 1. After exclusion of several studies for methodological reasons, our search yielded 33 eligible published studies for this meta-analysis.[16–48] All studies were published after 2005 and included a total of 4109 patients. Among these patients, 2126 (51.7%) underwent LAGB, and 1983 (48.3%) underwent LSG. The sample size of these trials ranged from 10 to 245 patients. Fourteen trials were nonrandomized, prospective observational studies,[16,19,22–25,27,28,33,37,39,41,45,47] 18 were retrospective cohort studies,[17,18,20,21,26,29–32,34–36,38,40,42–44,46] and 1 was a prospective RCT.[48] The improvement or remission of diabetes and hypertension is recorded in different follow-up periods, and all patients were followed for less than 4 years. The characteristics and the risk of bias in the included studies are shown in Table 1 and Fig. 2.
Figure 1.
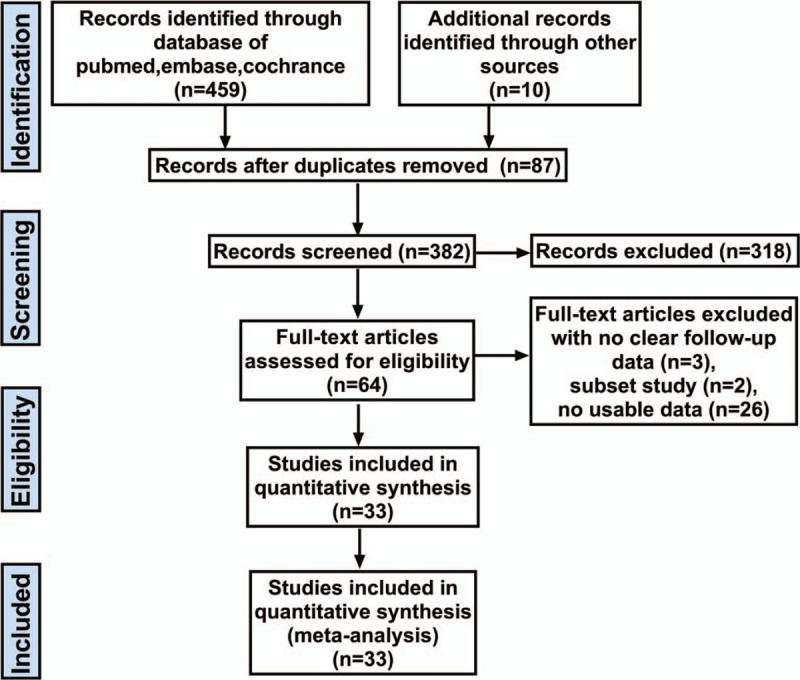
Study selection diagram for the meta-analysis of bariatric procedures.
Table 1.
Characteristics of the studies included in the meta-analysis.
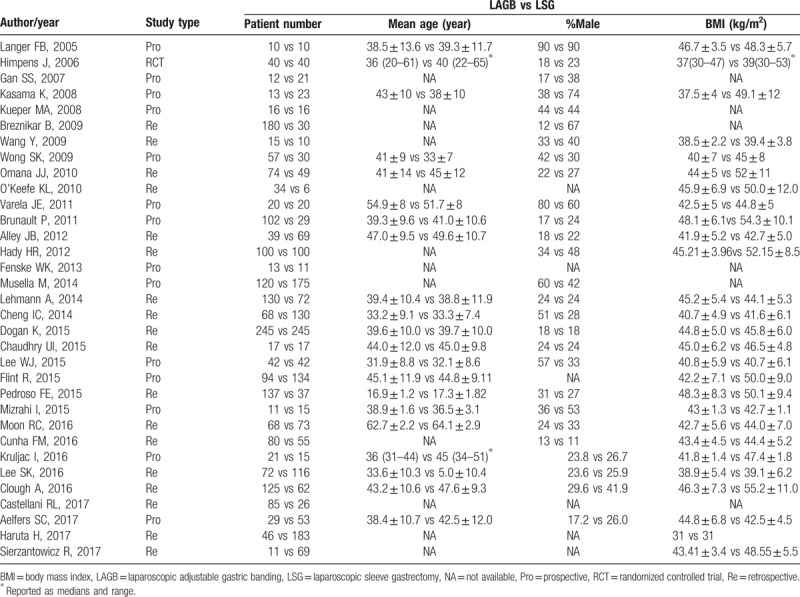
Figure 2.
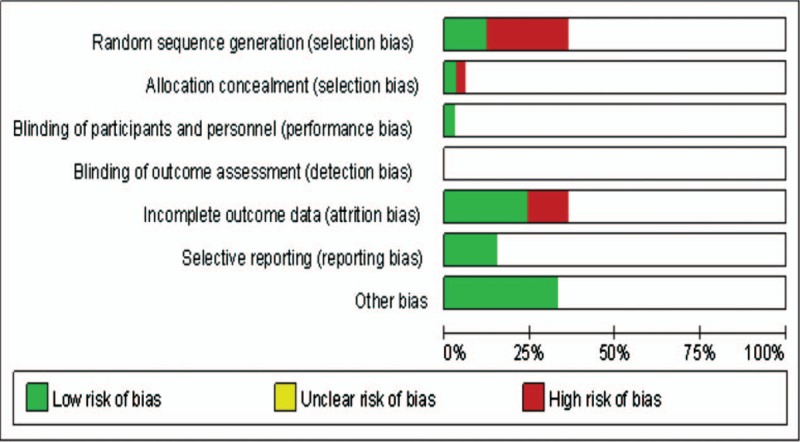
Risk of bias of included studies.
3.1. %EWL after LAGB versus LSG
The results indicated that patients receiving LSG had significantly higher scores at 6 months (WMD −9.56, 95% CI: −15.74 to −3.38, P = .002, I2 = 95%; Fig. 3B), 12 months (WMD −16.67 95% CI: −24.30 to −9.05, P < .0001, I2 = 97%; Fig. 4A), 24 months (WMD −19.63, 95% CI: −29.00 to −10.26, P < .0001, I2 = 95%; Fig. 4B), and 36 months (WMD −19.28, 95% CI: −27.09 to −11.47, P < .0001, I2 = 82%; Fig. 4C) after surgery. However, there was no significant difference between the LSG group and the LAGB group at 3 months (WMD −1.61, 95% CI: −9.96 to 6.73, P = .62, I2 = 98%; Fig. 3A).
Figure 3.
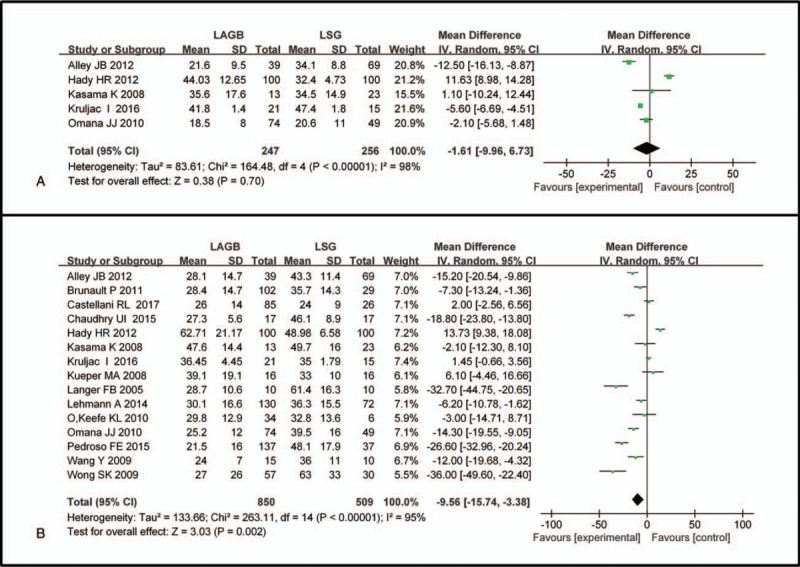
Forest plot of comparisons between LAGB and LSG for %EWL at (A) 3 months and (B) 6 months after surgery. Mean differences are shown with 95% CIs. LAGB = laparoscopic adjustable gastric banding, LSG = laparoscopic sleeve gastrectomy, CIs = confidence intervals, %EWL = percentage of excess weight loss.
Figure 4.
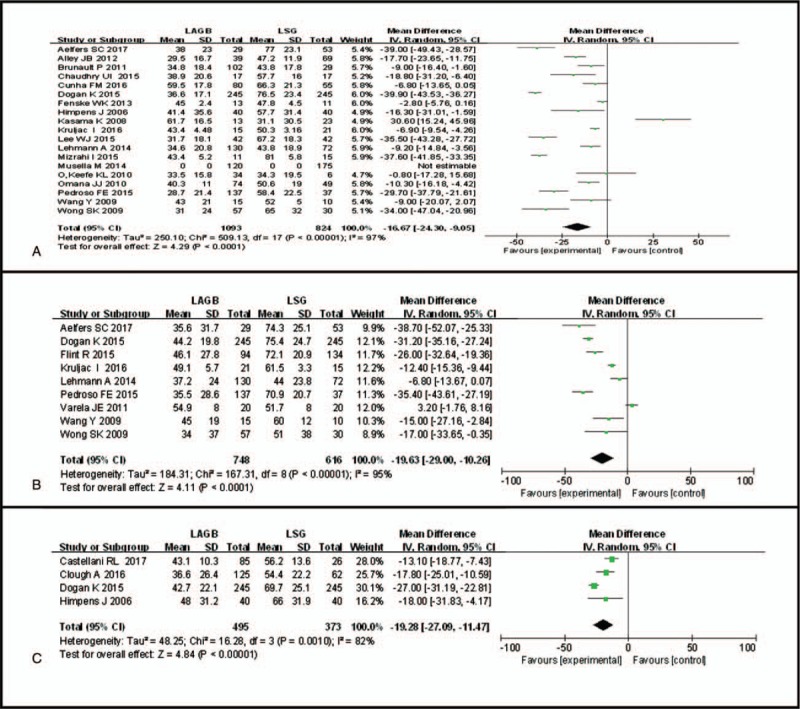
Forest plot of comparisons between LAGB and LSG for %EWL at (A) 12 months, (B) 24 months, and (C) 36 months after surgery. Mean differences are shown with 95% CIs. LAGB = laparoscopic adjustable gastric banding, LSG = laparoscopic sleeve gastrectomy, CIs = confidence intervals, %EWL = percentage of excess weight loss.
3.2. Improvement or remission of T2DM after LAGB versus LSG
Considering the significant heterogeneity between the 2 groups (P < .00001, I2 = 87%), the random-effect model was applied. LSG appeared to have a significant effect on improvement or remission of T2DM after the postoperative follow-up period (OR 0.22, 95% CI: 0.06–0.87, P = .03, Fig. 5A).
Figure 5.
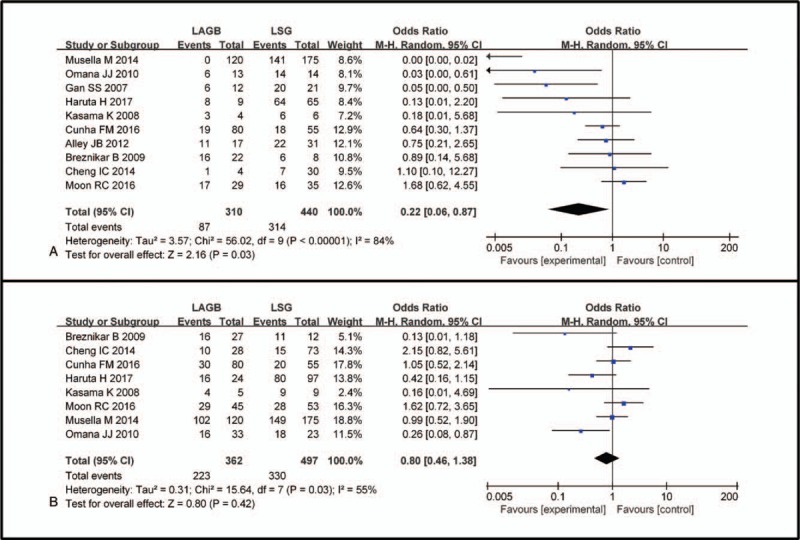
Forest plot of comparisons between LAGB and LSG in terms of the improvement or remission of T2DM and hypertension postoperatively. Odds ratios are shown with 95% CIs. LAGB = laparoscopic adjustable gastric banding, LSG = laparoscopic sleeve gastrectomy, CIs = confidence intervals, T2DM = type 2 diabetes mellitus.
3.3. Improvement or remission of hypertension after LAGB versus LSG
According to the pooled data, 223 of 362 (61%) patients with hypertension experienced improvement of their hypertension after LAGB, and 330 of 497 (63%) patients with hypertension improved after LSG. As expected, LSG had the same impact on hypertension as LAGB (OR 0.80, 95% CI: 0.46–1.38, P = .42, Fig. 5B) according to the meta-analysis of the 7 eligible studies. Due to the existence of heterogeneity (P = .03, I2 = 55%), a random effects model was used.
3.4. Publication bias and heterogeneity
The funnel plots used to detect publication bias in the meta-analysis are presented in Fig. 6. No evidence of publication bias was detected for %EWL at 6 months (Egger test, P = .458, Fig. 6A), %EWL at 12 months (Egger test, P = .622, Fig. 6B) after surgery, or the improvement/remission of hypertension (Egger test, P = .107, Fig. 6D) after surgery. Publication bias was present only for improvement/remission of T2DM after surgery (P = .044). After using the trim and fill’ method for improvement/remission of T2DM after surgery, there was no longer evidence of publication bias (P = .057, Fig. 6C). Publication bias was not calculated for the rest of the outcomes due to the small number of studies that were included. Substantial heterogeneity was present in the meta-analysis findings, with I2 values ranging from 55.0% to 98.0%. After stratification of data, heterogeneity decreased in some categories. The pooled effectiveness of LAGB versus LSG for obesity findings significantly varied by study type, publication date, and revision surgery (Table S1).
Figure 6.
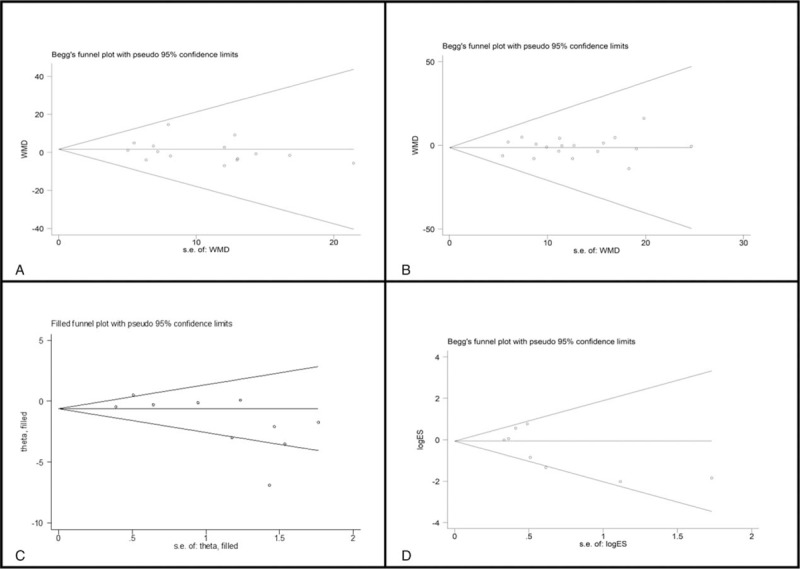
Funnel plot using Begg method for (A) %EWL at 6 months, (B) %EWL at 12 months, (D) improvement or remission of hypertension after surgery; Funnel plot using adjusted trim and fill method for (C) improvement or remission of diabetes.
4. Discussion
Bariatric surgery is currently the most effective therapy for long-term weight loss in morbidly obese patients. In addition, it is effective for the treatment of obesity-related diseases, such as T2DM and hypertension. Currently available bariatric procedures include LAGB, LRYGB, LSG, and BPD.[5,6] In this meta-analysis, we compared the efficacy of LAGB and LSG on obesity and related diseases by analyzing published studies.
Although an informative systematic review has been published, no previous studies have quantitatively analyzed such a robust dataset of LAGB versus LSG for obesity and related diseases. Wang et al[49] showed that LSG was a more effective procedure for morbid obesity than LAGB in a small meta-analysis using a fixed effect model for data analysis, while heterogeneity was evident. Our study incorporates and extensively updates these results and clarifies the difference between the effects of LAGB and LSG on %EWL. However, improvement or remission of diabetes and hypertension cannot be clarified because of insufficient data. We extracted data from 33 valid independent studies of 4109 patients interviewed for LAGB and LSG using a standardized method and sampled data without selection bias. This evaluation reinforced the finding that LSG led to significantly greater %EWL than LAGB over long-term periods. These findings provide strong evidence for long-term %EWL benefit among patients who undergo LSG. Only 2 studies included 5-year outcomes and recruited adolescent or adult patients. It is very likely that publication bias exists; we did not include the analysis for 5-year outcomes. To the best of our knowledge, this is the first meta-analysis comparing the long-term outcomes of LSG and LAGB. Furthermore, LSG is a more effective approach than LAGB for treating morbid obesity comorbidities such as diabetes mellitus.
Altieri et al[50] reported that at least one-fifth of patients who received LAGB in the state of New York between 2004 and 2010 underwent device revision or removal. The revision rate may be as high as 34.17%. However, 20.36% underwent more than 1 subsequent intervention. Revisional procedures had a higher rate of complications, most commonly digestive/intestinal complications, surgical errors, or pneumonia.[50] In contrast, the total number of LSG cases is increasing due to the simplicity of the procedure, its low risk, and good outcomes.[51,52] According to a new survey, LSG has been the most frequently performed surgery in Asia in recent years. Our meta-analysis indicated that LAGB has a similar effect as LSG on %EWL in the 3-month postoperative period. The reason for this similar result may be that surgeons often prescribe a low carbohydrate diet after LAGB, which may account for greater %EWL at 3 months than at other time points, as adherence to the diet declines. One interesting study by Chakravarty et al[53] compared LAGB with other bariatric procedures. The authors concluded that LAGB was not the most effective bariatric procedure for reducing weight compared with other procedures; nevertheless, LAGB was associated with fewer early complications, a shorter operative time, and a shorter length of hospital stay. However, our meta-analysis also demonstrated that patients who underwent LSG lost more excess weight by 6, 12, 24, and 36 months than those who underwent LAGB. The highest concentrations of ghrelin are found in the gastric fundus, and production stops when this area is removed after LSG, which may result in greater %EWL after LSG.[5,23,54] Ghrelin is the only gastrointestinal hormone that stimulates food intake, and the serum ghrelin level is inversely proportional to body weight. Langer et al[23] reported that ghrelin levels remained unchanged immediately after LAGB and increased after 1 and 6 months, whereas ghrelin decreased both immediately and at 1 and 6 months after LSG.
Because the prevalence of comorbidities was different among groups, performing a direct comparison of the procedures is difficult to determine which procedure is superior; therefore, we only compared the comorbidities of T2DM and hypertension. LSG was observed to achieve better T2DM control than LAGB. In addition to improved glucose metabolism being associated with %EWL after surgery, gut hormones play a major role in diabetes improvement or remission and, most likely, in %EWL after LSG.[5,55] The hindgut hypothesis proposes that stimulation of the distal ileum with the early arrival of undigested nutrients is responsible for the improvement in glucose tolerance after bariatric surgery.[56] Remarkable changes in factors such as glucagon-like peptide 1 are the basis of this hypothesis.[57] Gastric banding operations do not cause obvious alterations in gut hormones and seem to depend exclusively on restriction-derived weight loss for the antidiabetic and weight loss effects. Himpens et al[58] reported long-term outcomes after LAGB; preoperatively, 6.4% of the patients had DM, which increased to 14.1% at 12 years. Other mechanisms of %EWL involve bile acid, gut microflora, the vagus nerve, and other gut hormones.[59–61] LSG may be a better choice than LAGB for patients with T2DM. Due to complications such as LSG-like leaks and gastro-bronchial fistulas, LAGB can still be considered for the surgical treatment of morbid obesity and appears to be safer, especially in patients without obesity-related diseases. However, both procedures were equivalent in hypertension control. The antihypertensive effect of bariatric surgery has been attributed to the reduction in plasma aldosterone levels, particularly in those with visceral adiposity.[62] BP might often increase back to preoperative levels during the weight regain or even during the weight maintenance phase.[63,64]
There are some limitations of our meta-analysis that should be considered. First, our article is limited by a lack of RCTs with large sample sizes. Second, some patients in the included studies converted to open surgery, which may introduce bias in the final result. Third, due to fasting blood glucose < 126 mg/dL was defined as diabetes remission, there were patients who were defined as being in remission of T2DM who had prediabetes. In addition, there may be patients with blood pressure < 130 mm Hg but ≥ 120 mm Hg, systolic, and < 85 mm Hg but ≥ 80 mm Hg, diastolic. These patients may fall into the elevated blood pressure category according to the 2017 updated blood pressure guidelines, although these clinical reports included in the meta-analysis were completed before 2017.
Well-designed studies with larger sample sizes and longer follow-up periods are merited for future studies. Nevertheless, this meta-analysis was conducted at an appropriate time, and we provide the most up-to-date information in this area.
In conclusion, our meta-analysis emphasizes that LSG can lead to significantly greater sustained %EWL and T2DM remission than LAGB, while these 2 procedures have similar effects on hypertension. However, these conclusions should be validated in further RCTs with appropriate sample sizes, and long-term follow-up outcomes should be confirmed.
Author contributions
Data curation: Jinglin Liang, Yinyin Guo.
Formal analysis: Huichuan Yu, Shaoyong Peng.
Funding acquisition: Yanxin Luo, Jianping Wang.
Methodology: Yinyin Guo.
Project administration: Yanxin Luo.
Supervision: Jianping Wang.
Writing – original draft: Laiyuan Li.
Writing – review & editing: Laiyuan Li.
Laiyuan Li orcid: 0000-0002-3022-2005.
Supplementary Material
Footnotes
Abbreviations: %EWL = percentage of excess weight loss, BMI = body mass index, BP = blood pressure, BPD = biliopancreatic diversion, CIs = confidence intervals, LAGB = laparoscopic adjustable gastric banding, LRYGB = laparoscopic Roux-en-Y gastric bypass, LSG = laparoscopic sleeve gastrectomy, MeSH = medical subject heading, OR = odds ratio, RCTs = randomized controlled trials, T2DM = type 2 diabetes mellitus, WHO = World Health Organization, WMD = weighted mean difference.
Support for these studies was provided by the National Basic Research Program of China (973 Program) (No. 2015CB554001, JW), the National Natural Science Foundation of China (No. 81472257, YL; No. 81201920, YL), the Natural Science Fund for Distinguished Young Scholars of Guangdong Province (No. 2016A030306002, YL), the Tip-top Scientific and Technical Innovative Youth Talents of Guangdong special support program (No. 2015TQ01R454, YL), the Natural Science Foundation of Guangdong Province (No. S2013010013607, YL; No. 2016A030310222, HY), the Science and Technology Program of Guangzhou (No. 201506010099, YL; No. 2014Y2-00160, JW), and the Fundamental Research Funds for the Central Universities (Sun Yat-sen University) (No. 2015ykzd10, YL; No.13ykpy37, YL), National Key Clinical Discipline.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Rizzello M, Abbatini F, Casella G, et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg 2010;20:50–5. [DOI] [PubMed] [Google Scholar]
- [2].Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004;240:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dixon JB, le Roux CW, Rubino F, et al. Bariatric surgery for type 2 diabetes. Lancet 2012;379:2300–11. [DOI] [PubMed] [Google Scholar]
- [4].Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85. [DOI] [PubMed] [Google Scholar]
- [5].Thomas S, Schauer P. Bariatric surgery and the gut hormone response. Nutr Clin Pract 2010;25:175–82. [DOI] [PubMed] [Google Scholar]
- [6].Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013;23:427–36. [DOI] [PubMed] [Google Scholar]
- [7].Loy JJ, Youn HA, Schwack B, et al. Safety and efficacy of laparoscopic adjustable gastric banding in patients aged seventy and older. Surg Obes Relat Dis 2014;10:284–9. [DOI] [PubMed] [Google Scholar]
- [8].Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822–32. [DOI] [PubMed] [Google Scholar]
- [9].Lombardo V, Baratta R, Giannone G. Laparoscopic sleeve gastrectomy for morbid obesity. Our initial experience. Ann Ital Chir 2010;81:17–20. [PubMed] [Google Scholar]
- [10].Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 2013;258:690–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mas-Lorenzo A, Benaiges D, Flores-Le-Roux JA, et al. Impact of different criteria on type 2 diabetes remission rate after bariatric surgery. Obes Surg 2014;24:1881–7. [DOI] [PubMed] [Google Scholar]
- [12].Benaiges D, Sague M, Flores-Le Roux JA, et al. Predictors of hypertension remission and recurrence after bariatric surgery. Am J Hypertens 2016;29:653–9. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011: The Cochrane Collaboration 2018. Available at: http://www.handbook.cochrane.org (updated Mar 2011). [Google Scholar]
- [14].Clarke M, Horton R. Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet 2001;357:1728. [DOI] [PubMed] [Google Scholar]
- [15].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wong SK, Kong AP, Mui WL, et al. Laparoscopic bariatric surgery: a five-year review. Hong Kong Med J 2009;15:100–9. [PubMed] [Google Scholar]
- [17].Omana JJ, Nguyen SQ, Herron D, et al. Comparison of comorbidity resolution and improvement between laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding. Surg Endosc 2010;24:2513–7. [DOI] [PubMed] [Google Scholar]
- [18].O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg 2010;20:1199–205. [DOI] [PubMed] [Google Scholar]
- [19].Musella M, Milone M, Gaudioso D, et al. A decade of bariatric surgery. What have we learned? Outcome in 520 patients from a single institution. Int J Surg 2014;12suppl 1:S183–8. [DOI] [PubMed] [Google Scholar]
- [20].Moon RC, Kreimer F, Teixeira AF, et al. Morbidity rates and weight loss after roux-en-y gastric bypass, sleeve gastrectomy, and adjustable gastric banding in patients older than 60 years old: which procedure to choose? Obes Surg 2016;26:730–6. [DOI] [PubMed] [Google Scholar]
- [21].Lehmann A, Bobowicz M, Lech P, et al. Comparison of percentage excess weight loss after laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding. Wideochir Inne Tech Maloinwazyjne 2014;9:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee WJ, Lee KT, Ser KH, et al. Laparoscopic adjustable gastric banding (LAGB) with gastric plication: short-term results and comparison with LAGB alone and sleeve gastrectomy. Surg Obes Relat Dis 2015;11:125–30. [DOI] [PubMed] [Google Scholar]
- [23].Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg 15;15:1024–9. [DOI] [PubMed] [Google Scholar]
- [24].Kueper MA, Kramer KM, Kirschniak A, et al. Laparoscopic sleeve gastrectomy: standardized technique of a potential stand-alone bariatric procedure in morbidly obese patients. World J Surg 2008;32:1462–5. [DOI] [PubMed] [Google Scholar]
- [25].Kasama K, Tagaya N, Kanahira E, et al. Has laparoscopic bariatric surgery been accepted in Japan? The experience of a single surgeon. Obes Surg 2008;18:1473–8. [DOI] [PubMed] [Google Scholar]
- [26].Hady HR, Golaszewski P, Zbucki RL, et al. The influence of laparoscopic adjustable gastric banding and laparoscopic sleeve gastrectomy on weight loss, plasma ghrelin, insulin, glucose and lipids. Folia Histochem Cytobiol 2012;50:292–303. [DOI] [PubMed] [Google Scholar]
- [27].Gan SS, Talbot ML, Jorgensen JO. Efficacy of surgery in the management of obesity-related type 2 diabetes mellitus. ANZ J Surg 2007;77:958–62. [DOI] [PubMed] [Google Scholar]
- [28].Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and BP: a 12-month prospective study. Surg Obes Relat Dis 2013;9:559–68. [DOI] [PubMed] [Google Scholar]
- [29].Dogan K, Gadiot RP, Aarts EO, et al. Effectiveness and safety of sleeve gastrectomy, gastric bypass, and adjustable gastric banding in morbidly obese patients: a multicenter, retrospective, matched cohort study. Obes Surg 2015;25:1110–8. [DOI] [PubMed] [Google Scholar]
- [30].Cunha FM, Oliveira J, Preto J, et al. The effect of bariatric surgery type on lipid profile: an age, sex, body mass index and excess weight loss matched study. Obes Surg 2016;26:1041–7. [DOI] [PubMed] [Google Scholar]
- [31].Cheng IC, Wei SC, Yeh SL, et al. Comparison of weight loss and body composition changes in morbidly obese Taiwanese patients with different bariatric surgeries: a 1-year follow-up study. Obes Surg 2014;24:572–7. [DOI] [PubMed] [Google Scholar]
- [32].Chaudhry UI, Osayi SN, Suzo AJ, et al. Laparoscopic adjustable gastric banded plication: case-matched study from a single U.S. center. Surg Obes Relat Dis 2015;11:119–24. [DOI] [PubMed] [Google Scholar]
- [33].Brunault P, Jacobi D, Leger J, et al. Observations regarding ’quality of life’ and ’comfort with food’ after bariatric surgery: comparison between laparoscopic adjustable gastric banding and sleeve gastrectomy. Obes Surg 2011;21:1225–31. [DOI] [PubMed] [Google Scholar]
- [34].Breznikar B, Dinevski D. Bariatric surgery for morbid obesity: pre-operative assessment, surgical techniques and post-operative monitoring. J Int Med Res 2009;37:1632–45. [DOI] [PubMed] [Google Scholar]
- [35].Alley JB, Fenton SJ, Harnisch MC, et al. Quality of life after sleeve gastrectomy and adjustable gastric banding. Surg Obes Relat Dis 2012;8:31–40. [DOI] [PubMed] [Google Scholar]
- [36].Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg 2009;19:357–62. [DOI] [PubMed] [Google Scholar]
- [37].Flint R. A comparison of laparoscopic adjustable gastric band and laparoscopic sleeve gastrectomy: a single surgeon's experience. N Z Med J 2015;128:56–61. [PubMed] [Google Scholar]
- [38].Pedroso FE, Gander J, Oh PS, et al. Laparoscopic vertical sleeve gastrectomy significantly improves short term weight loss as compared to laparoscopic adjustable gastric band placement in morbidly obese adolescent patients. J Pediatr Surg 2015;50:115–22. [DOI] [PubMed] [Google Scholar]
- [39].Mizrahi I, Beglaibter N, Simanovsky N, et al. Ultrasound evaluation of visceral and subcutaneous fat reduction in morbidly obese subjects undergoing laparoscopic gastric banding, sleeve gastrectomy, and Roux-en-Y gastric bypass: a prospective comparison study. Obes Surg 2015;25:959–66. [DOI] [PubMed] [Google Scholar]
- [40].Lee SK, Heo Y, Park JM, et al. Roux-en-Y gastric bypass vs. sleeve gastrectomy vs. gastric banding: the first multicenter retrospective comparative cohort study in obese Korean patients. Yonsei Med J 2016;57:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kruljac I, Mirosevic G, Kirigin LS, et al. Changes in metabolic hormones after bariatric surgery and their predictive impact on weight loss. Clin Endocrinol (Oxf) 2016;85:852–60. [DOI] [PubMed] [Google Scholar]
- [42].Haruta H, Kasama K, Ohta M, et al. Long-term outcomes of bariatric and metabolic surgery in japan: results of a multi-institutional survey. Obes Surg 2017;27:754–62. [DOI] [PubMed] [Google Scholar]
- [43].Clough A, Hamill D, Jackson S, et al. Outcome of three common bariatric procedures in the public sector. ANZ J Surg 2017;87:930–4. [DOI] [PubMed] [Google Scholar]
- [44].Castellani RL, Toppino M, Favretti F, et al. National survey for bariatric procedures in adolescent: long time follow-up. J Pediatr Surg 2017;52:1602–5. [DOI] [PubMed] [Google Scholar]
- [45].Aelfers SCW, Schijns W, Ploeger N, et al. Patients’ preoperative estimate of target weight and actual outcome after bariatric surgery. Obes Surg 2017;27:1729–34. [DOI] [PubMed] [Google Scholar]
- [46].Sierzantowicz R, Lewko J, Trochimowicz L, et al. The effect of bariatric procedures on selected laboratory parameters of patients from rural areas in Poland. Adv Clin Exp Med 2017;26:679–86. [DOI] [PubMed] [Google Scholar]
- [47].Varela JE. Laparoscopic sleeve gastrectomy versus laparoscopic adjustable gastric banding for the treatment severe obesity in high risk patients. JSLS 2011;15:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg 2006;16:1450–6. [DOI] [PubMed] [Google Scholar]
- [49].Wang S, Li P, Sun XF, et al. Comparison between laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding for morbid obesity: a meta-analysis. Obes Surg 2013;23:980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Altieri MS, Yang J, Telem DA, et al. Lap band outcomes from 19,221 patients across centers and over a decade within the state of New York. Surg Endosc 2016;30:1725–32. [DOI] [PubMed] [Google Scholar]
- [51].Aarts EO, Dogan K, Koehestanie P, et al. Long-term results after laparoscopic adjustable gastric banding: a mean fourteen year follow-up study. Surg Obes Relat Dis 2014;10:633–40. [DOI] [PubMed] [Google Scholar]
- [52].Zellmer JD, Mathiason MA, Kallies KJ, et al. Is laparoscopic sleeve gastrectomy a lower risk bariatric procedure compared with laparoscopic Roux-en-Y gastric bypass? A meta-analysis. Am J Surg 2014;208:903–10. [DOI] [PubMed] [Google Scholar]
- [53].Chakravarty PD, McLaughlin E, Whittaker D, et al. Comparison of laparoscopic adjustable gastric banding (LAGB) with other bariatric procedures; a systematic review of the randomised controlled trials. Surgeon 2012;10:172–82. [DOI] [PubMed] [Google Scholar]
- [54].Karra E, Batterham RL. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol 2010;316:120–8. [DOI] [PubMed] [Google Scholar]
- [55].Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 2009;150:2518–25. [DOI] [PubMed] [Google Scholar]
- [56].Sala PC, Torrinhas RS, Heymsfield SB, et al. Type 2 diabetes mellitus: a possible surgically reversible intestinal dysfunction. Obes Surg 2012;22:167–76. [DOI] [PubMed] [Google Scholar]
- [57].Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery 2007;142:74–85. [DOI] [PubMed] [Google Scholar]
- [58].Himpens J, Cadiere GB, Bazi M, et al. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011;146:802–7. [DOI] [PubMed] [Google Scholar]
- [59].Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 2009;17:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011;60:1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Burton PR, Brown W, Laurie C, et al. The effect of laparoscopic adjustable gastric bands on esophageal motility and the gastroesophageal junction: analysis using high-resolution video manometry. Obes Surg 2009;19:905–14. [DOI] [PubMed] [Google Scholar]
- [62].Bueter M, Ahmed A, Ashrafian H, et al. Bariatric surgery and hypertension. Surg Obes Relat Dis 2009;5:615–20. [DOI] [PubMed] [Google Scholar]
- [63].Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- [64].Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


