Supplemental Digital Content is available in the text
Keywords: carpal tunnel syndrome, extracorporeal shock wave therapy, median neuropathy, meta-analysis
Abstract
Background:
Although several trials have reported the use of extracorporeal shock wave therapy (ESWT) for mild to moderate carpal tunnel syndrome (CTS), little is known about the efficacy of ESWT. Thus, we performed a meta-analysis to evaluate whether ESWT can improve symptoms, functional outcomes, and electrophysiologic parameters in CTS.
Methods:
Six randomized controlled trials investigating the effect of ESWT on CTS were retrieved from PubMed, Embase, and the Cochrane Library. We performed a pairwise meta-analysis using fixed- or random-effects models.
Results:
ESWT showed significant overall effect size compared to the control (overall Hedge g pooled standardized mean difference (SMD) = 1.447; 95% confidence interval [CI], 0.439–2.456; P = .005). Symptoms, functional outcomes, and electrophysiologic parameters all improved with ESWT treatment. However, there was no obvious difference between the efficacy of ESWT and local corticosteroid injection (pooled SMD = 0.418; 95% CI, −0.131 to 0.968; P = .135). A publication bias was not evident in this study.
Conclusion:
Our meta-analysis revealed that ESWT can improve symptoms, functional outcomes, and electrophysiologic parameters in patients with CTS. Further research is needed to confirm the long-term effects and the optimal ESWT protocol for CTS.
1. Introduction
Carpal tunnel syndrome (CTS) is a clinical syndrome caused by compression of the median nerve at the wrist. It is the most common entrapment neuropathy in adults.[1] Clinical features of CTS include nocturnal pain, numbness, tingling sensation in the median nerve dermatome, and the diagnosis is confirmed by these typical clinical symptoms, along with electrodiagnostic studies.[2,3] While the pathophysiology of CTS is not fully understood, ischemic injury due to increased carpal tunnel pressure is considered to be the most crucial factor.[3,4] Repetitive wrist movements, obesity, rheumatoid arthritis, diabetes mellitus, and menopause are known risk factors of CTS.[3,5]
Treatment options for CTS consist of wrist splints, physical modalities, local corticosteroid injections, and surgical treatments.[6,7,8] For mild to moderate CTS, conservative nonsurgical treatments are recommended prior to surgery.[9] When conservative treatment fails, surgical treatment can be considered.[10] The effects of a wrist splint, local corticosteroid injection, and surgical treatment have been demonstrated in multiple studies.[5] However, other conservative treatments such as oral steroids, therapeutic ultrasound, and low level laser therapy have limited evidence of being effective.[5,11]
Recently, extracorporeal shock wave therapy (ESWT) has been used for the treatment of CTS as a novel and noninvasive method. Since Romeo et al first used ESWT for pillar pain after carpal tunnel release,[12] a few randomized controlled studies have also reported that ESWT can improve functional outcomes and electrophysiologic parameters. However, there is still a lack of clear evidence regarding ESWT's effectiveness for CTS. Therefore, we performed this meta-analysis to evaluate the efficacy of ESWT for CTS. The aim of this study was to compare the improvement in symptoms, functional outcomes, and electrophysiologic parameters on patients with CTS between ESWT and control treatment groups. We hypothesized that ESWT would show better symptomatic, functional, and electrophysiologic improvements than other conservative treatment approaches.
2. Methods
2.1. Search strategy
The meta-analysis was conducted in line with the updated Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) guidelines.[13] PubMed-Medline, Embase, and Cochrane Library searches were performed in August 2018 using the following key terms: (Carpal tunnel syndrome OR Median neuropathy OR Entrapment neuropathy OR Median neuritis) AND (Extracorporeal Shockwave Therapy OR Shockwave Therapy OR Shock wave OR ESWT) AND Randomized controlled trial. An overview of the search strategy is presented in Supplementary Appendix 1. We included all randomized controlled trials investigating the effect of ESWT on CTS. We imposed no language restriction. We also searched for unpublished and grey literature using the databases/trial registries: World Health Organization Clinical Trial Register, EU clinical trials register, ClinicalTrials.gov, and OpenGrey.
2.2. Study selection criteria
Identified records were saved to EndNote software (X7.2; Thomson Reuters). Two independent reviewers (JCK, SYL) screened all titles and abstracts to identify relevant investigations. Inclusion criteria were articles reporting a randomized controlled trial with at least 3 months follow-up that described the effect of ESWT on CTS. There were no limitations in types of ESWT. Reviews, basic science articles, comments, letters, and protocols were excluded. When updates of earlier studies were available, we used only the most recent updates.
2.3. Outcome measures and data extraction
The primary outcome of interest was broadly defined any measure of symptoms which included pain, numbness, tingling sensation, or weakness. If a trial reported multiple measures of symptoms, the most composite measure of symptoms analyzed by a multidimensional instrument was chosen as the primary outcome measure. The secondary outcome of this study was a functional score, such as Boston Carpal Tunnel Syndrome Questionnaire (BCTQ) or Disabilities of the Arm, Shoulder, and Hand (DASH), motor component in electrophysiologic studies of the median nerve, such as distal motor latency or compound motor action potential amplitude, and sensory component in electrophysiologic studies of the median nerve, such as distal sensory latency, sensory nerve action potential amplitude, or sensory nerve conduction velocity. We combined the values of effect size in each study as one pooled effect size.[14] Because no differences between designs of the selected studies are found, effect sizes could be combined.[15] For every eligible study, the following data were extracted and entered into a spreadsheet by the 2 reviewers (JCK, SYL): first author's family name, year of publication, number of patients, mean age of participants, enrollment time, ESWT type and treatment intensity/frequency/duration, and follow-up duration.
2.4. Quality assessment and publication bias
Two authors (JCK, SYL) independently evaluated study quality using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions.[16] Criteria included the following 7 items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome data, incomplete outcome data addressed, selective reporting, and other biases. We assessed publication bias using Begg funnel plot[17] and Egger test.[18]
2.5. Statistical analysis
Effect sizes were computed as standardized mean difference (SMD) measures representing the magnitude of the pretest–posttest difference for each outcome. SMD was computed separately for all available control and treatment groups for each study. Heterogeneity between comparable studies was tested with the Chi-squared (χ 2) and I 2 tests. Values of P > .1 and I 2 < 50% were considered statistically significant. Because there was a significant heterogeneity among the 6 studies (P < .001 and I 2 = 91.9%), we used a random-effects meta-analysis to quantify the pooled effect size of the included studies. In each analysis by outcome parameters, symptoms (P < .001 and I 2 = 93.4%), functional score (P < .001 and I 2 = 95.0%), motor component (P = .039 and I 2 = 64.2%), and sensory component (P < .001 and I 2 = 83.5%) were also analyzed using the random-effects model. Additionally, we performed subgroup analyses by the type of control treatment (steroid injection and sham ESWT) and ESWT type (radial and focused). The Q test for heterogeneity was used when performing subgroup analyses. All analyses were performed using Comprehensive Meta-Analysis Software (version 3.3; Biostat, Englewood, NJ). This study was exempted from Institutional Review Board review since it did not involve human subjects.
3. Results
3.1. Description of included studies
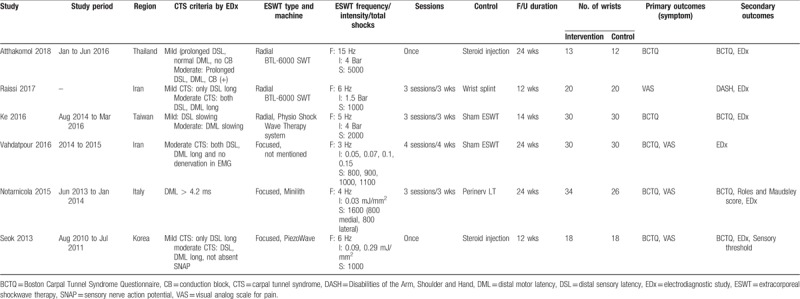
The primary database search yielded 48 records. After duplicates were removed, the titles and abstracts of 13 articles were initially screened, and only 8 were selected for full-text review. The full text articles were read, and 6 were considered relevant by qualitative analysis.[19,20,21,22,23,24] Studies selected for final inclusion (or exclusion) are shown in Figure 1, and the characteristics of the included studies are summarized in Table 1. In terms of quantitative analysis, these 6 studies (published from 2013 to 2018) fulfilled our inclusion criteria. Studies identified for meta-analysis included 281 participants. Study sample sizes varied from 25 to 60 wrists (13–34 cases and 12–30 controls). The selected studies represented a total of 145 wrists treated by ESWT and 136 wrists treated conservatively. The follow-up duration ranged from 12 to 24 weeks.
Figure 1.
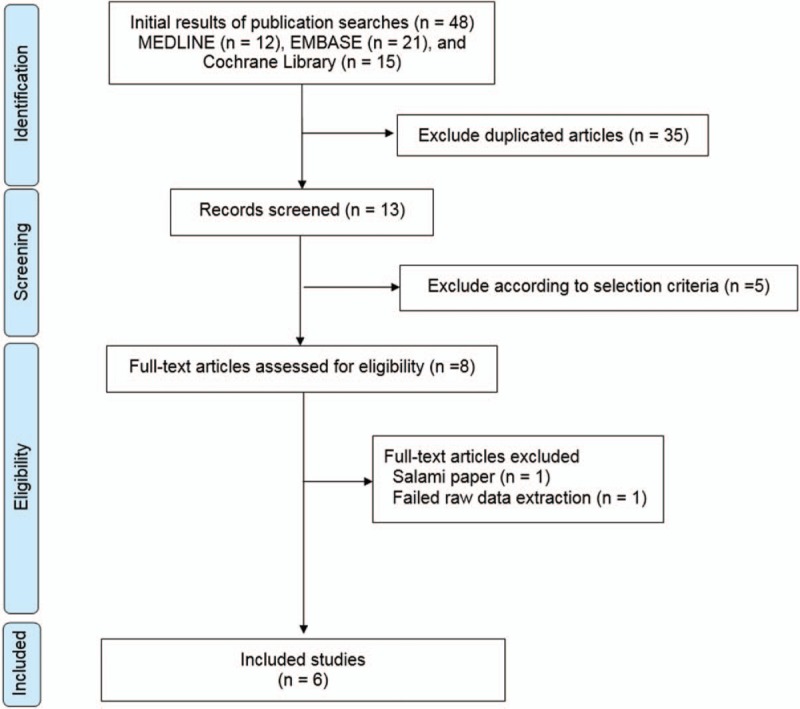
Preferred Reporting Items for Systematic review and Meta-Analysis flow diagram detailing the relevant clinical study selection process.
Table 1.
Characteristics of included individual studies.
3.2. Results after analysis
The ESWT showed significant overall effect size compared to the control (overall Hedge g pooled SMD = 1.447; 95% confidence interval [CI], 0.439–2.456; P = .005) (Fig. 2). ESWT also showed greater improvement in symptoms from CTS than the control group (pooled SMD = 1.604; 95% CI, 0.468–2.740; P = .006), as well as improvement in secondary outcomes such as functional scores (pooled SMD = 1.561; 95% CI, 0.181–2.940; P = .027), motor components of electrophysiology (pooled SMD = 0.945; 95% CI, 0.352–1.538; P = .002), and sensory components of electrophysiology (pooled SMD = 0.894; 95% CI, 0.163–1.624; P = .016) (Fig. 3).
Figure 2.
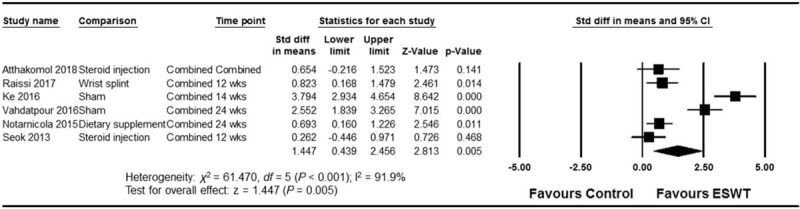
Forest plot of the overall effect of extracorporeal shock wave therapy (ESWT) on carpal tunnel syndrome (CTS) determined by a random-effects meta-analysis. Effect sizes are indicated as Hedge g standardized mean differences and 95% confidence intervals (CI).
Figure 3.
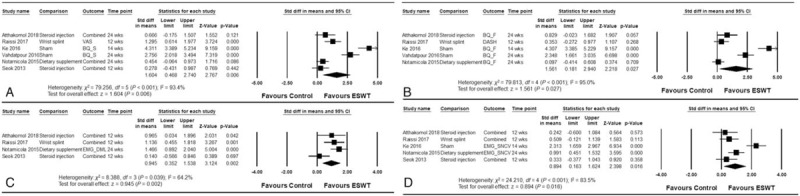
Forest plots of the effects of trial-level characteristics of extracorporeal shock wave therapy (ESWT) analyzed as outcome variables: (A) symptoms (primary outcome), (B) functional scores, (C) motor component, and (D) sensory component of electrodiagnostic study.
In subgroup analysis, ESWT showed greater effects than sham treatment (pooled SMD = 3.149; 95% CI, 1.933–4.365; P < .001). However, there was no obvious difference between the efficacies of ESWT and local corticosteroid injection (pooled SMD = 0.418; 95% CI, −0.131 to 0.968; P = .135). Both radial and focused ESWTs showed nonsignificant improvements (pooled SMD = 1.749; 95% CI, −0.159 to 3.656; P = .072 and pooled SMD = 1.161; 95% CI, −0.121 to 2.444; P = .076, respectively), and there were no apparent differences between the 2 groups (Q = 0.264 and P = .607) (Fig. 4).
Figure 4.
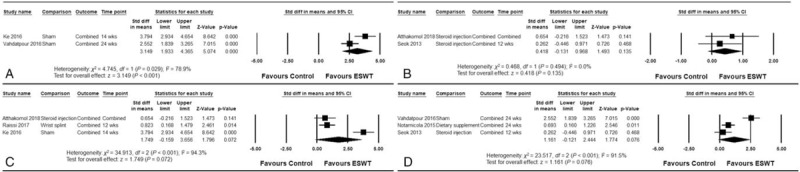
Forest plots of the subgroup analysis: (A) vs sham extracorporeal shock wave therapy (ESWT), (B) vs corticosteroid injection, (C) radial type ESWT, and (D) focused ESWT.
3.3. Quality assessment and publication bias
In terms of methodologic quality, all subjects were randomized appropriately, and all investigators and research assistants were blinded to the allocations. However, it is unclear whether the included trials met all quality-assessment criteria (Supplementary Appendix 2). Publication bias was not evident, as shown by the symmetrical Begg funnel plot (Supplementary Appendix 3), and the P-value for bias was 0.326 (Egger test; all 6 trials).
4. Discussion
Evidence has shown that ESWT improves symptoms, functional outcomes, and electrophysiologic parameters in patients with CTS. However, there was no obvious difference between the efficacies of ESWT and local corticosteroid injection. To the best of our knowledge, this is the 1st meta-analysis describing the overall impact of ESWT on patients with CTS.
The mechanism of ESWT in an entrapment neuropathy, such as CTS, is not fully understood. However, 2 main effects, the anti-inflammatory and neuronal regeneration effects, are potential mechanisms. The anti-inflammatory effect is similar to the mechanism of action noted in other musculoskeletal problems treated with ESWT.[25] Nitric oxide accumulation in the cell, which occurs when a decrease in nitric oxide is nullified by the stimulation of endothelial nitric oxide synthase in inflamed tissue, modulates NF kappa B activation, which in turn may prevent lipopolysaccharide/interferon-gamma-elicited induction of the inflammatory process.[26,27] Decreased inflammation of the carpal tunnel can reduce the perineural pressure and can improve symptoms. Recently, there has been increased interest in the effect of ESWT on peripheral nerve regeneration. After treatment with ESWT, neuronal regeneration may be induced by accelerating the elimination of the injured axon, increasing Schwann cell proliferation, and increasing axonal regeneration in animal experiments.[28] Improvement of electrophysiologic parameters, as observed in our review, might be explained by these mechanisms.
Local corticosteroid injection as a treatment option for CTS is supported by strong clinical evidence[5] and is preferred as the primary option for patients with mild to moderate CTS. In the subgroup analysis in this review, the comparison between local corticosteroid injection and ESWT did not show significant differences. However, perineural corticosteroid injection potentially has more risks than ESWT. Needle injection can lead to infection or median nerve injury, and corticosteroids can weaken the tendon by inhibiting activity of the tenocyte.[29] For this reason, unlike ESWT, corticosteroid injections cannot be used repeatedly for treating CTS. By contrast, no severe adverse effects of ESWT were reported in the trials included for this review. In other reviews of ESWT, only mild complications such as pain and redness have been reported, which resolved spontaneously.[30] ESWT is less invasive and more potent than local corticosteroid injection; therefore, it may be a better treatment option for mild to moderate CTS.
Trials analyzed in this review describe the use of 2 types of ESWT, radial and focused. Radial ESWT and focused ESWT were used in 3 studies each. The 2 types of ESWT are distinguished by the type of generators that use different physical characteristics of energy and use different methods for shock wave propagation. Radial ESWT produces a shock wave with relatively low energy, which is dispersed through the applicator tip and has a less penetrative depth.[31] A recent meta-analysis comparing radial and focused ESWT, reported that radial ESWT has the potential advantage of treating a larger area, lesser need for precise focusing, and low cost.[32] In our subgroup analysis, there was no difference in efficacy between radial and focused ESWT. Further studies should be performed to directly compare the 2 modalities in CTS management.
The ESWT protocol parameters such as frequency, intensity, and total shocks can affect the efficacy, potential adverse effects, and compliance of the treatment. However, the protocol was heterogeneous among the included trials. Raissi et al[22] tried a low-dose protocol, considering the procedure pain and the potential risk of nerve damage. Interestingly, Ke et al[20] compared a single session ESWT of 2000 shocks to a protocol using ESWT in 3 sessions, and showed a cumulative effect of ESWT on CTS. Atthakomol et al used a single session ESWT of 5000 shocks to improve patient compliance and reported a significant effect.[19] We tried to determine the dose-response of ESWT through a meta-regression analysis. However, as noted previously, the ESWT protocols, described in the trials included for this review, were too heterogeneous to analyze the regression. Thus, further studies should be performed to find the optimal and standardized ESWT protocol for CTS management.
This study has a few limitations. First, the number of studies that met the criteria was small. Since ESWT has been employed for the treatment of CTS for only a short span of time, the number of studies that met our criteria might be small. If an adequate number of studies had been included, more conclusive evidence could have been derived from comparisons of ESWT types or regarding the effect of corticosteroid injections in subgroup analyses. Second, the patient population was limited to those with mild to moderate CTS, as no studies attempted to investigate the effect of ESWT on severe CTS. Given that the primary option for severe cases of CTS (accompanied by motor weakness) is surgical treatment, studies examining the effect of ESWT on severe CTS would be lacking. However, if the efficacy and mechanisms of ESWT are clear and evident, it will be necessary to examine whether this technique can also be used in the management of severe CTS. Finally, data on the long-term effects of ESWT are lacking. The follow-up duration of the included trials ranged from 12 to 24 weeks. Clinical research should examine the long-term effects of more than 1-year follow-up.
5. Conclusion
The evidence summarized in this review suggests that treating CTS with ESWT can improve symptoms, functional outcomes, and electrophysiologic parameters. However, there was no significant difference in efficacies between ESWT and local corticosteroid injections. Further research is needed to confirm the long-term effects of ESWT and the optimal ESWT protocol for management of CTS.
Author contributions
Conceptualization: Shi-Uk Lee, Sang Yoon Lee.
Data curation: Se Hee Jung, Sang Yoon Lee.
Formal analysis: Ju Chan Kim, Se Hee Jung, Sang Yoon Lee.
Investigation: Ju Chan Kim, Sang Yoon Lee.
Methodology: Ju Chan Kim, Sang Yoon Lee.
Project administration: Se Hee Jung, Sang Yoon Lee.
Software: Sang Yoon Lee.
Supervision: Se Hee Jung, Shi-Uk Lee, Sang Yoon Lee.
Validation: Sang Yoon Lee.
Visualization: Sang Yoon Lee.
Writing – original draft: Ju Chan Kim, Sang Yoon Lee.
Writing – review & editing: Ju Chan Kim, Se Hee Jung, Shi-Uk Lee, Sang Yoon Lee.
Supplementary Material
Footnotes
Abbreviations: BCTQ = Boston Carpal Tunnel Syndrome Questionnaire, CI = confidence interval, CTS = carpal tunnel syndrome, DASH = disabilities of the arm, shoulder, and hand, ESWT = extracorporeal shock wave therapy, SMDs = standardized mean differences, PRISMA-P = Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1]. Dawson DM. Entrapment neuropathies of the upper extremities. N Engl J Med 1993;329:2013–8. [DOI] [PubMed] [Google Scholar]
- [2]. American Association of Electrodiagnostic Medicine AAoN, American Academy of Physical M, Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve 2002;25:918–22. [DOI] [PubMed] [Google Scholar]
- [3]. Werner RA, Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol 2002;113:1373–81. [DOI] [PubMed] [Google Scholar]
- [4]. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am 1981;63:380–3. [PubMed] [Google Scholar]
- [5]. Graham B, Peljovich AE, Afra R, et al. The American Academy of Orthopaedic Surgeons evidence-based clinical practice guideline on: management of carpal tunnel syndrome. J Bone Joint Surg Am 2016;98:1750–4. [DOI] [PubMed] [Google Scholar]
- [6]. Scholten RJ, Mink van der Molen A, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev 2007;CD003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev 2003;CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2007;CD001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Principles in the first aid competition for students in secondary medical school [in Czech]. Zdrav Prac 1975;25:73–5. [PubMed] [Google Scholar]
- [10]. Practice parameter for carpal tunnel syndrome (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1993;43:2406–9. [PubMed] [Google Scholar]
- [11]. Burger M, Kriel R, Damon A, et al. The effectiveness of low-level laser therapy on pain, self-reported hand function, and grip strength compared to placebo or “sham” treatment for adults with carpal tunnel syndrome: a systematic review. Physiother Theory Pract 2017;33:184–97. [DOI] [PubMed] [Google Scholar]
- [12]. Romeo P, d’Agostino MC, Lazzerini A, et al. Extracorporeal shock wave therapy in pillar pain after carpal tunnel release: a preliminary study. Ultrasound Med Biol 2011;37:1603–8. [DOI] [PubMed] [Google Scholar]
- [13]. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2016;354:i4086. [DOI] [PubMed] [Google Scholar]
- [14]. Lai MH, Kwok OM. Estimating standardized effect sizes for two- and three-level partially nested data. Multivariate Behav Res 2016;51:740–56. [DOI] [PubMed] [Google Scholar]
- [15]. Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods 2002;7:105–25. [DOI] [PubMed] [Google Scholar]
- [16]. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org. [Google Scholar]
- [17]. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [18]. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Atthakomol P, Manosroi W, Phanphaisarn A, et al. Comparison of single-dose radial extracorporeal shock wave and local corticosteroid injection for treatment of carpal tunnel syndrome including mid-term efficacy: a prospective randomized controlled trial. BMC Musculoskelet Disord 2018;19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Ke MJ, Chen LC, Chou YC, et al. The dose-dependent efficiency of radial shock wave therapy for patients with carpal tunnel syndrome: a prospective, randomized, single-blind, placebo-controlled trial. Sci Rep 2016;6:38344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Notarnicola A, Maccagnano G, Tafuri S, et al. Comparison of shock wave therapy and nutraceutical composed of Echinacea angustifolia, alpha lipoic acid, conjugated linoleic acid and quercetin (perinerv) in patients with carpal tunnel syndrome. Int J Immunopathol Pharmacol 2015;28:256–62. [DOI] [PubMed] [Google Scholar]
- [22]. Raissi GR, Ghazaei F, Forogh B, et al. The effectiveness of radial extracorporeal shock waves for treatment of carpal tunnel syndrome: a randomized clinical trial. Ultrasound Med Biol 2017;43:453–60. [DOI] [PubMed] [Google Scholar]
- [23]. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil 2013;92:327–34. [DOI] [PubMed] [Google Scholar]
- [24]. Vahdatpour B, Kiyani A, Dehghan F. Effect of extracorporeal shock wave therapy on the treatment of patients with carpal tunnel syndrome. Adv Biomed Res 2016;5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Ioppolo F, Tattoli M, Di Sante L, et al. Clinical improvement and resorption of calcifications in calcific tendinitis of the shoulder after shock wave therapy at 6 months’ follow-up: a systematic review and meta-analysis. Arch Phys Med Rehabil 2013;94:1699–706. [DOI] [PubMed] [Google Scholar]
- [26]. Ciampa AR, de Prati AC, Amelio E, et al. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett 2005;579:6839–45. [DOI] [PubMed] [Google Scholar]
- [27]. Mariotto S, Cavalieri E, Amelio E, et al. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide 2005;12:89–96. [DOI] [PubMed] [Google Scholar]
- [28]. Hausner T, Nogradi A. The use of shock waves in peripheral nerve regeneration: new perspectives? Int Rev Neurobiol 2013;109:85–98. [DOI] [PubMed] [Google Scholar]
- [29]. Tucci M, Freeland A, Mohamed A, et al. The role of proteoglycans in idiopathic carpal tunnel syndrome. Biomed Sci Instrum 2005;41:141–6. [PubMed] [Google Scholar]
- [30]. Wild C, Khene M, Wanke S. Extracorporeal shock wave therapy in orthopedics. Assessment of an emerging health technology. Int J Technol Assess Health Care 2000;16:199–209. [DOI] [PubMed] [Google Scholar]
- [31]. Lohrer H, Nauck T, Dorn-Lange NV, et al. Comparison of radial versus focused extracorporeal shock waves in plantar fasciitis using functional measures. Foot Ankle Int 2010;31:1–9. [DOI] [PubMed] [Google Scholar]
- [32]. Chang KV, Chen SY, Chen WS, et al. Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: a systematic review and network meta-analysis. Arch Phys Med Rehabil 2012;93:1259–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


