Supplemental Digital Content is available in the text
Keywords: bronchiectasis, exacerbations, macrolides, meta-analysis
Abstract
Background:
Macrolide maintenance treatment remains controversial for patients with noncystic fibrosis (non-CF) bronchiectasis, we performed a meta-analysis to estimate the benefits and safety of macrolides therapy in adults and children with non-CF bronchiectasis.
Methods:
PubMed, Embase, the Cochrane Library, and Web of Science databases were searched for all the randomized controlled trials of macrolides for treating non-CF bronchiectasis. The primary outcome was improvement of bronchiectasis exacerbations. Secondary outcomes included adverse events and macrolide resistance.
Results:
A total of 10 studies involving 602 patients were included in the analysis. Pooled results showed that macrolide therapy significantly reduced the number of patients who suffered from exacerbations (RR = 1.56, 95% CI = 1.14–2.14, P = .006, I2 = 72%), number of patients who experienced at least 3 exacerbations (RR = 0.55, 95% CI = 0.39–0.77, P = .0005, I2 = 40%), average exacerbations per patient during the observation time (SMD = −0.69, 95% CI = −1.06 to −0.32, P = .0002, I2 = 60%), and bronchiectasis exacerbation-related admissions (RR = 0.46, 95% CI = 0.23–0.96, P = .04, I2 = 0%). Specified subgroup analyses of the number of patients free from exacerbations were further performed; macrolide therapy showed a significant benefit in both children (RR 5.03, 95% CI 2.02–12.50, P = .0005, I2 = 45%) and adults (RR = 1.66, 95% CI = 1.37–2.02, P < .00001, I2 = 79%); azithromycin showed a significant reduction on the number of patients who suffered from exacerbations (RR = 2.25, 95% CI = 1.67–3.02, P < .00001, I2 = 0%), was different from erythromycin (RR = 1.33, 95% CI = 0.92–1.94, P = .13, I2 = 0%) and roxithromycin (RR = 1.14, 95% CI = 0.97–1.35, P = .11, I2 = 0%). The pooled results also showed no higher risk of adverse events (RR = 0.98, 95% CI = 0.85–1.13, P = .80, I2 = 8%), even a lower risk of severe adverse events (RR = 0.53, 95% CI = 0.33–0.85, P = .009, I2 = 0%). However, a higher risk of macrolide resistance (RR = 3.59, 95% CI 2.6–4.96, P < .00001, I2 = 0%) was observed.
Conclusion:
For both children and adults with non-CF bronchiectasis, macrolide maintenance therapy can effectively reduce bronchiectasis exacerbations, especially for patients with more frequent exacerbations and needing hospital treatment. Azithromycin was more effective than other macrolides. Macrolide maintenance therapy did not increase the risk of adverse events, but may increase the risk of macrolide resistance.
1. Introduction
Noncystic fibrosis (non-CF) bronchiectasis is a chronic respiratory disease characterized by abnormal dilatation and distortion of the bronchi and bronchioles,[1] mainly due to the vicious circle of frequent bacterial infections, chronic airway inflammation, retention of secretions, and airway destruction.[2,3] During the last 2 decades, the prevalence of bronchiectasis has not decreased as expected with a better control of airway infections. From 2000 to 2007, the prevalence of bronchiectasis in the United States has increased with an annual percentage of 8.74%.[4] The prevalence of bronchiectasis is particularly high among indigenous children in Central Australian, Maori, and Pacific Island in New Zealand, with at least 1470 cases for every 100,000 children.[5] Therefore, there is still a heavy health care burden associated with bronchiectasis worldwide.
Patients with non-CF bronchiectasis suffer from recurrent exacerbations, resulting in the destruction of airways and reduced lung function, life quality and lifespan.[6] There is a strong interest in developing approaches that can mitigate this substantial problem. The current therapy of non-CF bronchiectasis mainly includes airway clearance techniques, exercise, inhaled hyperosmolar agents and mucolytic agents, and other anti-inflammatory agents.[2] While the available non-CF treatments can provide some benefits, the above strategies are insufficient and the specific indications of the above strategies are not clearly defined. Better strategies are urgently needed.
To the best of our knowledge, the important step of preventing exacerbations is to interrupt the vicious circle of infection, obstruction, inflammation and destruction. Macrolide antibiotics are antibacterial agents with anti-inflammatory and immunomodulatory properties,[2] which indicates that macrolide maintenance treatment may be effective in preventing exacerbations of patients with non-CF bronchiectasis. Since the 1980s, macrolide antibiotics have been used to reduce the incidence of non-CF bronchiectasis exacerbations. In addition, macrolide antibiotics have the advantages of high plasma concentration, long half-life, and broad antimicrobial spectrum.[7] All of these provide the rationale for using macrolide maintenance therapy in patients with non-CF bronchiectasis, which would be the first choice for prevention of non-CF bronchiectasis exacerbations. However, antibiotic maintenance therapy is not currently recommended as part of conventional management to control the disease and macrolides are only recommended in selected patients (e.g., those with frequent exacerbations, ≥3 exacerbations and/or ≥2 hospitalizations in the previous 12 months).[5] Recently, several randomized controlled trials (RCTs) focusing on the benefits of reducing exacerbations have been carried out to evaluate the benefits and safety on macrolides maintenance therapy in non-CF bronchiectasis. However, individual studies obtained varied results. The current meta-analysis aimed to determine the benefits and safety of macrolide maintenance treatment for non-CF bronchiectasis exacerbations.
2. Methods
2.1. Search strategy
We operated a literature search using PubMed, Embase, Web of Science, and the Cochrane Library, with the last report up to May 2018. No language and date restriction were applied. Searches were limited to human only and RCTs. We searched the following MeSH terms: “Macrolides,” “Macrolide,” “Azithromycin,” “Erythromycin,” “Clarithromycin,” “Roxithromycin,” “Bronchiectasis,” “noncystic fibrosis bronchiectasis,” “non-CF bronchiectasis,” “NCFB.” In addition, the reference lists of the articles were manually searched for other potentially eligible studies. Abstracts published in academic conferences or website materials were excluded. The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[8]
2.2. Inclusion criteria, exclusion criteria, and study selection
The inclusion criteria were: a clinical randomized controlled trial (RCT); aimed to evaluate the benefits or safety of macrolides in comparison with control group (placebo, another class of antibiotic or blank control) in the treatment of patients with non-CF bronchiectasis; reported the number of exacerbations as the outcome; published in peer-reviewed journal. A study was excluded if: it is presented as a review article or protocol; involved patients with other chronic respiratory conditions, such as cystic fibrosis, COPD, or asthma; data could not be obtained by original manuscripts or e-mail to authors. Two reviewers independently managed the study selection, differences were resolved by consensus after discussion. Ethical approval was not necessary because this was a meta-analysis.
2.3. Assessment of risk of bias in included studies
The risk of bias of each study was assessed by 2 authors independently according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. Disagreements were resolved by discussion or arbitration involving a third reviewer. The detail domains of risk of bias included: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias. Each potential source of bias was graded as high, low, or unclear and the assessment outcome was noted in the “Risk of bias” table.
2.4. Data extraction and outcome measures
The following data were extracted: the year when the study was performed, location, age range of patients, number of patients, diagnostic criteria, total duration of study, macrolides intervention (the type of macrolide, medicine dose, interval of drug administration), reported outcomes, adverse events. We extracted data from the original manuscripts when possible; we also contacted the corresponding authors for original data.
The primary outcome measurement was the number of patients stratifying by different exacerbation times, the rate of exacerbation per patient per year and exacerbation-related admissions. A bronchiectasis exacerbation was defined using the study author's criteria, almost all of the criteria included intense coughing, dyspnea, wheezing, fever, chest pain, increased purulent sputum, requirement for oral or intravenous antibiotics. Secondary outcomes were adverse events and macrolide resistance.
2.5. Data analysis
Relative risk (RR) with 95% confidence intervals (95% CI) was used to evaluate dichotomous variables and standard mean differences (SMD) with 95% CI were used to evaluate continuous variables. Sensitivity analysis was performed for missing data. Cochran's X2 statistics with P value and I2 was used to measure the heterogeneity among studies. If P < .10 or I2 > 50%, suggesting significant heterogeneity, a random-effects model was chosen. Otherwise, calculations were performed with a fixed-effects model. Subgroup analyses were performed to explore heterogeneity when substantial heterogeneity was presented. For the primary outcome, subgroup analyses were also performed based on: type of macrolides: azithromycin vs erythromycin; children vs adults. For the data that cannot be merged, a descriptive analysis was performed. P<.05 was considered statistically significant. Publication bias was assessed by the funnel plot. All statistical analyses were performed using Review Manager Software (version 5.3; Cochrane Collaboration, Oxford, United Kingdom).
3. Result
3.1. Literature review and selection
Initial literature searches retrieved 541 articles, 85 articles were included after initial title and abstract screening. Another 75 articles were excluded for various reasons, and 10 RCTs were finally identified for meta-analysis (see figure, supplemental digital content S1, which illustrates the flowchart of the study selection process).
3.2. Characteristics of included trials
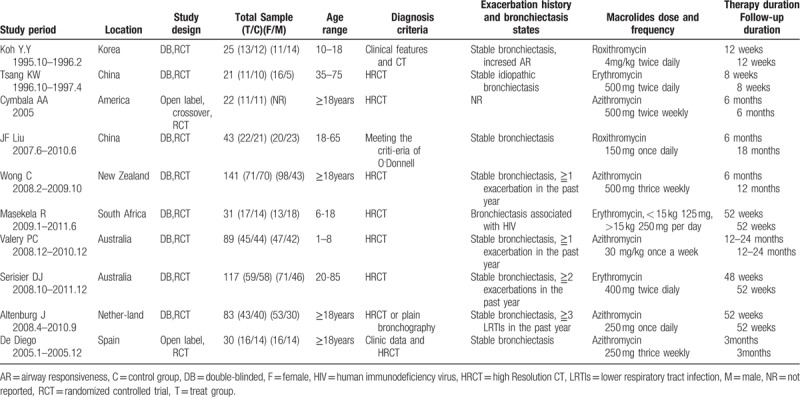
The year of publication for eligible studies ranged from 1997 to 2013. Except one cross-over study with 1 month washout period,[9] others were all parallel group studies.[10–18] Both children and adult patients were included. Seven trials recruited adult patients[9,11–13,16–18] and 3 trials recruited children.[10,14,15] Five trials[9,13,15,17,18] used azithromycin as the therapy intervention, 3 trials[11,14,16] used erythromycin, the other 2 trials[10,12] used roxithromycin, no trial on clarithromycin met inclusion criteria. Duration of the observation period ranged from 8 weeks to 24 months, the observation period of 4 trials[9–11,18] were <6 months, others were more than 6 months. The observation period refers to the whole of “treatment period” and “follow-up after finishing treatment period.” The characteristics of the included trials are shown in Table 1.
Table 1.
Characteristics of randomized clinical trials included in the meta-analysis.
The methodological quality of the included studies was evaluated using the risk of bias (see figure, supplemental digital content S2, which illustrates the quality assessment of the studies). Seven trials described random sequence generation, 5 trials described allocation concealment, all the trials reported incomplete outcome data and described the selective reporting, only 4 indicated no other bias. Three trials did not describe blinding of the patients or executors, one trial described no blinding of outcome assessment.
3.3. Outcomes
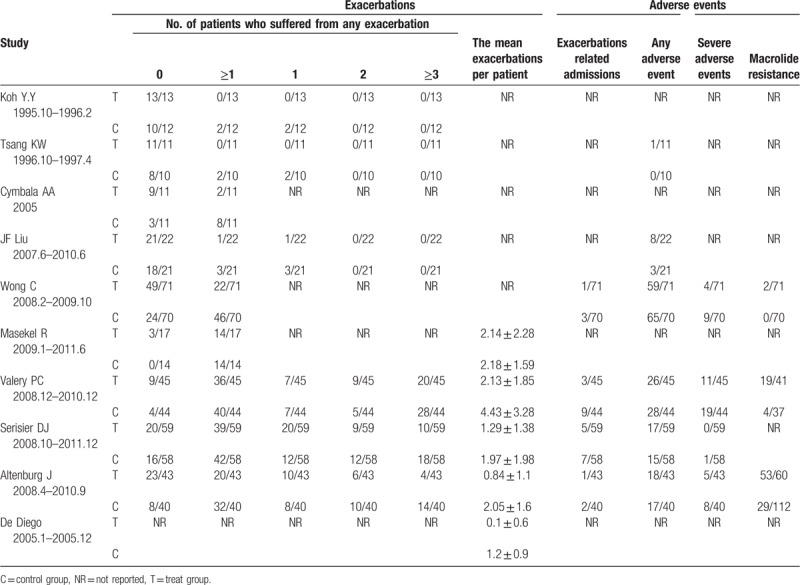
The detailed outcome measurements of the included trials are shown in Table 2.
Table 2.
The non-CF exacerbations of the trials included in the meta-analysis.
3.3.1. Primary outcome: clinic exacerbations
3.3.1.1. Number of patients free from exacerbations
Nine trials (n = 572) reported the number of patients free from exacerbations. Six trials were conducted in adults and the other 3 in children; 4 trials used azithromycin as the intervention, 3 used erythromycin, and the other 2 used roxithromycin. Compared with the standard group, the number of patients without exacerbation in the macrolides group significantly increased during the observation time (RR = 1.56, 95% CI = 1.14–2.14, P = .006; Fig. 1). Since significant heterogeneity was detected (I2 = 72%), random-effects model were chosen. To identify and measure heterogeneity, specified subgroup analyses were further performed (Fig. 2). There was a significant benefit in azithromycin group (RR = 2.25, 95% CI = 1.67–3.02, P < .00001), but no significant benefit for neither erythromycin (RR = 1.33, 95% CI = 0.92–1.94, P = .13) nor roxithromycin (RR = 1.14, 95% CI = 0.97–1.35, P = .11). The heterogeneity in the subgroup of azithromycin, erythromycin, and roxithromycin exhibited was all unremarkable (I2 = 0%), suggesting the result in azithromycin, erythromycin, roxithromycin was reliable. There was a significantly benefit of macrolide therapy in reducing the exacerbations both in children (RR 5.03, 95% CI 2.02–12.50, P = .0005) and adults (RR = 1.66, 95% CI = 1.37–2.02, P < .00001). The heterogeneity in the subgroup of children was not significant (I2 = 45%), suggesting the result in children may be credible; while the heterogeneity exhibited in the subgroup of adults was significant (I2 = 79%), indicating the result in adults may be not reliable. It can be concluded that there was a significant benefit of macrolide therapy in preventing the exacerbation for patients with non-CF bronchiectasis and reducing the number of patients experiencing one or more exacerbations in children, while in adults more evidences were needed; azithromycin showed significant benefit, while erythromycin and roxithromycin did not show significant benefit.
Figure 1.
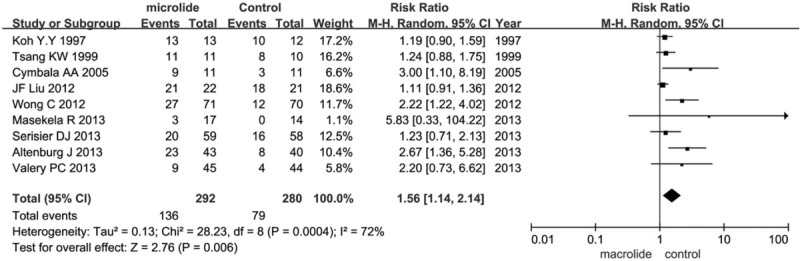
Effects of macrolide therapy on the number of patients with non-CF bronchiectasis free from exacerbations.
Figure 2.
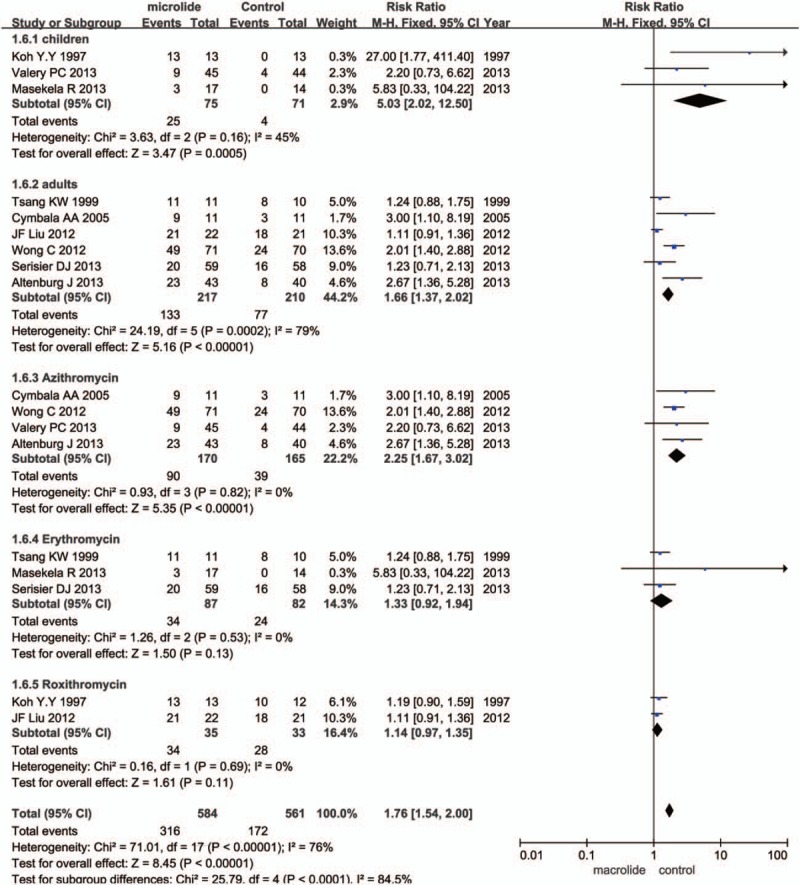
Subgroup analysis of effects of macrolide therapy on the number of patients with non-CF bronchiectasis free from exacerbations.
3.3.1.2. Number of patients who experienced only one exacerbation during the observation time
Six trials (n = 378) reported the number of patients who experienced only one exacerbation. Compared with the control group, the macrolides group exhibited no significant reduction in the number of patients experienced only one exacerbation (RR = 1.07, 95% CI = 0.71–1.60, P = .74) (see figure, supplemental digital content S3, which illustrates the effects of macrolide therapy on the number of patients who experienced only one exacerbation during the observation time). No significant heterogeneity was detected (I2 = 14%), thus fixed-effects model was chosen. It can be concluded that there was no significant benefit for patients with non-CF bronchiectasis who experienced only one exacerbation.
3.3.1.3. Number of patients who experienced 2 exacerbations during the observation time
Three trials (n = 293) reported the number of patients who experienced 2 exacerbations. Compared with the control group, the macrolides group exhibited no significant reduction in the number of patients who experienced 2 exacerbations (RR = 0.88, 95% CI = 0.53–1.46, P = .63) (see figure, supplemental digital content S4, which illustrates the effects of macrolide therapy on the number of patients who experienced 2 exacerbations during the observation time). Since no significant heterogeneity was detected (I2 = 23%), fixed-effects model was chosen. It can be concluded that there was no significant benefit for patients with non-CF bronchiectasis who experienced 2 exacerbations.
3.3.1.4. Number of patients who experienced at least 3 exacerbations during the observation time
Three trials (n = 289) reported the number of patients who experienced at least 3 exacerbations. Compared with the control group, the macrolides group exhibited significant reduction in the number of patients who experienced at least 3 exacerbations (RR = 0.55, 95% CI = 0.39–0.77, P = .0005; Fig. 3). Since no significant heterogeneity was detected (I2 = 40%), fixed-effects model was chosen. It can be concluded that there was a significant benefit for patients with non-CF bronchiectasis who experienced frequent exacerbations.
Figure 3.

Effects of macrolide therapy on the number of patients who experienced at least 3 exacerbations during the observation time.
3.3.1.5. Average exacerbations per patient during the observation period
Five trials (n = 350) reported the average exacerbations per patient. Three trials were conducted in adults and the other 2 in children; 3 trials used azithromycin as the intervention, and the other 2 used erythromycin. Compared with the control group, the macrolides group exhibited significant reduction in the frequency of exacerbations (SMD = −0.69, 95% CI = −1.06 to −0.32, P = .0002; Fig. 4). Since significant heterogeneity was detected (I2 = 60%), random-effects model was chosen. To identify and measure heterogeneity, specified subgroup analyses were further performed (see figure, supplemental digital content S5, which illustrates the subgroup analysis of effects of macrolide therapy on the frequency of exacerbations). There was a significant benefit of macrolide therapy in reducing the frequency of exacerbations for both adults (SMD = −1.01, 95% CI = −1.35 to −0.67, P < .00001) and children (SMD = −1.09, 95% CI = −1.85 to −0.33, P = .005). The heterogeneity in the subgroup of children was significant (I2 = 88%), suggesting the result in children may be not precisely credible; while the heterogeneity in the subgroup of adults was not significant (I2 = 0%). The heterogeneity in the subgroup of both azithromycin and erythromycin was not significant (I2 = 46%, I2 = 8%), suggesting the results in azithromycin and erythromycin exhibited may be reliable. Azithromycin showed significant benefit (P < .00001), while erythromycin did not show significant benefit (P = .06).
Figure 4.
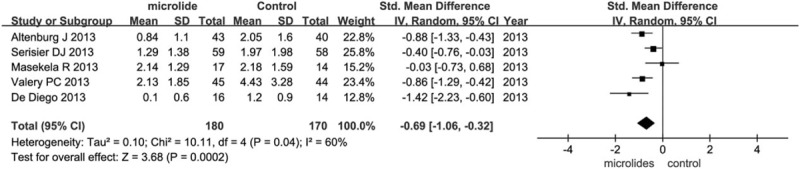
Effects of macrolide therapy on the frequency of exacerbations.
3.3.1.6. Bronchiectasis exacerbation-related admissions
Four trials (n = 430) reported admission associated with bronchiectasis exacerbation. Pooled analyses showed that macrolide therapy reduced the risk of admissions for infective exacerbations compared with the control group (RR = 0.46, 95% CI = 0.23–0.96, P = .04; Fig. 5). As no significant heterogeneity was detected (I2 = 0%), fixed-effects model was chosen.
Figure 5.
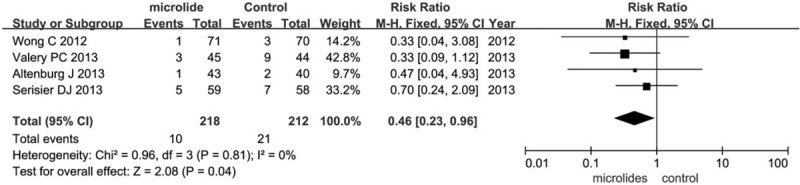
Effects of macrolide therapy on bronchiectasis exacerbation-related admissions.
3.3.1.7. Secondary outcome: adverse events and macrolide resistance
Six studies with a total of 494 patients reported clinical and laboratory monitoring for adverse events. The main side effects included diarrhea, nausea, vomiting, abdominal discomfort, and headache, rash, or sinusitis. Six trials (n = 494) reported the number of patients with one or more adverse events. The number of patients who experienced any adverse event was found to have no statistical difference among participants receiving macrolides compared to those receiving placebo (RR = 0.98, 95% CI = 0.85–1.13, P = .80; Fig. 6). No significant heterogeneity was detected (I2 = 8%), fixed-effects model was chosen. Three trials reported the gastrointestinal symptoms (diarrhea, nausea, vomiting, abdominal discomfort, etc.) of the adverse events. Gastrointestinal symptoms were more common in the macrolide therapy group (RR = 3.62, 95% CI = 1.93–6.79, P < .0001, I2 = 0%) (see figure, supplemental digital content S6, which illustrates the effects of macrolide therapy on the number of patients suffering from gastrointestinal adverse events). Four trials (n = 430) reported the number of patients with one or more severe adverse events. The severe adverse events mainly included disease needed surgery, heart failure, stroke, corrected QT interval (QTc) prolongation, and even death. The number of patients who experienced any severe adverse event was significantly reduced in the macrolides group, compared with the control group (RR = 0.53, 95% CI = 0.33–0.85, P = .009; Fig. 7). No significant heterogeneity was detected (I2 = 0%), fixed-effects model was chosen. Three trials reported the macrolide resistance and the rate in macrolides group is higher than the control group (RR = 3.59, 95% CI = 2.60–4.96, P < .00001; Fig. 8). No significant heterogeneity was detected (I2 = 0%), fixed-effects model was chosen. The 3 trials were all about azithromycin. Unfortunately, no data on erythromycin and roxithromycin was reported about the macrolide resistance. So we could draw a conclusion that azithromycin was associated with a higher risk of developing resistance, but that no data was available for other macrolide agents to allow a comparison.
Figure 6.
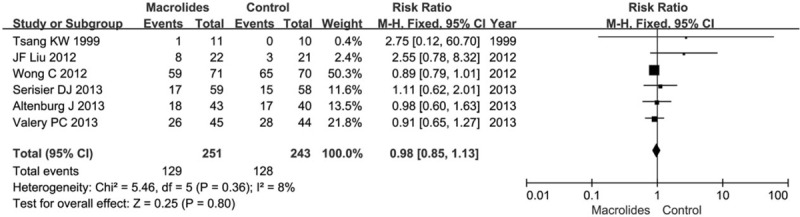
Effects of macrolide therapy on the number of patients suffered from any adverse event.
Figure 7.

Effects of macrolide therapy on the number of patients suffered from any severe adverse event.
Figure 8.

Effects of macrolide therapy on the macrolide resistance.
It can be concluded that, compared with the control group, macrolides therapy would not increase the risk of adverse events, would decrease the risk of severe adverse events, but would increase the gastrointestinal complications and the risk of macrolide resistance.
3.4. Publication bias
There is an asymmetrical appearance of the funnel plot with a gap in a bottom corner for the number of patients free from bronchiectasis exacerbations. (see figure, supplemental digital content S7, which illustrates the funnel plot of included trials for the number of patients free from bronchiectasis exacerbations). However, Egger's test did not show a significant publication bias (P = .09). Publication bias for the rate of exacerbation was not assessed due to the limited number of studies.
4. Discussion
The overall aim of the treatment of patients with non-CF bronchiectasis is to reduce symptoms, to prevent exacerbations, and thereby maintain lung function and improve the quality of life.[1] As a group of antibiotics with a wide antimicrobial spectrum, macrolides has been demonstrated to have the effect of preventing exacerbations in patients with CF[19] and COPD.[20] We performed the systematic review and meta-analysis to explore the evidence for a beneficial therapy of macrolide treatment on non-CF bronchiectasis.
In spite of the 2 meta-analyses conducted by Gao et al[21] and Fan et al[22] assessing the benefits and safety of macrolides in patients with non-CF bronchiectasis, the current study utilized different perspectives in assessing macrolide effect and included more eligible articles. For primary outcome, our study concluded that macrolide therapy could significantly decrease the number of patients with exacerbations and the average exacerbations per patient during the observation time. However, we further analyzed the number of patients stratifying by different exacerbations (0, 1, 2, ≥3), and found that there is a significant decrease of the number of participants with 0 and ≥3 exacerbations in the macrolide-treated group compared with the control group. There is no significant decrease of the number of participants with 1 and 2 exacerbations. Different from the result of Gao's study, we found that macrolides therapy can reduce the risk of admissions for infective exacerbations compared with the control group. Our results suggested that macrolides seem to have a positive net effect among individuals with more frequent exacerbations of non-CF. In addition, to address the degree of heterogeneity in the findings, subgroup analyses were performed for the number of patients free from exacerbations and the average exacerbations per patient by adults or children, azithromycin or erythromycin or roxithromycin. We concluded that exacerbations could be prevented in children as well as adults, but we cannot eliminate the heterogeneity in adults about the number of patients with 0 exacerbation and in children about the average exacerbations per patient. Due to the limited number of studies, we cannot employ further analysis to decrease the heterogeneity. We also concluded that only azithromycin could prevent the exacerbations, erythromycin and roxithromycin did not show significant effect in patients with non-CF bronchiectasis, no trial on clarithromycin met inclusion criteria.
Except the benefits, the safety is another important matter of concern for the widespread implementation of the macrolides. So we collected the data about the adverse events of included studies. Macrolides might have gastrointestinal reaction, ototoxicity, and cardiovascular risks, even proarrhythmic effect.[23] The main adverse events included gastrointestinal complications, such as nausea, vomiting, diarrhea, and abdominal discomfort, as well as headache, sinusitis, and rash in some participants. In our study, the gastrointestinal adverse events of the macrolide antibiotic treatment group were significantly more common than the control group. Diarrhea was the most common side effect, followed by nausea and vomiting. A few patients had abdominal pain. But the symptoms were mild and can be relieved by expectant treatment, which would not result in patient withdrawal.
Neither ototoxicity nor cardiovascular risks were systematically assessed in our selected studies, and only one study[16] monitored electrocardiogram changes and reported the QTc prolongation with one participant (1/59) in the macrolides group. However, clinicians should pay attention to monitor the ECG to prevent cardiovascular events. According to meta-analysis results, the pooled estimate of the number of patients suffering from any adverse event was not significantly different in the macrolides treatment group than that in the control group (P = .92). Compared with the control group, there was a significant decrease of the number of patients who experiencd any severe adverse event in the macrolides treatment group (P = .02). Severe adverse events mainly included disease needed admission or surgery, heart failure, stroke, QTc prolongation, even death. Both Li and Gao did not report severe adverse events in their meta-analyses. Due to the emergence of multidrug resistant strains, macrolide resistance is another critical issue for the use of long-term macrolide therapy. Comparing with the control group, we observed a significant increase of antimicrobial resistance in the macrolides group. This is in accordance with Li's study, but there was an obvious mistake in the forest plot of antimicrobial resistance in Li's study. In Li's study, he extracted the data reported by Valery et al[15] as the same as reported by Wong et al[13] (macrolides group 2/71 vs control group 0/70). However, the exact data reported by Valery et al[15] was macrolides group 19/41 vs control group 4/37. Macrolide resistance has become an increasing concern worldwide,[24] particularly in Asia,[25] which probably is the most hazardous shortcoming of the widespread use of macrolides.
Certain limitations of our study should be noted. Our analyses were based on pooled data from various trials with different macrolide antibiotics, populations, duration, in particular, of heterogeneous illness states (different exacerbation history, age and disease severity) before the participants were recruited, and without more details, we ca not employ meta-regression to detect whether the effect size is related to clinical heterogeneity. In addition, the random-effect model and subgroup analyses were used to account for the heterogeneity. Finally, studies recruited participants without CT scan to confirm the presence of bronchiectasis, which may induce bias.
5. Conclusion
In conclusion, the result from this meta-analysis strongly supported that macrolide maintenance treatment had a benefit in preventing exacerbations both in adults and children with bronchiectasis compared with controls. Azithromycin showed significant benefits comparing with other macrolides. In addition, macrolides would not increase the risk of adverse events, could decrease the risk of severe adverse events, but would increase the risk of antimicrobial resistance. Nonetheless, randomized controlled trials involving larger patient samples are needed to confirm the optimal agents, dose, duration, and the potential antimicrobial resistance following macrolide therapy for patients with non-CF bronchiectasis.
Author contributions
Donghai Wang and Jihong Dai conceived and designed the study, Donghai Wang and Wenlong Fu carried out the databases and did the data extraction and analysis, Donghai Wang wrote the first draft of the manuscript, and all the authors read and approved the final manuscript.
Conceptualization: Donghai Wang, Jihong Dai.
Data curation: Donghai Wang, Jihong Dai, Wenlong Fu.
Formal analysis: Donghai Wang, Jihong Dai, Wenlong Fu.
Funding acquisition: Jihong Dai.
Investigation: Donghai Wang, Wenlong Fu.
Methodology: Wenlong Fu.
Project administration: Jihong Dai.
Supervision: Jihong Dai.
Writing – original draft: Donghai Wang, Wenlong Fu.
Writing – review & editing: Jihong Dai.
Supplementary Material
Footnotes
Abbreviations: CF = cystic fibrosis, CI = confidence intervals, COPD = chronic obstructive pulmonary disease, ECG = electrocardiograph, non-CF = noncystic fibrosis, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized controlled trials, RR = relative risk, SMD = standard mean differences.
The authors have no funding and no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65suppl 1:i1–58. [DOI] [PubMed] [Google Scholar]
- [2].Khoo JK, Venning V, Wong C, et al. Bronchiectasis in the last five years: new developments. J Clin Med 2016;5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fjaellegaard K, Sin MD, Browatzki A, et al. Antibiotic therapy for stable non-CF bronchiectasis in adults—a systematic review. Chron Respir Dis 2017;14:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among medicare beneficiaries in The United States, 2000–2007. Chest 2012;142:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chang AB, Bell SC, Torzillo PJ, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med J Aust 2015;202:130. [DOI] [PubMed] [Google Scholar]
- [6].McDonnell M, Ward C, Lordan J, et al. Non-cystic fibrosis bronchiectasis. QJM 2013;106:709–15. [DOI] [PubMed] [Google Scholar]
- [7].Haworth CS, Bilton D, Elborn JS. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir Med 2014;108:1397–408. [DOI] [PubMed] [Google Scholar]
- [8].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [9].Cymbala AA, Edmonds LC, Bauer MA, et al. The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treat Respir Med 2005;4:117–22. [DOI] [PubMed] [Google Scholar]
- [10].Koh Y, Lee M, Sun Y, et al. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled study. Eur Respir J 1997;10:994–9. [DOI] [PubMed] [Google Scholar]
- [11].Tsang K, Ho PI, Chan K, et al. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J 1999;13:361–4. [DOI] [PubMed] [Google Scholar]
- [12].Liu J, Zhong X, He Z, et al. Effect of low-dose, long-term roxithromycin on airway inflammation and remodeling of stable noncystic fibrosis bronchiectasis. Mediat Inflamm 2014;2014:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:660–7. [DOI] [PubMed] [Google Scholar]
- [14].Masekela R, Anderson R, Gongxeka H, et al. Lack of efficacy of an immunomodulatory macrolide in childhood HIV related bronchiectasis: a randomised, placebo-controlled trial. J Antivir Antiretrovir 2013;5:44–9. [Google Scholar]
- [15].Valery PC, Morris PS, Byrnes CA, et al. Long-term azithromycin for indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. Lancet Resp Med 2013;1:610–20. [DOI] [PubMed] [Google Scholar]
- [16].Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309:1260–7. [DOI] [PubMed] [Google Scholar]
- [17].Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251–9. [DOI] [PubMed] [Google Scholar]
- [18].Diego AD, Milara J, Martinez-Moragón E, et al. Effects of long-term azithromycin therapy on airway oxidative stress markers in noncystic fibrosis bronchiectasis. Respirology 2013;18:1056–62. [DOI] [PubMed] [Google Scholar]
- [19].Cai Y, Chai D, Wang R, et al. Effectiveness and safety of macrolides in cystic fibrosis patients: a meta-analysis and systematic review. J Antimicrob Chemother 2011;66:968–78. [DOI] [PubMed] [Google Scholar]
- [20].Yao G-Y, Ma Y-L, Zhang M-Q, et al. Macrolide therapy decreases chronic obstructive pulmonary disease exacerbation: a meta-analysis. Respiration 2013;86:254–60. [DOI] [PubMed] [Google Scholar]
- [21].Gao Y-H, Guan W-J, Xu G, et al. Macrolide therapy in adults and children with noncystic fibrosis bronchiectasis: a systematic review and meta-analysis. PLoS One 2014;9:e90047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fan L-C, Lu H-W, Wei P, et al. Effects of long-term use of macrolides in patients with non-cystic fibrosis bronchiectasis: a meta-analysis of randomized controlled trials. BMC Infect Dis 2015;15:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dumke R, Von Baum H, Lück P, et al. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect 2010;16:613–6. [DOI] [PubMed] [Google Scholar]
- [25].Morozumi M, Iwata S, Hasegawa K, et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother 2008;52:348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


