Abstract
Rationale:
Histoplasmosis occurs most commonly in Northern and Central America and Southeast Asia. Increased international travel in Japan has led to a few annual reports of imported histoplasmosis. Healed sites of histoplasmosis lung infection may remain as nodules and are often accompanied by calcification. Previous studies in endemic areas supported the hypothesis that new infection/reinfection, rather than reactivation, is the main etiology of symptomatic histoplasmosis. No previous reports have presented clinical evidence of reactivation.
Patient concerns:
An 83-year-old Japanese man was hospitalized with general fatigue and high fever. He had been treated with prednisolone at 13 mg/d for 7 years because of an eczematous skin disease. He had a history of travel to Los Angeles, Egypt, and Malaysia 10 to 15 years prior to admission. Five years earlier, computed tomography (CT) identified a solitary calcified nodule in the left lingual lung segment. The nodule size remained unchanged throughout a 5-year observation period. Upon admission, his respiratory condition remained stable while breathing room air. CT revealed small, randomly distributed nodular shadows in the bilateral lungs, in addition to the solitary nodule.
Diagnosis:
Disseminated histoplasmosis, based on fungal staining and cultures of autopsy specimens.
Interventions:
The patient's fever continued despite several days of treatment with meropenem, minocycline, and micafungin. Although he refused bone marrow aspiration, isoniazid, rifampicin, ethambutol, and prednisolone were administered for a tentative diagnosis of miliary tuberculosis.
Outcomes:
His fever persisted, and a laboratory examination indicated severe thrombocytopenia with disseminated intravascular coagulation. He died on day 43 postadmission. During autopsy, the fungal burden was noted to be higher in the calcified nodule than in the disseminated nodules of the lung, suggesting a pathogenesis involving endogenous reactivation of the nodule and subsequent hematogenous and lymphatic spread.
Lessons:
Physicians should consider histoplasmosis in patients with calcified nodules because the infection may reactivate during long-term corticosteroid therapy.
Keywords: calcification, hemophagocytic syndrome (HPS), Japanese, corticosteroid therapy, histoplasmosis, immunocompromised, lung nodules, relapse
1. Introduction
Histoplasmosis most commonly occurs in North and Central America and Southeast Asia not including Japan.[1] The increase in international travel in Japan over the last 20 years has led to a few reports indicating imported histoplasmosis.[2] Upon exposure to Histoplasma, most individuals remain asymptomatic or develop only mild illness; however, a few develop symptoms.[3] Healed Histoplasma lung infection sites may remain as nodules or become calcified.[4] Meanwhile, immunocompromised individuals (e.g., HIV-infected patients with low CD4 counts, solid organ or hematopoietic stem cell transplantation recipients, and patients using immunosuppressive therapies, including biologics) may develop disseminated histoplasmosis.[3] Previous studies in endemic areas supported new infection/reinfection, rather than reactivation, as the main pathogenesis of symptomatic histoplasmosis,[5] and no previous reports have presented evidence of reactivation. Here, we present an autopsy case of disseminated histoplasmosis that developed from a calcified nodule after long-term corticosteroid therapy in Japan, a nonendemic area.
2. Case report
An 83-year-old Japanese man was hospitalized with general fatigue and a high fever. He had a 7-year history of an eczematous skin disease of unknown cause, despite 2 skin biopsies, and was treated with prednisolone 13 mg/d. He also had a history of travel to Los Angeles, Egypt, and Malaysia 10 to 15 years prior to admission. Five years earlier, computed tomography (CT) identified a solitary calcified nodule in the lingual left lung segment (Fig. 1A). The nodule size remained unchanged throughout a 5-year observation. Upon admission, the patient's respiratory condition remained stable while breathing room air. CT revealed small, randomly distributed nodular shadows in the bilateral lungs, in addition to the solitary nodule (Fig. 1B). Laboratory examinations revealed pancytopenia (white blood cells, 1900/μL; hemoglobin, 9.2 g/dL; platelets, 32,000/μL) and elevated C-reactive protein (8.25 mg/dL; normal range <0.35 mg/dL) and β-D-glucan levels (42.4 pg/mL; normal range, 0–11 pg/mL), but negative results from an IFN-γ release assay (T-SPOT.TB), Aspergillus and Cryptococcus antigen tests, and anti-HIV antibody tests. Blood, urine, sputum, and pleural effusion cultures were negative for all tested pathogens.
Figure 1.
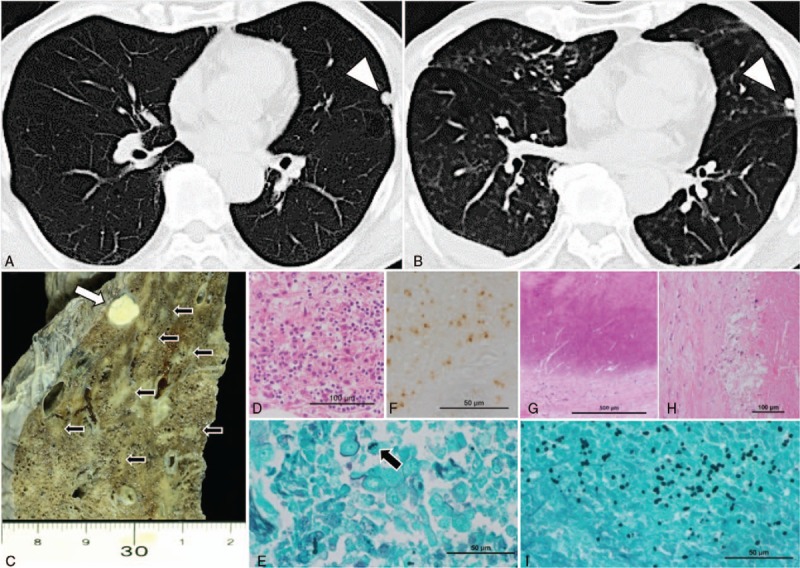
Chest computed tomography images revealing a solitary pulmonary nodule in the lingular segment (arrowhead) 5 years prior to the currently discussed admission (A), randomly distributed diffuse small nodular shadows at the time of admission (B). Macroscopic findings from the lingular segment of the lung revealed a calcified nodule (white arrow) and small necrotizing lesions (black arrow) (C). Microscopic analysis of the diffuse small nodular lesions revealed granulomatous areas with necrosis (D) and yeast-like fungi detected using Grocott stain (black arrow) (E) and immunohistochemistry with a Histoplasma-specific antibody (Meridian Bioscience) (F). The calcified nodule underlying the visceral pleura was surrounded and coated with fibrous material (G) and exhibited necrosis (H) and many yeast-like fungi (I).
The patient's fever continued, despite several days of treatment with meropenem (3 g/d), minocycline (400 mg/d), and micafungin (150 mg/d). Although he refused bone marrow aspiration, isoniazid (300 mg/d), rifampicin (450 mg/d), ethambutol (750 mg/d), and prednisolone (increased from 13–25 mg/d) were administered for a tentative diagnosis of miliary tuberculosis. His fever persisted, and a laboratory examination indicated severe thrombocytopenia (5000/μL) with disseminated intravascular coagulation. He died on day 43 postadmission (Fig. 2).
Figure 2.
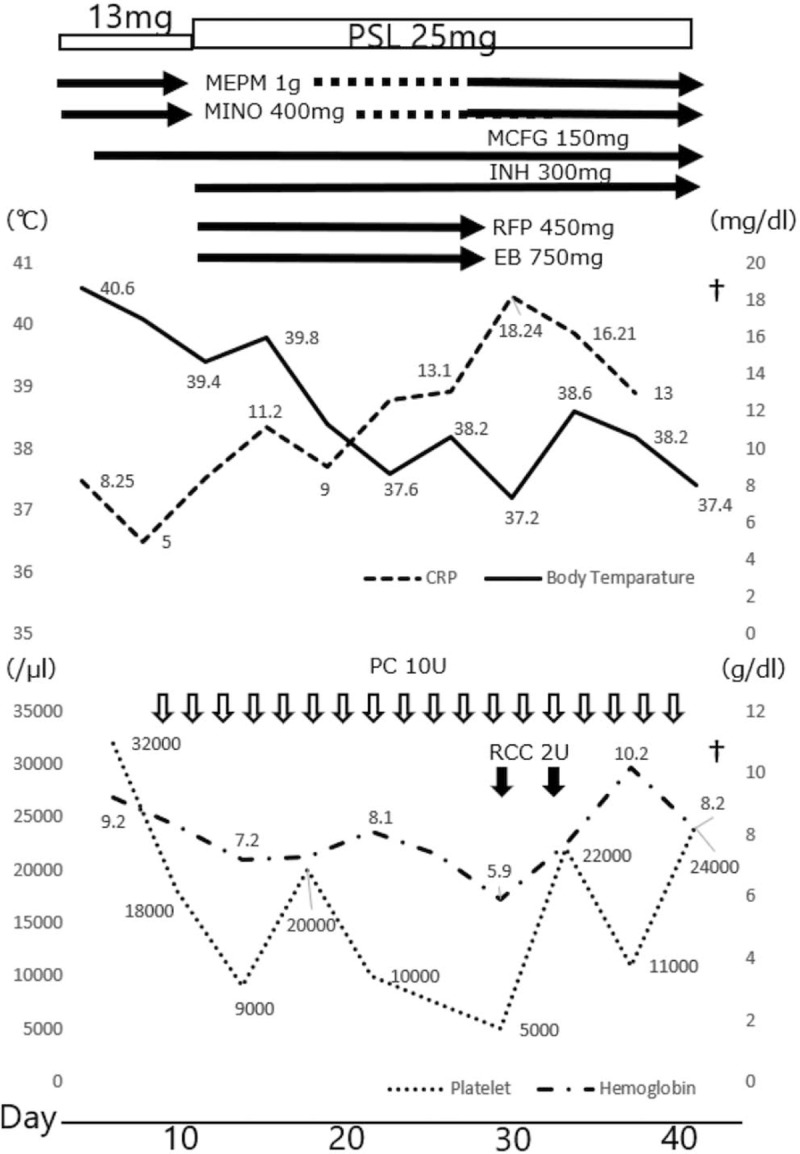
Clinical course of our case. The serum C-reactive protein level, body temperature, and transfusions are shown. EB = ethambutol, INH = isoniazid, MCFG = micafungin, MEPM = meropenem, MINO = minocycline, PC = platelet concentrate, PSL = prednisolone, RCC = red cell concentrate, RFP = rifampicin.
During an autopsy, macroscopic findings included diffuse large and small necrotizing lesions throughout the bilateral lungs and a calcified nodule in the lingular segment (Fig. 1C). Microscopically, diffuse granulomatous lesions with necrosis and many yeast-like fungi were detected using Grocott's staining and identified as Histoplasma capsulatum by immunohistochemistry (Fig. 1D–F) and culture. The calcified nodule underlying the visceral pleura was coated with fibrous material and exhibited necrosis with many yeast-like fungi (Fig. 1G–I). Histopathologically, the fungal burden was higher in the calcified nodule than in the disseminated nodules. Histoplasma was detected in multiple organs, including the liver, spleen, pancreas, skin, and lymph nodes. Bone marrow evaluation revealed many macrophages that had phagocytosed other blood cells, indicating hemophagocytic syndrome (Fig. 3).
Figure 3.
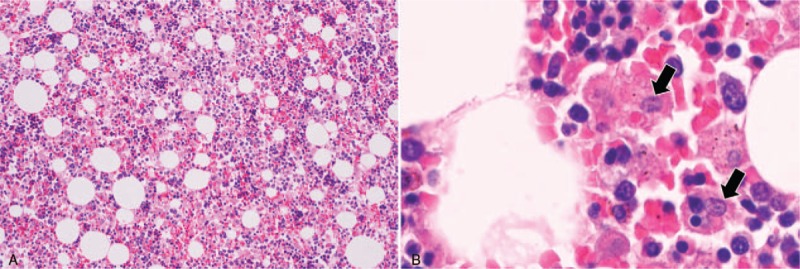
Microscopic findings of the bone marrow. Hypercellularity was apparent (A), and many macrophages that had phagocytosed blood cells were observed (B, arrows).
3. Discussion
Currently, cultures and fungal stains of tissues or body fluids and tests for antibodies and antigens have been proven as useful modalities for diagnosing Histoplasma. However, an immunosuppressive status can lead to a low level of antibody positivity in patients with disseminated disease.[6] However, as a high fungal burden facilitates antigen production in a patient with disseminated disease, antigen detection is useful for a rapid diagnosis. Notably, previous studies suggested the presence of higher antigen levels in bronchoalveolar lavage fluid, compared with those in blood or urine.[7] As our patient refused any other testing, including bronchoscopy, we finally made a diagnosis based on fungal staining and cultures of autopsy specimens.
The low incidence of histoplasmosis among immunocompromised patients, even in endemic areas, suggests that new infection/reinfection, rather than reactivation, is the main etiological mechanism of histoplasmosis.[8–10] One report described a clear seasonal pattern in incident cases of histoplasmosis among HIV patients in an endemic area.[8] Another study of nearly 600 allogeneic bone marrow or solid organ transplantation recipients in a hyperendemic area found no cases of active histoplasmosis, although many patients exhibited radiographic and/or serologic evidence of previous histoplasmosis.[9] In the same region, only 3 of 469 adult patients (0.64%) treated with tumor necrosis factor blockers for inflammatory bowel disease developed histoplasmosis.[10] Furthermore, the incidence of post-transplant histoplasmosis was higher during or after community outbreaks.[11]
In our case, the histopathologically determined fungal burden of Histoplasma was higher in the calcified nodule that was first detected 5 years earlier than in the disseminated lesions in the absence of an opportunity for reinfection. This suggests a pathogenesis involving endogenous reactivation of the nodule and subsequent hematogenous and lymphatic spread. Regarding Histoplasma immunity, the pathogen is internalized by immune cells in the lungs, including macrophages, dendritic cells, and neutrophils. Although neutrophils and dendritic cells can kill Histoplasma via fungistatic and fungicidal activity, macrophages require cellular activation regulating the pH of the intraphagosomal environment to kill the pathogen. Phagocytes promote the dissemination of Histoplasma to other organs, such as the liver, spleen, and lymph nodes.[5] Therefore, cellular immunity is an essential component of host defense and the control of intracellular Histoplasma growth. On the other hand, a previous study in an endemic area found that 70 of 105 (67%) autopsies consequent to various diseases involved calcified pulmonary granulomas that contained yeast forms resembling H capsulatum,[12] and yeast cells were cultured from 40 granulomas and injected into mice. However, all culture results were negative for histoplasmosis. Although the mechanism underlying the reactivation of the calcified nodule in our case remains unknown, an immunocompromised status might have enabled Histoplasma to survive in the nodule, and long-term impairment of cellular immunity may have resulted in hematological dissemination.
4. Conclusion
In summary, we report a non-HIV-infected, nontransplanted Japanese patient with disseminated histoplasmosis that was reactivated by long-term steroid exposure in a nonendemic area. Accordingly, physicians should consider histoplasmosis in patients with calcified nodules, as these can cause reactivation during long-term corticosteroid therapy.
Author contributions
KK, TA, and IK drafted the manuscript and were responsible for patient care. HH, SC, HS, and SF provided patient care and supervised the revision of the manuscript. KO, JK, SI, TU, HK, YU, YM, and KK performed the patient autopsy, including the pathological examination and fungus identification. NH and TB supervised the manuscript revision. All authors read and approved the final manuscript.
Writing – original draft: Keigo Kobayashi, Takanori Asakura.
Writing – review & editing: Ichiro Kawada, Hanako Hasegawa, Shotaro Chubachi, Kentaro Ohara, Junko Kuramoto, Hiroaki Sugiura, Seitaro Fujishima, Satoshi Iwata, Takashi Umeyama, Harutaka Katano, Yoshifumi Uwamino, Yoshitsugu Miyazaki, Katsuhiko Kamei, Naoki Hasegawa, Tomoko Betsuyaku.
Footnotes
Abbreviation: CT = computed tomography.
Tomoko Betsuyaku: Deceased September 2018.
Informed consent was obtained from the patient's wife for the publication of this study.
Ethical Approval: Not applicable.
The authors have no conflicts of interest to disclose.
References
- [1].Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45:807–25. [DOI] [PubMed] [Google Scholar]
- [2].Medical Mycology Reseach Center CU. The trend of importedmycoses in Japan. Date retrieved: August 2015. http://www.pf.chiba-u.ac.jp/clinical/mycosis.html [in Japanese] (Accessed April 6, 2019). [Google Scholar]
- [3].Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007;20:115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ulbright TM, Katzenstein AL. Solitary necrotizing granulomas of the lung: differentiating features and etiology. Am J Surg Pathol 1980;4:13–28. [PubMed] [Google Scholar]
- [5].Adenis AA, Aznar C, Couppie P. Histoplasmosis in HIV-infected patients: a review of new developments and remaining gaps. Curr Trop Med Rep 2014;1:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Williams B, Fojtasek M, Connolly-Stringfield P, et al. Diagnosis of histoplasmosis by antigen detection during an outbreak in Indianapolis. Ind Arch Pathol Lab Med 1994;118:1205–8. [PubMed] [Google Scholar]
- [7].Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003;11:488–94. [DOI] [PubMed] [Google Scholar]
- [8].Hanf M, Adenis A, Couppie P, et al. HIV-associated histoplasmosis in French Guiana: recent infection or reactivation? Aids 2010;24:1777–8. [DOI] [PubMed] [Google Scholar]
- [9].Vail GM, Young RS, Wheat LJ, et al. Incidence of histoplasmosis following allogeneic bone marrow transplant or solid organ transplant in a hyperendemic area. Transpl Infect Dis 2002;4:148–51. [DOI] [PubMed] [Google Scholar]
- [10].Wheat LJ, Smith EJ, Sathapatayavongs B, et al. Histoplasmosis in renal allograft recipients. Two large urban outbreaks. Arch Intern Med 1983;143:703–7. [PubMed] [Google Scholar]
- [11].Freifeld AG, Iwen PC, Lesiak BL, et al. Histoplasmosis in solid organ transplant recipients at a large Midwestern university transplant center. Transpl Infect Dis 2005;7:109–15. [DOI] [PubMed] [Google Scholar]
- [12].Straub M, Schwarz J. The healed primary complex in histoplasmosis. Am J Clin Pathol 1955;25:727–41. [DOI] [PubMed] [Google Scholar]


