Supplemental Digital Content is available in the text
Keywords: circadian rhythm, direct current, electrodermal activity, ryodoraku
Abstract
Human body undergoes the 24-hour daily rhythm in response primarily to light and darkness. The circadian rhythms of biomarkers reflect bodily conditions including the prognosis of some pathologies. As a sensitive index of sympathetic nervous response (SNR), electrodermal activity (EDA) is a recent research topic in healthcare industry as a noninvasive and easy-access biosignal. The EDA response at Ryodoraku points (RPs) is of potential clinical usage in relation to SNR and meridian theory, but still remains in its primitive development stage.
In this study, the 24-hour variations of EDA for 14 hospitalized participants were monitored over 3 days using a Ryodoraku device, and a circadian model of EDA was constructed using a cosinor analysis based on the linear mixed effect model.
As a result, EDA at every RP showed a circadian cycle with its value the lowest in the morning and increased gradually until the late afternoon, and monotonically decreased again until the next morning. Circadian variations were observed in EDAs of all 12 RPs. However, Ryodoraku-specific features were not detected. Midline estimating statistic of rhythm (MESOR) values in men and young group were higher than in women and old group, and cosinor analysis showed significant circadian rhythms, especially for men and young groups. Especially, circadian variation in EDA in the young group went above 35% of the MESOR value.
It implies that the circadian rhythm should be considered for the Ryodoraku analysis to examine bodily conditions or the prognosis of some pathologies.
1. Introduction
The human body produces diverse responses to various stimuli during the day that affect the human circadian rhythm, which is the 24-hour daily rhythm.[1,2] The importance of the circadian rhythm has been growing more apparent in recent years because it reflects body conditions, including the prognosis of some pathologies that play a significant role in medicine and health care.[3–8] Circadian rhythm is controlled by the suprachiasmatic nucleus of the hypothalamus, and it is generally influenced by exogenous and endogenous factors.[2] Exogenous factors include physical activity, mental and emotional stress, eating behavior, caffeine, vitamin D3 synthesis and environmental elements such as light, temperature and sound. Endogenous factors include the sympathetic and parasympathetic nervous system, plasma melatonin and adrenaline concentrations, vascular total peripheral resistance, vasoconstriction, blood volume, and the renin-angiotensin-aldosterone system.[9]
Several studies have verified that various indicators and hormones in the body show circadian variation. For example, sympathetic nervous system activation and vasoconstriction are dominant in the daytime, whereas plasma melatonin concentration and vasodilatory activity are elevated at night.[10] It has been confirmed that the heart rate, modulated by the autonomic nervous system, is increased during REM sleep in the morning,[11] and blood pressure is rapidly increased in the morning, as is the heart rate.[12] In addition, many studies have been conducted to understand the relationship between circadian variations and various diseases such as dementia, insomnia, and heart disease.[13–17] Jonghe et al[18] showed that the circadian protein clock, through which the circadian rhythm is controlled by a biological clock, is obstructed by diseases such as dementia, and melatonin treatment is an effective treatment for this obstruction.
Metabolism and cardiovascular activation affect the circadian rhythm, and their effects can be detected by observing variation of certain hormones or bio-signals through techniques such as electrocardiography (ECG) and electroencephalography (EEG). In addition, electrodermal activity (EDA), another bioelectrical signal, can be used to observe daily changes. EDA can be estimated using a Ryodoraku device to measure the direct current (DC) at Ryodoraku points (RPs), the spots of high electrical conductance on each side of the body, as discovered by Nakatani.[19] When a DC voltage of 12 V is applied to the body, it is possible to measure the amount of current at RPs across a range of 0 to 200 μA that is inversely proportional to the magnitude of the skin resistance. Ryodoraku devices have been used to investigate the functions of vitality, stamina of physical strength, and circulation and to treat various diagnoses with therapy in traditional medicine.[20] The Ryodoraku method has been applied to classify respiratory disease patients and healthy people, and patients with functional indigestion and asthma were categorized using DC at RPs.[21] In addition, several clinical studies based on RPs have been carried out to analyze back pain, idiopathic Parkinson's, menopause syndrome, cardiac disorders and cerebrovascular disease.[22–25]
Although Ryodoraku may reflect the functions of the meridians and their corresponding organs due to similarities between Ryodoraku and meridian pathways, this phenomenon was originally explained as an index of autonomic nervous responses.[19] DC at RPs is particularly affected by sweat glands of the skin that are innervated by the sympathetic nervous system. When the sympathetic nerves become excited due to the function of the sweat glands, the moisture content of the horny layer of the epidermis is increased, and skin resistance is decreased.[26] In addition, perspiration allows high conductivity of the skin because of the abundance of various electrolytes in sweat. Therefore, sympathetic nervous activity can be assessed by the Ryodoraku method. Changes in Ryodoraku characteristics derived from sympathetic nerve activation have been observed, confirming that DC at RPs may reflect autonomic nervous responses.[27]
Despite vigorous study, however, understanding the scientific and clinical basis of Ryodoraku remains a challenge. In particular, unlike other biosignals, the circadian rhythm of EDA measured at RPs has been rarely reported. In previous studies, Ryodoraku currents were measured daily in the morning and afternoon to monitor the effects of a qigong workshop on body energy,[28] and the Ryodoraku currents and urine volume of just 3 participants were observed throughout a day without control in measurement time or daily schedule.[29] Although rough tendencies were shown in previous studies, a well-organized clinical study under controlled environment and physiological input has never been reported. The aim of this study was to observe the circadian rhythm of EDA under a controlled environment encompassing eating, sleeping and daily activities. We monitored 24-hour variations of the EDA in healthy people over 3 days using a commercial Ryodoraku device, and examined the daily variation of Ryodoraku EDAs in the hope of correction factors to improve its clinical usage.
2. Methods
2.1. Ethics and participants
This study was designed as a pilot study and observational study conducted at the Medical Department of Otorhinolaryngology, Gacheon University Medical Center, from September to December 2015. The research protocol received approval from the Institutional Review Board (GDIRB2015-229), and the clinical study was registered at the Clinical Research Information Service (KCT0001679) retrospectively. Written informed consent was obtained from all participants prior to the onset of the study. All participants were recruited according to the following criteria:
2.1.1. Inclusion criteria
Age 20 to 69 years
Receipt of a clear explanation of the purpose and characteristics of the study by the research staff and signing of the consent form
2.1.2. Exclusion criteria
Patients with history of abnormal thyroid function or other endocrine system anomaly
Those with heart disease such as heart failure, angina pectoris or myocardial infarction
Patients with malignant tumors or malignant tumors complaining of complications (registration is possible if there was no recurrence for more than 5 years)
Those with a history of serious liver disease, blood disease, respiratory disease, gastrointestinal disease, cerebellar or cerebral disease
Those who are pregnant, lactating, or taking contraceptives
Patients with mental illnesses such as depression, anxiety, neurosis or schizophrenia
Patients who abuse drugs or alcohol or are current smokers
Heart pacemaker and electronic device recipients
Those who have experienced high intensity physical activity or exercise for more than one hour within the last week
Those who previously participated in other clinical studies within the last 3 months
Those who have experienced hypersensitivity reactions after a basic pathology test
Any person who was considered ineligible for participation in this study
Eligibility was assessed by physical examination, standard biochemistry, hematology urinalysis and thyroid blood tests, as well as medical history and history of concomitant medications at the screening phase (visit 0).
2.2. Study procedures
Eligible participants completing the admission procedure on the day of visit 1 (day 1) were hospitalized for 3 days in a designated room after 16:00 prior to the measurement of circadian variation of DC at RPs. Water intakes of each participant was regulated to 2L/day through intravenous catheter except through taking meals. During hospitalization, all participants were scheduled to consume the identical calories and were strictly prohibited to taking extra foods. The duration of each participant's sleep was fixed to 8 hours (11:00 pm–7:00 am) and the measurement during sleep hours was not performed. The first DC measurement on RPs was conducted at 21:00 on the first day of hospitalization and the measurement was proceeded every 3 hours from 7:00 am to 21:00 pm on the next day (day 2). Immediately after the wake-up at the third day (day 3) of hospitalization, the final measurement was completed at 7:00 am. With this procedure, the EDA measurements over 24 hours in a standardized environmental condition was fulfilled.[30]
2.3. Measurement
Rebon Skin Check (Uracle Co., South Korea), one of a commercial Ryodoraku measurement device which is available with remote connection to Window-based PC via Bluetooth wireless technology was used to obtain EDA responses at RPs. This device consists of the following components: a probe (18.5 × 2.3 cm) and grip (10.8 × 3.2 cm) in a total weight of 240 g. A probe is equipped with a conductor for DC measurement located on the edge with the diameter size of 7 mm, LED status indicator, on/off switch and grip-connection cable. A grip consists of a current adjustment volume, LED charge-status indicator, cable connector, power switch and electrodes wrapped up around the body of grip. The output voltage of the device is 12 V and the power consumption is 5 W.
The method for calibration and preparation for the RP measurement are described in Cha et al.[31] Before the measurement, initial DC intensity of the device was set to 200 μA by contacting the conductor at the edge of the probe and the electrode in the main body of the grip. The room temperature and humidity were maintained at 26°C and 70%, respectively, because a change in external temperature can affect the moisture content on the stratum corneum through perspiration. The participants took a break for 10 to 15 minutes before measurement and removed metal objects such as watches and rings. An investigator wore cotton gloves as insulation between the body of the investigator and the participants. In the measurement process, an investigator set up the probe at right angles to the measurement point and applied pressure until a beep was heard. Participants held the electrode of the grip in the opposite direction of the measurement point. DC at each of 24 RPs was measured in the following order: left hand, right hand, left foot, and right foot. Slightly wet conductor of the probe was used to maintain moisture in the body of the participant. The details of RPs are described in Table 1 and Figure 1. A total of 8 measurements were made per participant.
Table 1.
Description of Ryodoraku measurement points.

Figure 1.

Ryodoraku measurement on the hands and feet.
2.4. Statistical analysis
Demographic characteristics were summarized by the mean and standard deviation, frequency and percentage for each continuous and categorical variable. The measured DC at RPs can be separated by the following factors: lateral direction (2 levels: left and right), RPs (6 levels: 1 to 6) nested within the measured location (2 levels: hands and feet), and circadian time sequences (7:00 to 7:00 during the next day at 3-hour intervals). Based on this data structure, a linear mixed effect (LME) model was applied for the gender and age groups independently. In both LME model analyses, RPs nested within the location and circadian time were considered as fixed effect terms. The variation of DC due to the participants and lateral direction were considered as random effects. As a preliminary analysis, the circadian time sequence was regarded as a categorical factor with 7 levels to compare the mean difference between the time points of Ryodoraku currents measured at the hands and feet with respect to the baseline values (measured at the beginning of 7:00 on day 2). Both LME models corresponding to the gender and age group allows the third-order interaction between fixed effects. In order to verify the circadian rhythm of DC on the RPs, cosinor analysis[32–34] was conducted based on the LME model framework[35,36] to estimate the midline estimating statistic of rhythm (MESOR, the rhythm adjusted mean), amplitude (AMP, the peak-to-mean difference within a cycle) and acrophase (ACP, time at the peak within a cycle) of the circadian model. Let yij be the jth observation of a participant i at the time tij with ni repeated measurement, where i = 1, …, m and j = 1, …, ni. The total number of observations from all participants is  and the length of the fundamental period τ is known. For a participant i, let μi be the MESOR, Ai be the AMP, ϕi be the ACP, and
and the length of the fundamental period τ is known. For a participant i, let μi be the MESOR, Ai be the AMP, ϕi be the ACP, and 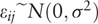 be the error term related to the jth observation of participant i. For a brief illustration, the simple single-harmonic cosinor model is described by the following formula,
be the error term related to the jth observation of participant i. For a brief illustration, the simple single-harmonic cosinor model is described by the following formula,
 |
where  , and
, and 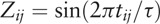 . Then, Ai and ϕi can be derived by trigonometric function identities as follows:
. Then, Ai and ϕi can be derived by trigonometric function identities as follows:
 |
The single-harmonic cosinor model can be easily extended to the multiple-harmonic component model. Since the number of harmonics in the circadian rhythm of Ryodoraku currents is unclear, the selection of optimal number of harmonics were performed based on Akaike information criteria (AIC). The cosinor models with the LME model framework initially included all combination of interaction terms and those models were reduced by the stepwise variable selection procedures based on AIC to obtain the parsimonious models. The circadian phase was binned into 45° bins and the first measurement at 7:00 on day 2 was assigned to 0°. Because the 2 parameters, AMP and ACP, were nonlinear functions of β1 and β2, the delta method was used to obtain the standard errors of the parameters to construct 95% confidence intervals and to perform the hypothesis test for comparing the differences in the gender and age group.[36,37] The statistical significance in the analysis was set to α = 0.05 and all analyses were conducted using R statistical software with version 3.5.0.[38]
3. Results
A flow diagram of the study is shown in Figure 2. A total of 15 healthy adults were enrolled, and we acquired DC data for 14 participants. One participant was excluded from the analysis due to the refusal to participate. The participants’ demographic data are shown in Table 2. The ages of the participants ranged from 20 to 69 years with 8 in the 20–30 years group (young age group; 5 men, 3 women) and 6 in the 50–69 years group (old age group; 4 men, 2 women). Height, weight, BMI and body temperature were averaged. At the time of the experiment, none of the participants were smokers, but all of the participants ingested caffeine regularly. All 14 participants had normal blood pressure levels.
Figure 2.

Flow diagram of the study. A total of 15 healthy adults were enrolled, and data acquisition from 14 participants were completed.
Table 2.
Demographic characteristics of participants.

Time-specific variations in the DC are shown in Figure 3. Average DC values at the 12 RPs and average Ryodoraku values according to each circadian time sequence were calculated. Mean differences in DC contrasted with the reference value measured at the first 7:00 time point according to gender and age groups were calculated. In the case of men, mean differences at 16:00 and 19:00 were significantly different on the hands (P < .1 and P < .05, respectively) and on the feet (P < .1 and P < .01, respectively). In the case of the young age group, significant differences in mean differences were observed at 16:00 and 19:00 on the hands (P < .1 and P < .01, respectively) and at 19:00 on the feet (P < .001).
Figure 3.

Mean differences in DC compared to the reference values measured at the first 7:00 time point on the hands (first row) and feet (second row) according to the gender (left column) and age groups (right column). Each bar in the panels represents the mean difference from the reference, and lines indicate the standard error (SE) of the mean difference derived from the full model of the LME model for a preliminary analysis. The axes on the bottom represent the circadian time sequence, and the top axes represent the corresponding circadian phase (0°=7:00). Dunnett's test was applied for the adjustment of P-values obtained from the multiple comparison in time sequence. The statistical significance was indicated by following notations: ∗∗∗, P < .001; ∗∗, P < .01; ∗, P < .05; #, P < .10. DC = direct current, LME model = linear mixed effects model.
The circadian model of average Ryodoraku current on the hands and feet according to gender and age group is shown in Figure 4. The circadian rhythm and standard error band were estimated using cosinor analysis based on the LME model. The circadian cycles were the lowest at 7:00 and increased gradually until 16:00 or 19:00. Then, the cycle tended to be lower again until the next morning. Parameters determining the circadian model of average Ryodoraku current on the hands and feet according to gender and age group are represented in Table 3. The MESOR, AMP and ACP were calculated, and significant differences between the calculated values and MESOR were represented with 95% confidence intervals that were derived from the delta method. Higher MESOR values were seen in men (64.5 μA on the hands and 64.6 μA on the feet) than in women (50.7 μA on the hands and 49.2 μA on the feet). In case of the men, the AMP and ACP of the circadian model in the hands were 7.82 μA and 141.0°, respectively. There was a significant difference between the sinusoidal circadian pattern and MESOR derived from the average Ryodoraku current during the day (P < .001), suggesting there was a certain circadian rhythm. The AMP of the circadian model in feet was 4.83 μA, and a significantly different circadian rhythm was also observed (P < .01). In the case of women, the AMPs of the circadian model in the hands and feet were 4.01 and 6.46 μA, respectively, and circadian rhythms were observed in both locations (P < .1 and P < .01, respectively). The young age group had markedly higher MESOR values (67.8 μA on the hands and 65.7 μA on the feet) than the old age group (48.5 μA on the hands and 50.3 μA on the feet). The AMPs of the circadian model in the young group were 8.60 μA on the hands and 6.46 μA on the feet. Circadian rhythms were clearly found with significant differences in the sinusoidal circadian pattern at both measurement sites (P < .001 for both). However, there was no such significant difference in the old age group.
Figure 4.

Circadian model of the average Ryodoraku current on the hands (the top row) and feet (the bottom row) according to the gender (the first column) and age groups (the second column). For illustrative purposes, the results were repeated over 2 circadian cycles. The vertical dotted line indicates folding at 360° (7:00). The circadian rhythm and standard error bands corresponding to each group were estimated using cosinor analysis based on the LME model. The circles and lines indicate the marginal mean and standard error at each circadian time point. The axes on the bottom represent the circadian time sequence, and the top axes represent the corresponding circadian phase (0° = 7:00).
Table 3.
Parameters determining the circadian model of the average Ryodoraku current on the hands and feet according to the gender and age group.

The marginal means and standard errors of DC at the 12 RPs according to the gender and age groups are shown in Figures 5 and 6, respectively. The DC values at the RPs were calculated by averaging the left and right sides, and the circadian variations according to the circadian time sequence were represented. All RPs had a similar DC pattern depicting a 24-hour rhythm, in which the trend showed the highest current in the afternoon and decreasing current from the afternoon until the next morning. The highest values were consistently observed at H5 and F3 and the lowest values were observed at H2 and F4 regardless of the gender or age group. DC values that were significantly different from reference values measured at the first 7:00 time point are represented by a red color, and significant differences during the day were observed at H1, H2, H3, F1, F2, F3 and F4.
Figure 5.

Mean profiles of DC at the 12 RPs according to the gender. Each circle, triangle, square and error bar in the circadian time sequences respectively represent the estimated mean and standard error of men, women and total group, derived from the full model of the preliminary LME model. DC values that differ significantly from reference values measured at the first 7:00 time point are indicated by a red color. DC = direct current, LME model = linear mixed effects model.
Figure 6.

Mean profiles of DC at the 12 RPs according to the age group. Each circle, triangle, square and error bar in the circadian time sequences respectively represent the estimated mean and standard error of young, old, and total group, derived from the full model of the preliminary LME model. DC values that differ significantly from the reference values measured at the first 7:00 time point are indicated by a red color. DC = direct current, LME model = linear mixed effects model.
4. Discussion
We developed a strict experimental design for a clinical study to investigate the consistent circadian patterns of EDA in a noninvasive manner using a commercial Ryodoraku device. The participants in this study were hospitalized for 3 days to ensure equal exposure to various environmental factors that may affect the EDA. This stringent control of the experimental environment guaranteed the reliability of the data and results. Unlike previous studies, it was possible to compare 2 consecutive measurements at night and the next morning. In addition, participants in different gender and age groups were recruited for a more detailed analysis of the circadian model of EDA.
We first examined EDA derived from the average DC at RPs in a circadian time sequence. The DC values measured in the morning were the lowest, and the current increased gradually until the late afternoon. Then, the values decreased again. In addition, the values measured at 7:00 were always lower than those measured at 21:00 the night before. EDA is widely used as a sensitive index of sympathetic activity that is affected by the central nervous system (CNS).[39,40] We observed sinusoidal wave patterns over 24 hours that were similar to autonomic nerve system. Because the sympathetic nervous system is generally active when the body is tense, the sympathetic nerves become activated in the morning with arousal stress and higher vascular resistance.[41] The sympathetic nerves are also influenced by daily activities such as food intake, bowel movements, walking and bathing. Accordingly, it is thought that the highest EDA values occur in the afternoon. The trend shown in this study is in accordance with the results of a previous report.[29] Nobuhiko reported that variations in Ryodoraku currents were roughly similar to changes in urinary volume per hour. Because an anti-diuretic hormone controlled by the CNS regulates urinary volume, Nobuhiko explained that Ryodoraku might be modulated by the CNS. Taken together, the EDA pattern in this study was similar to autonomic nerve patterns; we believe that EDA might reflect daily body conditions controlled by the CNS and therefore excessive or deficient physical and mental stresses will cause the EDA responses abnormal.
There are 2 major viewpoints defining the physiologically normal current range at RPs. First, less than 40 μA indicates a hypofunction state, and more than 80 μA indicates a hyperfunction state.[42] Alternative view is that the physiologically normal range has been defined as a ± 20% value relative to an individual's average Ryodoraku current.[43] Both suggestions did not consider the natural circadian rhythm revealed in this work. For example, the AMP of the circadian model in the young age group was 8.6 μA, and the circadian variation between the highest and lowest value over a day was 17.2 μA. This variation increased to 24.9 μA considering the 95% confidence interval, and this variation was 36.7% of the MESOR with an average Ryodoraku current of 67.8 μA in the young age group. In other words, the changes of EDA occurring naturally due to circadian rhythm can lead to misdiagnose and should be took into account to be utilized in the clinics.
Differences in muscle mass and body water between men and women may affect electrical current in the body.[44,45] Because men have more muscle mass and body water than women, the electrical resistance values of men are generally low; this explains the higher MESOR values in men than in women shown in this study. In addition, men generally have more eccrine sweat glands than do women, which might partially explain the increased Ryodoraku current in men.[46–48] Similarly, muscle mass and the function of sweat gland decreases at old ages, which explains why the young age group had markedly higher MESOR values than the old age group.[49,50] Regardless of the measurement site, MESOR values in men and the young age group were consistently high, corresponding to Ryodoraku theory: the higher the average value, the greater the stamina.
Hands and feet have long been regarded as suitable parts for measuring bioelectrical signals. Circadian behaviors of EDA at hands and feet were slightly different in men and women. The AMPs of the circadian variations in men were higher in the hands than in the feet. The reasons for this behavior can be inferred from previous studies. It is known that the density of the eccrine sweat glands on the back of the hand is higher than that on top of the foot, and the hands consistently produce greater EDA than the feet.[51] In addition, autonomic nervous activation derived by treadmill exercise causes more sweat gland activity on the hands than on the feet, and this suggests that the change in EDA caused by autonomic nervous activation on the hands is generally more sensitive than that of the feet.[27] On the contrary, in the case of women, the current variation in circadian model on the hands was lower than on the feet. Future studies that include analyses of the muscle mass, cellular water, relevant ion concentrations and skin temperature will be needed to examine this contrasting phenomenon for women in more detail. In the analysis of circadian behaviors according to age, young age group showed statistically significant circadian rhythms at both measurement sites (P < .001 at both hands and feet). However, there was no specific circadian rhythm on the hands in the old age group, and only a slight circadian pattern was observed (P < .1) on the feet. Less muscle mass and body water as well as decreased sweat gland density in the older group were suspected reasons for lower sensitivity and its daily variation in EDA.
We observed no Ryodoraku-specific features of DC responses in its circadian pattern; some RPs showed pronounced circadian rhythm, while others showed no such rhythm. However, it commonly showed that DC responses increased from the morning to the afternoon and then lowered again until the next morning. The values measured at night were higher than those measured in the next morning. The highest values were measured on H5 and F3 and the lowest values were measured on H2 and F4 regardless of gender or age group (see Figure S1, Supplemental Content, which illustrates the marginal mean and its standard error of DC responses at the RPs). It is consistent with previous studies; H5 had the highest and H2 had the lowest values during the course of a day, F3 had the highest value and was most sensitive to autonomic nervous activation and F4 had the lowest value and was least sensitive to autonomic nervous activation.[27,29] According to the Ryodoraku theory, each Ryodoraku point has its own corresponding organ along with the meridian pathway, the current response outside the normal range at a specific Ryodoraku point may be partially explained by a disorder in the corresponding organ. Clinical usefulness of the EDA responses at RPs will be determined by the verification of the functional relationship of the RPs with its corresponding organ.
Studies of human body rhythms have endeavored to clarify the relationship between daily rhythm and physiological and pathological phenomena. The circadian rhythm of EDA, a bioelectrical signal, can be estimated using a Ryodoraku device to measure the DC at RPs. Although the reproducibility of the Ryodoraku method has been verified, more studies are required to demonstrate the basic characteristics and clinical meaning of EDA measured at RPs.[31,52,53] To explore the fundamental properties of EDA variations, it is important to obtain reliable data from well-organized clinical studies and investigate consistent circadian rhythms from multiple perspectives. Prior to human study, the underlying mechanism of circadian variation of EDA can be understood with an animal model including laboratory test (melatonin, cortisol and so on), DEXA, ECG and EDA for young/old/male/female compositions.[54] We summarize the contributions of our study as follows. We controlled environmental factors by hospitalizing participants over 3 days and measured EDA responses over RPs, which guaranteed the reliability of our study results. Based on this clinically controlled environment, we constructed a circadian model of EDA using cosinor analysis based on the LME Furthermore, the circadian rhythm of EDA was compared in gender and age groups that has not been reported previously. Additional studies with large sample size are needed to find clearer physiological or environmental factors affecting the circadian variation in EDA and the relationships between the Ryodoraku current and other bio-signals such as ECG or EEG.
Author contributions
Conceptualization: Jaeuk U. Kim, Boncho Ku.
Data curation & Investigation: Jang-Han Bae.
Methodology & Formal analysis: Boncho Ku.
Preliminary analysis: Se-Eun Bae.
Supervision: Jaeuk U. Kim.
Writing – original draft: Jang-Han Bae.
Writing – review & editing: Boncho Ku, Jaeuk U. Kim.
Boncho Ku orcid: 0000-0001-5520-9782.
Supplementary Material
Footnotes
Abbreviations: ACP = acrophase, AIC = Akaike information criteria, AMP = amplitude, CNS = central nervous system, DC = direct current, ECG = electrocardiography, EDA = electrodermal activity, EEG = electroencephalography, LME model = linear mixed effects model, MESOR = midline estimating statistic of rhythm, RP = Ryodoraku point, SNR = sympathetic nervous response.
J-HB and BK contributed equally to this work.
Clinical Research Information Service (KCT0001679). Registered at 28 October 2015 (retrospectively registered).
The study was approved by the Institutional Review Board of Gacheon University Medical Center, Incheon, South Korea (GDIRB2015-229).
Written informed consent was obtained from each participant. All subjects were participated in this study under the ethics, consent and permissions.
The data used in the analyses are available on request by contacting the corresponding author.
This study was supported by grants (KSN1812170, K17012) from the Korea Institute of Oriental Medicine funded by the Korean government.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Scheer FA, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 2010;107:20541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 2014;24:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mormont MC, Levi F. Cancer chronotherapy: principles, applications, and perspectives. Cancer 2003;97:155–69. [DOI] [PubMed] [Google Scholar]
- [4].Hermida RC, Ayala DE, Calvo C. Administration-time-dependent effects of antihypertensive treatment on the circadian pattern of blood pressure. Curr Opin Nephrol Hypertens 2005;14:453–9. [DOI] [PubMed] [Google Scholar]
- [5].Minutolo R, Gabbai FB, Borrelli S, et al. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis 2007;50:908–17. [DOI] [PubMed] [Google Scholar]
- [6].Keijzer H, Snitselaar MA, Smits MG, et al. Precision medicine in circadian rhythm sleep–wake disorders: current state and future perspectives. Pers Med 2017;14:171–82. [DOI] [PubMed] [Google Scholar]
- [7].Innominato P, Komarzynski S, Karaboué A, et al. Home-based e-health platform for multidimensional telemonitoring of symptoms, body weight, sleep, and circadian activity: relevance for chronomodulated administration of irinotecan, fluorouracil-leucovorin, and oxaliplatin at home—results from a pilot study. JCO Clin Cancer Inform 2018;2:1–5. [DOI] [PubMed] [Google Scholar]
- [8].Pressman A, Hernandez A, Sikka SC. Lifestyle stress and its impact on male reproductive health. Bioenvironmental Issues Affecting Men's Reproductive and Sexual Health: Elsevier 2018. 73–83. [Google Scholar]
- [9].Smolensky MH, Hermida RC, Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med Rev 2017;33:4–16. [DOI] [PubMed] [Google Scholar]
- [10].Arendt J. The pineal gland, circadian rhythms and photoperiodism. Physiology and Pharmacology of Biological Rhythms: Springer 1997. 375–414. [Google Scholar]
- [11].Boudreau P, Yeh W-H, Dumont GA, et al. Circadian variation of heart rate variability across sleep stages. Sleep 2013;36:1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 1989;79:733–43. [DOI] [PubMed] [Google Scholar]
- [13].Muller JE, Ludmer PL, Willich SN, et al. Circadian variation in the frequency of sudden cardiac death. Circulation 1987;75:131–8. [DOI] [PubMed] [Google Scholar]
- [14].Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 2015;47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okawa M, Mishima K, Hishikawa Y, et al. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep 1991;14:478–85. [DOI] [PubMed] [Google Scholar]
- [16].Wilson SJ, Nutt D, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol 2010;24:1577–601. [DOI] [PubMed] [Google Scholar]
- [17].Zhao R, Li D, Zuo P, et al. Influences of age, gender, and circadian rhythm on deceleration capacity in subjects without evident heart diseases. Ann Noninvasive Electrocardiol 2015;20:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].de Jonghe A, Korevaar JC, van Munster BC, et al. Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia. Are there implications for delirium? A systematic review. Int J Geriatr Psychiatry 2010;25:1201–8. [DOI] [PubMed] [Google Scholar]
- [19].Nakatani Y. Skin electric resistance and ryodoraku. J Autonomic Nerve 1956;6: [Google Scholar]
- [20].Kim T, Park Y, Park Y, et al. Biofunctional Medicine, Seoul. 2008;Korea: Koonja Press, 81–101. [Google Scholar]
- [21].Hwang J-H, Jung S-Y, Jung S-K. The diagnostic values of Ryodoraku and pulse analysis for respiratory disease patients. J Korean Oriental Internal Med 2007;28:560–9. [Google Scholar]
- [22].Moon Y, Bae H, Moon S, et al. Clinical investigation about the interrelationship between differentiation of syndromes and numerical value of measurement (Yang-do-rack diagnosis) in acute stroke patients. Korean J Orient Int Med 1998;19:2. [Google Scholar]
- [23].Bang J-K, Park Y-C, Lee S-H, et al. The study on the characteristics of Yangdorak in the patients with idiopathic parkinson's disease. Acupuncture 2006;23:153–64. [Google Scholar]
- [24].Kim E-S, Lee J-M, Lee C-H, et al. A study on characters of Yangdorak in climacteric women. J Korean Obstetrics Gynecol 2008;21:159–68. [Google Scholar]
- [25].Kim K, Chung S, Kim S, et al. The study on characteristics of Ryodoraku score in the chronic low back pain patients. J Oriental Rehab Med 2009;19:145–54. [Google Scholar]
- [26].Noh S-H, Kim K-H, Yoon Y-J, et al. Ryodoraku application for diagnosis: a review of Korean literature. Korean J Acupunct 2011;28:125–35. [Google Scholar]
- [27].Bae J-H, Oh YJ, Kim JU. A feasibility study about change of characteristics caused by treadmill exercise test. J Korean Med 2017;38:1–2. [Google Scholar]
- [28].Sancier KM. Electrodermal measurements for monitoring the effects of a qigong workshop. J Altern Complement Med 2003;9:235–41. [DOI] [PubMed] [Google Scholar]
- [29].Takahashi N. Circadian rhythm of Ryodoraku. JJRM 2007;52:121–35. [Google Scholar]
- [30].Clark L, Denby L, Pregibon D, et al. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J Clin Epidemiol 1987;40:671–81. [DOI] [PubMed] [Google Scholar]
- [31].Cha J-H, Kim Y-B, Shin Y-J, et al. A clinical study on the repeatability and reproducibility of Ryodoraku score. J Korean Med Soc 2009;30:76–82. [Google Scholar]
- [32].Halberg F, Tong YL, Johnson E. Circadian system phase—an aspect of temporal morphology; procedures and illustrative examples. The Cellular Aspects of Biorhythms: Springer 1967. 20–48. [Google Scholar]
- [33].Tong YL. Parameter estimation in studying circadian rhythms. Biometrics 1976. 85–94. [PubMed] [Google Scholar]
- [34].Cornelissen G. Cosinor-based rhythmometry. Theoretical Biology and Medical Modelling 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mikulich SK, Zerbe GO, Jones RH, et al. Relating the classical covariance adjustment techniques of multivariate growth curve models to modern univariate mixed effects models. Biometrics 1999;55:957–64. [DOI] [PubMed] [Google Scholar]
- [36].Mikulich SK, Zerbe GO, Jones RH, et al. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat Med 2003;22:3195–211. [DOI] [PubMed] [Google Scholar]
- [37].Fontana A, Copetti M, Mazzoccoli G, et al. A linear mixed model approach to compare the evolution of multiple biological rhythms. Stat Med 2013;32:1125–35. [DOI] [PubMed] [Google Scholar]
- [38].R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/. [Google Scholar]
- [39].Cambridge University Press, Cacioppo JT, Tassinary LG, Berntson G. Handbook of Psychophysiology. 2007. [Google Scholar]
- [40].Dawson ME, Schell AM, Filion DL. The electrodermal system. Handbook Psychophysiol 2007;2:200–23. [Google Scholar]
- [41].Atkinson G, Jones H, Ainslie PN. Circadian variation in the circulatory responses to exercise: relevance to the morning peaks in strokes and cardiac events. Eur J Appl Physiol 2010;108:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Division KHUA. A Practical Approach to Traditional Korean Medicine Diagnosis. Seoul: Iljoongsa; 1997. [Google Scholar]
- [43].Kim K. Study on clinical establish direction for oriental medicine diagnosis methods. J Orient Physiol Pathol 2006;20:245–56. [Google Scholar]
- [44].Lukaski HC, Johnson PE, Bolonchuk WW, et al. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr 1985;41:810–7. [DOI] [PubMed] [Google Scholar]
- [45].Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 2008;11:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Buss DM, Larsen RJ, Westen D, et al. Sex differences in jealousy: evolution, physiology, and psychology. Psychol Sci 1992;3:251–6. [Google Scholar]
- [47].Hare R, Wood K, Britain S, et al. Autonomic responses to affective visual stimulation: Sex differences. J Exp Res Personality 1971;5:14–22. [Google Scholar]
- [48].Roman F, Martinez-Selva JM, Garcia-Sanchez FA, et al. Sex differences, activation level, and bilateral electrodermal activity. Pavlovian J Biol Sci 1987;22:113–7. [DOI] [PubMed] [Google Scholar]
- [49].Garwood MK, Engel BT, Quilter RE. Age differences in the effect of epidermal hydration on electrodermal activity. Psychophysiology 1979;16:311–7. [DOI] [PubMed] [Google Scholar]
- [50].Gavazzeni J, Wiens S, Fischer H. Age effects to negative arousal differ for self-report and electrodermal activity. Psychophysiology 2008;45:148–51. [DOI] [PubMed] [Google Scholar]
- [51].Payne AF, Dawson ME, Schell AM, et al. Can you give me a hand? A comparison of hands and feet as optimal anatomical sites for skin conductance recording. Psychophysiology 2013;50:1065–9. [DOI] [PubMed] [Google Scholar]
- [52].Yoon S-H. An association of Kyung–Rak principle and autonomic nerve theory related with Ryodoraku of patients with gastric dysmotility and gastric ulcer. J Korean Oriental Internal Med 2010. 31. [Google Scholar]
- [53].Lee JI, Ko SC, Song HS. A clinical study on the repeatability and reproducibility of portable ryodoraku devicefx1. J Korean Acupunct Moxibustion Soc 2013;30:135–40. [Google Scholar]
- [54].Eckel-Mahan K, Sassone-Corsi P. Phenotyping circadian rhythms in mice. Curr Protocols Mouse Biol 2015;5:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


