Abstract
Background:
The goal of the current meta-analysis and systematic review was to explore the efficacy of tiotropium in treating patients with moderate-to-severe asthma on the basis of qualified randomized controlled trials (RCTs).
Methods:
The following online electronic databases, such as Cochrane, PubMed, and Embase database were screened to identify qualified studies updated to January 2019 through the use of index words. Several literatures that were relevant to the present analysis were also included. To further analyze the main outcomes, we utilized the odds rations (OR), and mean difference (MD) along with its 95% confidence interval (95% CI).
Results:
A total of 14 RCTs with 4998 patients in the tiotropium group and 5074 patients in the control group were included in the present study. On the basis of the pooled results, tiotropium was significantly associated with improved morning PEF (SMD: 3.29, 95%CI: 2.03–4.55), evening PEF (SMD: 3.36, 95%CI: 2.24–4.48), peak FEV (SMD: 2.67, 95%CI: 1.47–3.88), and trough FEV (SMD: 1.90, 95%CI: 0.87–2.92) vs the control group. Nevertheless, no significant difference was observed in peak FVC (SMD: 0.77, 95%CI: −0.21–1.76), trough FVC (SMD: 0.67, 95%CI: −0.18–1.53), AE (RR: 0.98, 95%CI: 0.94–1.02) and serious AE (RR: 1.08, 95%CI: 0.77–1.52) between the 2 groups.
Conclusions:
In this review, we summarized the significant effect of tiotropium for the treatment of moderate-to-severe asthma, mainly in increasing morning PEF, evening PEF, peak FEV and trough FEV based on high-quality RCTs. Nevertheless, no significant difference in peak FVC, trough FVC, AE and serious AE was found between the 2 groups. A close comparison of the 2 groups revealed that more high-quality larger-sample RCTs are needed to gather more strong evidence on the therapeutic efficacy and safety of tiotropium for clinical practice.
Keywords: asthma, meta-analysis, randomized controlled trial, tiotropium
1. Introduction
Asthma is a type of respiratory disease that occurs due to chronic airway inflammation. It is generally characterized by infiltration of eosinophils, activated mast cells and T lymphocytes into the bronchial wall. Cytokines released by TH2 cells and leukotrienes play a key role in mediating airway inflammation. Another feature of asthma is reversible ventilation dysfunction caused by excessive contraction of bronchial smooth muscle. Given the present understanding of the pathogenesis of asthma, clinical treatment mainly focuses on anti-inflammatory approaches and dilating the airway. According to the recommendation from the latest GINA Guidelines in 2015, anti-inflammatory drugs mainly include inhaled corticosteroids (ICS) and leukotriene antagonists, while bronchodilators mainly include inhaled long-acting beta 2 agonists (LABA).[1,2,3,4]
Tiotropium is a specific selective anticholinergic drug with a muscarinic receptor-like affinity and has been approved to treat chronic obstructive pulmonary disease. By binding to the muscarinic receptor on the bronchial smooth muscle, it can inhibit bronchoconstriction, bronchial vasodilation and mucus secretion caused by acetylcholine released from the parasympathetic nerve endings. Theoretically, tiotropium bromide is able to alleviate asthma symptoms, but debates exist concerning whether it can be used for routine treatment in clinical practice.[5,6,7,8,9] Hence, the current meta-analysis was conducted based on updated data and literature to gather available evidence to explore the clinical efficacy of tiotropium in treating patients suffering from moderate-to-severe asthma.
2. Methods
2.1. Ethics approval
The ethics approval was waived because the study does not involve any human participants and animals.
2.2. Search strategy
An electronic search of literature using Embase, Cochrane, and PubMed was conducted by 2 reviewers up to January 2019 in terms of the efficacy of tiotropium in treating patients with moderate-to-severe asthma. References from relevant studies and reviews were further searched to confirm retrieval of all possible pertinent trials. The following search terms were used: tiotropium, tiotropium bromide, asthma, cough variant asthma, wheezing, CVA, randomized, randomized controlled trial, and RCT. These terms were used in combination with “AND” or “OR”. The search process was carried out separately by 2 reviewers. Any differences were settled through the aid of a third party.
2.3. Selection criteria
To be included in the current meta-analysis, studies should meet the following criteria:
-
1.
RCTs;
-
2.
patients diagnosed with asthma as the research subjects;
-
3.
the interventions of the experiment group were tiotropium; the interventions of the control groups were standard therapy or ICS or LABA;
-
4.
only English publications were eligible.
Studies were excluded due to the following criteria:
-
1.
duplicate publications, or shared contents and results;
-
2.
data with obvious errors;
-
3.
case report, case-control studies, conference report, theoretical research, meta-analysis, systematic review, economic analysis, expert comment, or real world study;
-
4.
irrelevant outcomes.
The literature selection process was performed by 2 reviewers separately based on predefined criteria and any arising differences were settled by discussion with a third party.
2.4. Data extraction and quality assessment
We examined the data based on the included studies with 2 parts consisted, including the following 2 parameters:
-
1.
basic information such as name of author, year of publication, interventions of the experiment group and the control group, sample size, gender, age, severity of asthma, and the Jadad score;
-
2.
clinical outcomes including morning peak expiratory flow (PEF), evening PEF, peak forced expiratory volume (FEV), trough FEV, peak forced vital capacity (FVC), trough FVC, adverse event (AE), and serious AE as clinical outcomes.
To appraise the quality of the involved studies, we used the Jadad score checklist. In addition, all RCTs were under assessment from the 5 items: appropriateness of generation of randomized sequence, statement of randomization, detail of withdrawals and dropouts, use of double blinding, as well as the description of double blinding method. A score less than 3 in the included studies represented a low-quality and high bias risk, and a score greater than 3 represents a trial with high quality. The abovementioned process was separately conducted by 2 investigators; arising differences were settled by discussion to reach a consensus.
2.5. Statistics analysis
The meta-analysis was conducted through the use of the STATA 10.0 (TX). Heterogeneity of the trial results was assessed with the Chi-squared and I2 tests to select ideal analysis model (random-effects model or fixed-effects model): I2 > 50% and Chi-squared test P ≤ .05 reflected a high heterogeneity and the random-effects model was utilized; I2 ≤ 50% and Chi-squared test P > .05 reflected an acceptable heterogeneity data with the assessment of the fixed-effects model. As for continuous variables, they were initially expressed as mean ± standard deviation and then analyzed through the use of mean difference (MD). We presented categorical data by percentages and further analyzed it through odds ratio (OR) or relative risk (RR). RR along with 95% CI was used to analyze AE and serious AE. Morning PEF, evening PEF, peak FEV, trough FEV, peak FVC and trough FVC were analyzed by MD along with 95%CI.
3. Results
3.1. Study characteristics
Totally, 1175 publications were included based on the indexes. During initial screening of the abstracts and titles, 1124 publications were eliminated, leaving 51 publications for further assessment. During full-text screening and searching, 37 publications were excluded because of failure to meet the inclusion criteria: theoretical or economic research (6), lack of clinical outcomes (17), non-RCT (8), or duplicate articles (6). Therefore, a final total of 14 RCTs[8,10,11,12,13,14,15,16,17,18,19,20,21,22] were presented in the current meta-analysis, of which there were 4998 patients in the tiotropium group and 5074 patients in the control group (See Fig. 1). Table 1 shows the major characteristics of studies.
Figure 1.

Flow diagram of the literature search and selection process.
Table 1.
The basic characteristics description of included studies.
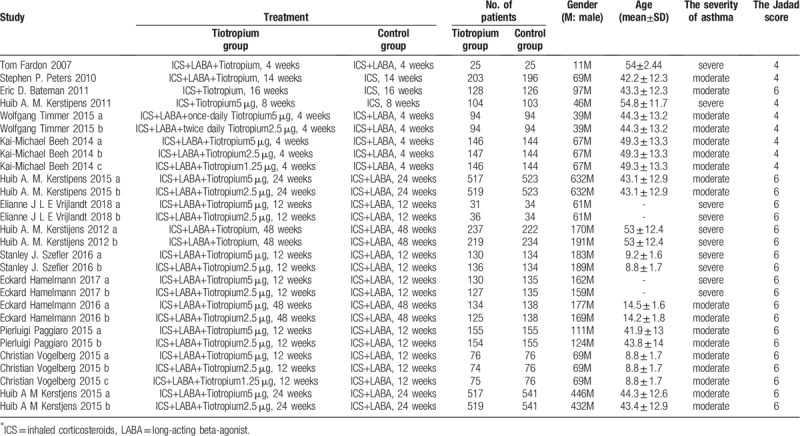
The basic information consisted of sample, gender, age, treatment, severity and the Jadad score. The main Jadad score of the included studies was 5.43 which was greater than 3; thus, all the included studies were of high quality.
3.2. PEF
Seven trials with 2146 patients in the tiotropium group and 2197 patients in the control group studied morning PEF. On the basis of the I2 test-value (99.6% > 50%) and Chi-squared test P value (.000 < .05), the random effects model was selected for analysis of morning PEF. The pooled result showed that the tiotropium group was associated with significant effect in improving morning PEF vs the control group (SMD: 3.29, 95%CI: 2.03–4.55, Fig. 2).
Figure 2.
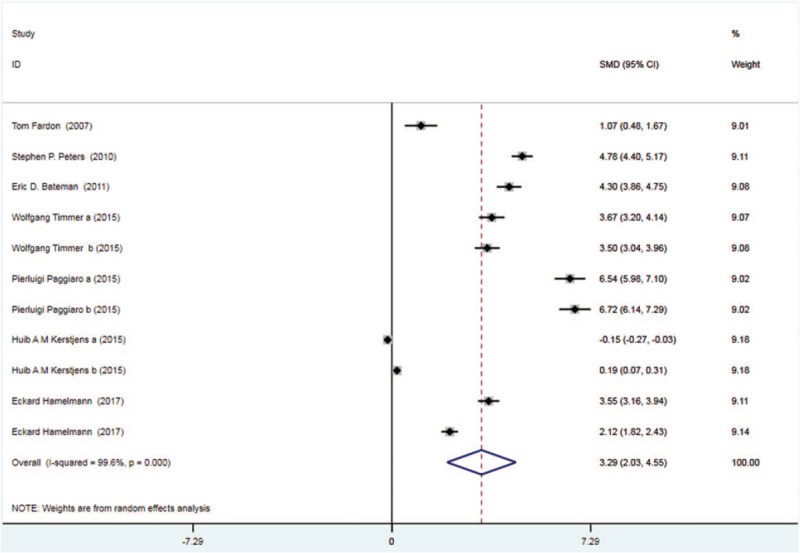
Forest plot for the result of morning PEF. PEF = peak expiratory flow.
Eight trials with 2412 patients in the tiotropium and 2465 patients in the control group reported evening PEF. On the basis of the I2 test-value (99.5% > 50%) and Chi-squared test P value (.000 < .05), the random effects model was applied to analyze evening PEF. Based on the pooled results, evening PEF was remarkably improved in the tiotropium group vs the control group (SMD: 3.36, 95%CI: 2.24–4.48, Fig. 3).
Figure 3.
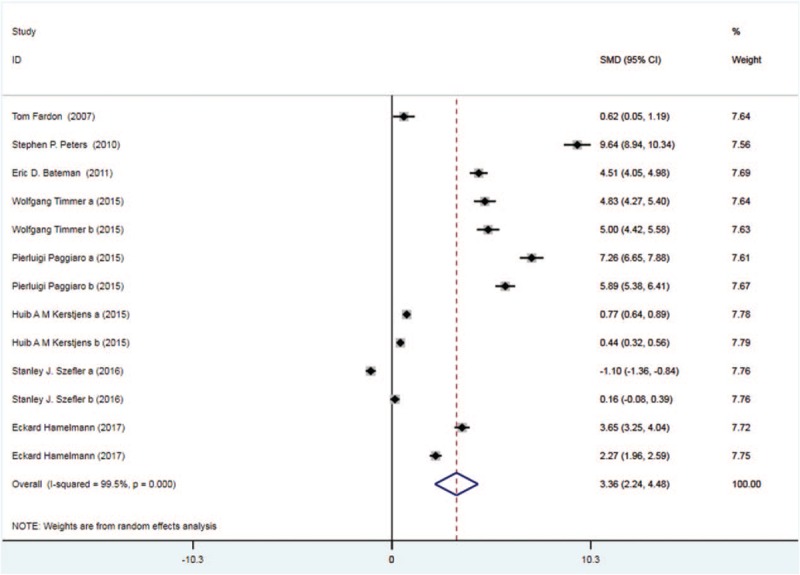
Forest plot for the result of evening PEF. PEF = peak expiratory flow.
3.3. FEV
Six trials with 2162 patients in the tiotropium and 2227 patients in the control group reported peak FEV. On the basis of the I2 test value (99.6% > 50%) and Chi-squared test P value (.000 < .05), the random effects model was selected for analysis of peak FEV. The pooled result showed significantly improved trend of peak FEV in the tiotropium group vs the control group (SMD: 2.67, 95%CI: 1.47–3.88, Fig. 4).
Figure 4.
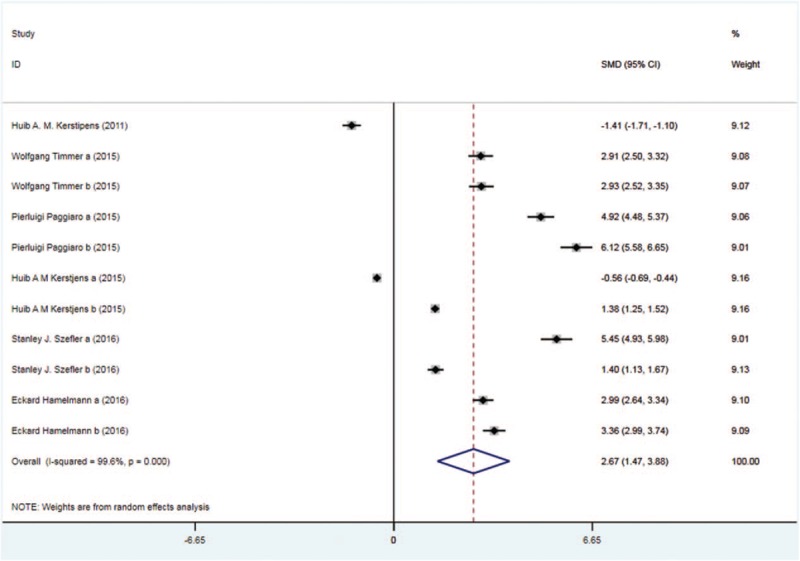
Forest plot for the result of peak FEV. FEV = forced expiratory volume.
Five trials with 1896 patients in the tiotropium and 1959 patients in the control group reported the trough FEV. On the basis of the I2 test value (99.3% > 50%) and Chi-squared test P value (.000 < .05), the random effects model was selected for analysis of trough FEV. According to the pooled results, there was remarkable improvement of trough FEV in the tiotropium group vs the control group (SMD: 1.90, 95%CI: 0.87–2.92, Fig. 5).
Figure 5.
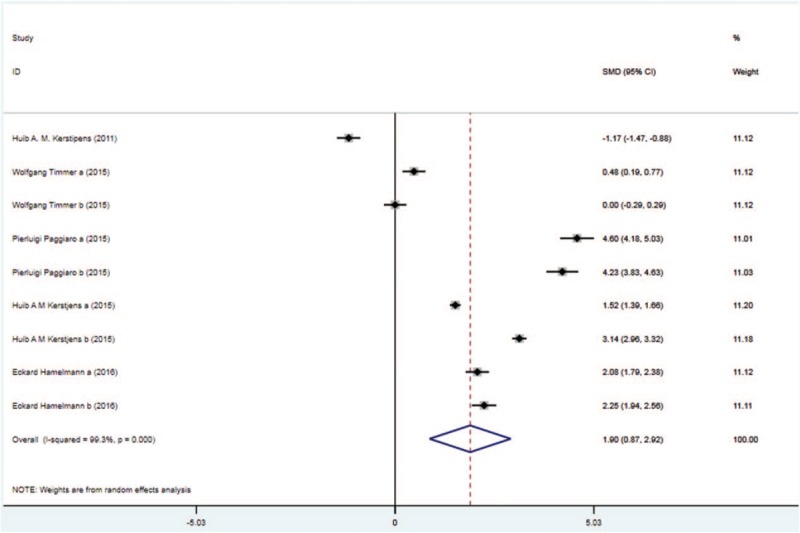
Forest plot for the result of trough FEV. FEV = forced expiratory volume.
3.4. FVC
Four trials with 1818 patients in the tiotropium and 1896 patients in the control group reported peak FVC. On the basis of the I2 test value (99.4% > 50%) and Chi-squared test P value (.000 < .05), the random effects model was selected for analysis of peak FVC. Based on the pooled results, no significant difference in peak FVC was found between the 2 groups (SMD: 0.77, 95%CI: −0.21–1.76, Fig. 6).
Figure 6.
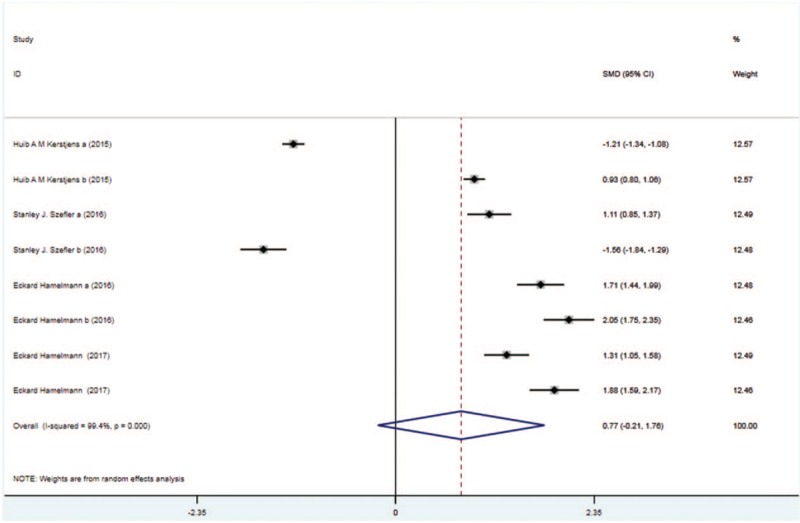
Forest plot for the result of peak FVC. FVC = forced vital capacity.
Four trials with 1818 patients in the tiotropium and 1896 patients in the control group showed the results of trough FVC. On the basis of the I2 test value (99.1% > 50%) and Chi-squared test P value (.000 < .05), the fixed effects model was selected for analysis of trough FVC. Based on the pooled result, no significant difference in trough FVC was identified between the two groups (SMD: 0.67, 95%CI: −0.18–1.53, Fig. 7).
Figure 7.
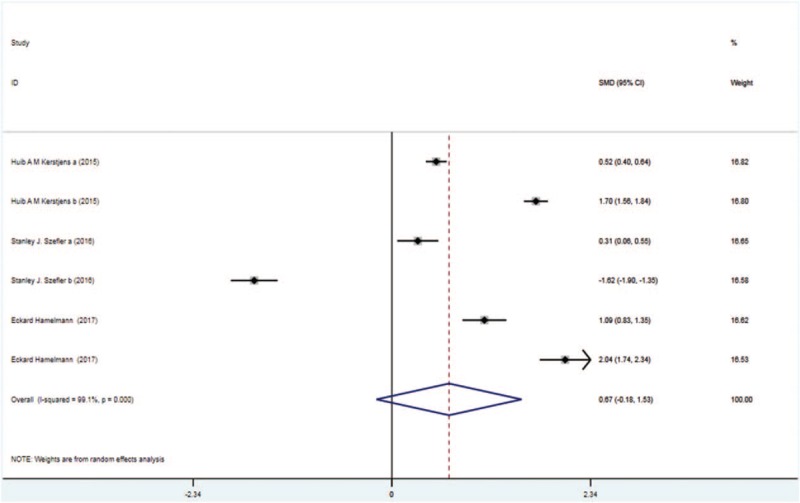
Forest plot for the result of trough FVC. FVC = forced vital capacity.
3.5. AE
Thirteen trials with 4973 patients in the tiotropium and 5049 patients in the control group reported AE. On the basis of the I2 test value (0.0% < 50%) and Chi-squared test P value (.817 > .05), the fixed effects model was selected for analysis of AE. According to the pooled result, no significant difference in the incidence of AE was observed between the 2 groups (RR: 0.98, 95%CI: 0.94–1.02, Fig. 8).
Figure 8.
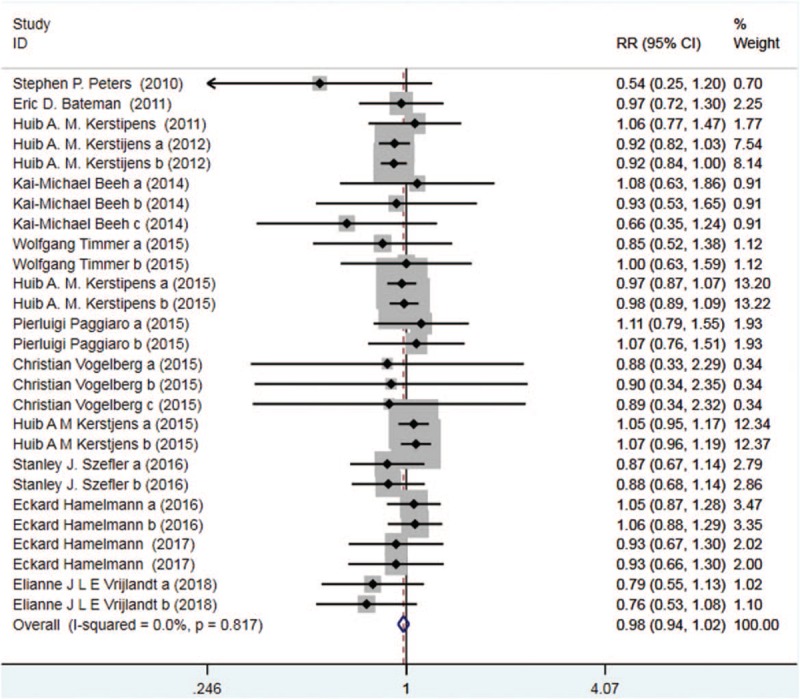
Forest plot for the result of AE. AE = adverse event.
Nine trials with 2212 patients in the tiotropium and 2234 patients in the control group reported serious AE. On the basis of the I2 test value (0.0% < 50%) and Chi-squared test P value (.967 > .05), the fixed effects model was selected for analysis of serious AE. Based on the pooled result, no significant difference in the incidence of serious AE was found between the two groups (RR: 1.08, 95%CI: 0.77–1.52, Fig. 9).
Figure 9.
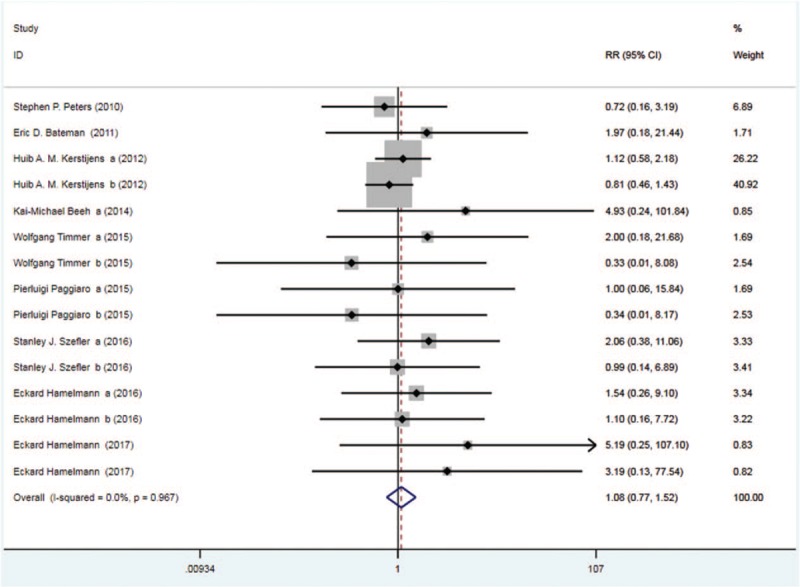
Forest plot for the result of serious AE. AE = adverse event.
3.6. Quality assessment and potential bias
According to the predefined criteria, a total of 20 publications were included for analysis in the present meta-analysis. We performed quality assessment for potential bias through the use of funnel plot, Begg and Mazumdar rank test, and Egger test. Notable symmetry was found through the funnel plot for log RR in serious AE of the included studies, indicating no remarkable publication bias (Fig. 10). In addition, we identified significant symmetry by the use of Begg and Mazumdar rank test (Z = −2.85, P = .009). Nonetheless, no significant publication bias was observed according to the Egger test results (P = .009).
Figure 10.
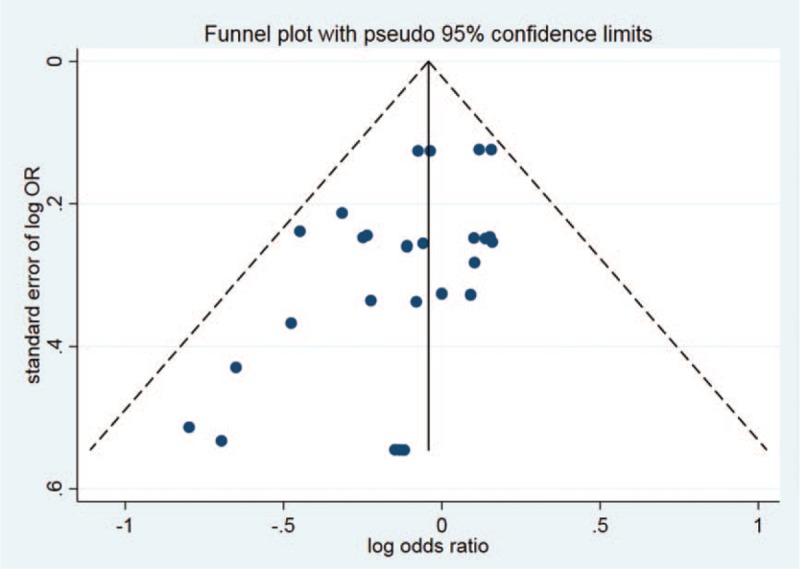
Funnel plot of studies included in the meta-analysis.
4. Discussion
On the basis of earlier studies supported by Rodrigo et al,[23] 3 studies identified the remarkable effect of tiotropium in improving asthma by 100 mL (P < .001) and FEV1 peak (mean change from baseline) by 120 mL (P < .001) vs the placebo. The percentage of patients was decreased significantly by tiotropium who were suffering from an Asthma Control Questionnaire 7 worsening episode (a change from trial baseline of 0.5 points or more) vs the placebo (2.1% vs 4.8%, number needed to treat 1/4 38). Tiotropium also significantly reduced the number of patients with one or more exacerbation vs the placebo (17.6 vs 23.8%, number needed to treat 1/4 16). Nevertheless, there were no significant differences between the 2 groups in AEs (27.3% vs 27.1%), serious AEs (6.5% vs 7.1%), rescue medication use, withdrawals, and withdrawals due to AEs. Tiotropium 2.5 mg and 5.0 mg once daily contributed to the equivalent effects. Ian et al[24] concluded that, compared with the placebo, the additional use of tiotropium was associated with significant improvement in all spirometric indices, including trough and peak FEV1 (WMD 0.13 L, 95% CI 0.09–0.18 L, P < .001; and WMD 0.10 L, 95% CI 0.06–0.14 L, P < .001, respectively), morning and evening PEF (WMD 20.59 L/min, 95% CI 15.36 –25.81 L/min, P < .001; and WMD 24.95 L/min, 95% CI 19.22–30.69 L/min, P < .001, respectively), the area under the curve of the first 3 hours of FVC (WMD 0.11 L, 95% CI 0.06–0.15 L, P < .001), trough and peak FVC (WMD 0.1 L, 95% CI 0.05–0.15 L, P < .001; and WMD 0.08 L, 95% CI 0.04–0.13 L, P < .001, respectively), and the area under the curve of the first 3 hours of FEV1 (WMD 0.13 L, 95% CI 0.08–0.18 L, P < .001). The mean change in the 7-point Asthma Control Questionnaire score (WMD 0.12, 95% CI 0.21–0.03, P < .01) was markedly lower in tiotropium group, but it was not clinically important. There were no significant differences in Asthma Quality of Life Questionnaire score (WMD 0.09, 95% CI 0.01–0.20, P < .09), night awakenings (WMD 0.00, 95% CI 0.05–0.05, P < .99) or rescue medication use (WMD 0.18, 95% CI 0.36–0.00, P < .06). We noticed no significant increase in AEs based on the results of the tiotropium group (OR 0.80, 95% CI 0.62–1.03, P < .08).
Asthma is known as a common clinical airway obstructive disease and bronchodilators are commonly used for controlling asthma symptoms. The ultimate goal of asthma management is to not only control asthma symptoms but also to reduce the incidence of acute attacks and AEs. The pathogenesis of asthma explains why inhalation of ICS and short-acting β-2 receptor antagonist (SABA) are recommended for the treatment of persistent asthma. ICS controls airway inflammation and SABA relieves airway spasm to control acute asthma symptoms. To reduce the side effects of the drug, the guidelines state that the more asthma is treated, the higher the dose of inhaled ICS should be taken. Despite with the largest doses of inhaled ICS, some asthma still cannot be controlled. Therefore, new valid and alternative drugs are in great demand to treat asthma in clinical practice. Tiotropium bromide is a highly selective receptor antagonist for the muscarinic acetylcholine (Ach) receptor, and it has the effect of continuous airway dilation for 16 hours due to slow dissociation from the Ach M3 receptor.
The current meta-analysis extends efforts with an attempt to confirm the efficacy of tiotropium for asthma. We conclude the following advantages of the current study: the findings obtained from the current systemic review might be more convincing than any individual study among all included studies because the efficacy of tiotropium in treating patients suffering from moderate-to-severe asthma was quantitatively determined using pooled large sample size; the strict inclusion and exclusion criterion were used to select qualified studies; all the data were analyzed by standard statistical analysis to make sure that the results were accurate. All the included studies were high-quality RCTs, making the conclusion clinically significant.
Admittedly, several limitations of the present analysis should be acknowledged. It was possible to include the following factors:
-
1.
only RCTs were included;
-
2.
the predefined criteria were different for patients among various studies;
-
3.
types of drug and dosage were various;
-
4.
different severities of asthma;
-
5.
we used I2 to assess the heterogeneity of the included studies and decided to select which kind of model (the random effects model or fixed effects model). If I2 > 50%, meaning that there was a high heterogeneity, and the index was analyzed by the random effects model. If I2 < 50%, meaning that there was a low heterogeneity, and the index was analyzed by the fixed effects model. Morning PEF, evening PEF, peak FEV, trough FEV, and peak FVC were analyzed by the random effects model. So the included studies on morning PEF, evening PEF, peak FEV, trough FEV, and peak FVC has high heterogeneity;
-
6.
we used the pooled data for analysis; data of individual patients were unavailable, which limit more comprehensive analyses.
Considering the limitation of number among included studies based on earlier meta-analyses, we conduct a new and more comprehensive meta-analysis to further explore the accurate efficacy of tiotropium in treating moderate-to-severe asthma. Based on the available evidence, tiotropium could increase morning PEF, evening PEF, peak FEV and trough FEV vs the control group based on high-quality RCTs. Besides, there was no significant difference in peak FVC, trough FVC, AE and serious AE between the 2 groups. Given that there was no significant publication bias and the main Jadad score of included studies was 5.43, high quality of all the included studies should be confirmed. Furthermore high-quality, larger-sample RCTs are warranted to gather more solid evidence of the safety profile of tiotropium in clinical practice.
Author contributions
Formal analysis: Hua Li, Hai-Bin Li.
Investigation: Hua Li.
Methodology: Ming-Jie Luo.
Resources: Ming-Jie Luo.
Supervision: Jian-Feng Meng.
Validation: Ming-Jie Luo.
Writing – original draft: Jian-Feng Meng.
Writing – review & editing: Hai-Bin Li.
Footnotes
Abbreviations: 95% CI 95% = confidence interval, AE = adverse event, FEV = forced expiratory volume, FVC = forced vital capacity, ICS = inhaled corticosteroids, LABA = inhaled long-acting beta 2 agonists, MD = mean difference, OR = odds rations, PEF = peak expiratory flow, RCTs = randomized controlled trials, RR = relative risk.
The authors have no funding and conflicts of interest to disclose.
References
- [1]. Meurs H, Dekkers BG, Maarsingh H, et al. Muscarinic receptors on airway mesenchymal cells: novel findings for an ancient target. Pulm Pharmacol Ther 2013;26:145–55. [DOI] [PubMed] [Google Scholar]
- [2]. Long-Ai Z, Hou-Kun XU, Hua-Ming LI, et al. Asthma management and prevention. Global Initiative for Asthma. Irish Med J 2000;Suppl (Suppl):i: [PubMed] [Google Scholar]
- [3]. Rodrigo GJ, Rodrigo C. First-line therapy for adult patients with acute asthma receiving a multiple-dose protocol of ipratropium bromide plus albuterol in the emergency department. Am J Respir Crit Care Med 2000;161:1862–8. [DOI] [PubMed] [Google Scholar]
- [4]. Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta-analysis. Thorax 2005;60:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Ohta S, Oda N, Yokoe T, et al. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin Exp Allergy 2010;40:1266–75. [DOI] [PubMed] [Google Scholar]
- [6]. Alagha K, Palot A, Sofalvi T, et al. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther Adv Chronic Dis 2014;5:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Gross NJ. Tiotropium bromide. Chest 2004;126:1946–53. [DOI] [PubMed] [Google Scholar]
- [8]. Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012;367:1198–207. [DOI] [PubMed] [Google Scholar]
- [9]. Celli B, Decramer M, Leimer I, et al. Cardiovascular safety of tiotropium in patients with COPD. Chest 2010;137:20–30. [DOI] [PubMed] [Google Scholar]
- [10]. Fardon T, Haggart K, Lee DK, et al. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respir Med 2007;101:1218–28. [DOI] [PubMed] [Google Scholar]
- [11]. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med 2010;363:1715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Bateman ED, Kornmann O, Schmidt P, et al. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol 2011;128:315–22. [DOI] [PubMed] [Google Scholar]
- [13]. Kerstjens HA, Disse B, Schroder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol 2011;128:308–14. [DOI] [PubMed] [Google Scholar]
- [14]. Timmer W, Moroni-Zentgraf P, Cornelissen P, et al. Once-daily tiotropium Respimat ((R)) 5 mug is an efficacious 24-h bronchodilator in adults with symptomatic asthma. Respir Med 2015;109:329–38. [DOI] [PubMed] [Google Scholar]
- [15]. Beeh KM, Moroni-Zentgraf P, Ablinger O, et al. Tiotropium Respimat (R) in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res 2014;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med 2015;3:367–76. [DOI] [PubMed] [Google Scholar]
- [17]. Vrijlandt E, El Azzi G, Vandewalker M, et al. Safety and efficacy of tiotropium in children aged 1-5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2018;6:127–37. [DOI] [PubMed] [Google Scholar]
- [18]. Szefler SJ, Murphy K, Harper T, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol 2017;140:1277–87. [DOI] [PubMed] [Google Scholar]
- [19]. Hamelmann E, Bernstein JA, Vandewalker M, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J 2017;49 (1.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol 2016;138:441–50. e448. [DOI] [PubMed] [Google Scholar]
- [21]. Paggiaro P, Halpin DM, Buhl R, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract 2016;4:104–13. e102. [DOI] [PubMed] [Google Scholar]
- [22]. Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, et al. A randomised dose-ranging study of tiotropium Respimat(R) in children with symptomatic asthma despite inhaled corticosteroids. Respir Res 2015;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Rodrigo GJ, Castro-Rodriguez JA. Tiotropium for the treatment of adolescents with moderate to severe symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2015;115:211–6. [DOI] [PubMed] [Google Scholar]
- [24]. Tian JW, Chen JW, Chen R, et al. Tiotropium versus placebo for inadequately controlled asthma: a meta-analysis. Respir Care 2014;59:654–66. [DOI] [PubMed] [Google Scholar]


