Abstract
Background:
Multiple myeloma (MM) is a clonal plasma cell malignancy associated with hypercalcemia, bone lesions, and renal failure. The prognostic significance of the mutation of miRNAs, one kind of small noncoding RNA molecules that can modulate gene expression, should be confirmed in non-Hodgkin lymphomas (NHL). This study aimed to identify the prognostic value of miRNAs in patients with MM.
Methods:
A meta-analysis was performed to estimate the pooled hazard ratios and their corresponding 95% confidence intervals for the associations between levels of miRNA expression (predictive factors) and outcomes in patients with MM. We systematically searched the PubMed, Web of Science, and China National Knowledge Infrastructure databases (final search conducted January 1, 2018) to identify eligible studies. Eligible studies were included by certain inclusion and exclusion criteria, whose quality was assessed by Newcastle-Ottawa Scale.
Results:
After performing the literature search and review, 10 relevant studies, including 1214 cases, were identified. The results of our meta-analysis revealed that upregulated miR-92a level and downregulated miR-16, miR-25, miR-744, miR-15a, let-7e, and miR-19b expression were associated with poor prognosis in MM.
Conclusions:
This study identified miRNAs could serve as potential prognostic biomarkers in MM. Given the limited research available, the clinical application of these findings has yet to be verified.
Keywords: biomarker, meta-analysis, microRNA, multiple myeloma, prognosis
1. Introduction
Multiple myeloma (MM) is a malignant clonal proliferative disease of plasma cells, which is characterized by extensive proliferation of pathological plasma cells and infiltration of other tissues and organs in the bone marrow, leading to extensive bone destruction, anemia, renal failure, and hypercalcemia. Although MM is currently incurable, patient's overall survival (OS) and progression free survival (PFS) prolonged with the application of new molecule-targeted drugs such as immunomodulatory factors (lenalidomide, pomamide) or proteasome inhibitors (bortezomib, carfilzomib), in which case the prognostic evaluation and risk stratification become extremely critical as they influence the choice of treatment.
Over the past few years, the prognosis of MM has been widely discussed and changed. Numerous parameters have been examined for their value as prognosis features, among which the most widely used prognostic factors in newly diagnosed MM are the International Staging System (ISS)[1] and currently revised ISS (R-ISS) system.[2] As a result, several sophisticated technologies such as interphase fluorescence in situ hybridization and gene-expression profiling analysis should be applied to clinical treatment. Although these examination methods have the advantages in accuracy, they are very complicated and inconvenient to use. Investigators are devoted to finding a specific biomarker that can be easily detected as the outcome indicator for myeloma.
Many advances in in-depth MM-related research on biomarkers, such as microRNAs (miRNAs), have promoted the utility of miRNAs in the prognosis of myeloma. MiRNAs are one kind of small noncoding RNA molecules that are 19 to 25 nucleotides in length and can modulate gene expression through degrading target mRNAs and/or suppressing their translation by binding to the 3′-untranslated region of target genes.[3] Bioinformatics projections indicate that 30% of all human genes are regulated by miRNAs. Therefore, miRNAs are involved in a variety of biological processes and have the endless potential as biomarkers. In addition to their role in normal biological processes, growing evidence indicates that aberrant expression of miRNAs might be related to the progression of human cancers,[4] including pancreatic ductal adenocarcinoma,[5] renal cell carcinoma,[6] brain tumors[7] and so on. The tissue levels of specific miRNAs correlate well with several hematological malignancies, including MM.[8,9,10,11,12]
Several investigators have paid attention to the prognostic role of miRNAs in MM.[13,14] However, to date, no systematic review or meta-analysis on the role of particular miRNAs in the survival of patients with MM has been performed. In this study, we systematically reviewed relevant studies on the prognostic value of miRNAs in MM and performed a meta-analysis to better understand the prognostic value of miRNAs in MM. The present study aimed to investigate the relationship between the expression of several miRNAs and the outcome of MM disease to provide a rationale for miRNA-based therapeutics.
2. Materials and methods
This meta-analysis was performed following the Meta-analysis of Observational Studies in Epidemiology guidelines.[12]
2.1. Search strategy
Literature searches were conducted using the PubMed, Web of Science, and China National Knowledge Infrastructure databases (final search conducted January 1, 2018). The keyword combinations in the search strategy were “microRNA OR microRNAs OR miR OR miRNA” (all fields), “myeloma OR MM” (all fields), and “prognosis OR prognostic OR survival” (all fields). Searches were limited to English language publications.
2.2. Inclusion and exclusion criteria
Eligible studies included in the meta-analysis met the following criteria:
-
(1)
focused on patients with MM,
-
(2)
assayed type either blood or tissue samples,
-
(3)
investigated the prognostic value of miRNA,
-
(4)
clearly defined the cut-off, and
-
(5)
clearly described the miRNA measurement method.
Studies were excluded if they met one of the following criteria:
-
(1)
single study focused on a miRNA not investigated by another study,
-
(2)
failure to extract the data,
-
(3)
and lack of basic data for aggregate calculation.
2.3. Data extraction and quality assessment
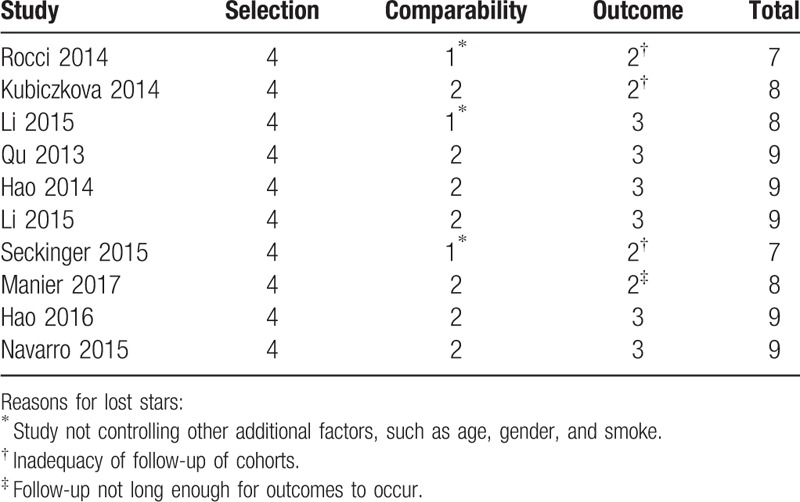
The database search was independently reviewed by 2 authors (T. Xia and X. Liu). Basic information was independently pooled by 2 investigators. We calculated from the available numerical data in the articles by using the methods described by Tierney[15] when the statistical variables were not described. The data from Kaplan–Meier survival curves were read by Engauge Digitizer version 4.1. We sent e-mails to the corresponding authors of eligible articles requesting additional information and original data needed for the meta-analysis. The quality of included studies was assessed by Newcastle-Ottawa Scale (NOS) according to the following categories: selection (descriptions on the derivation of the cohort, derivation of the non-exposed cohort, exposure ascertainment, presence of the outcome of interest at the start of the study), comparability (study controlled the most important factor and other factors), and outcome of interest (description of outcome assessment, adequacy of follow-up of cohorts, follow-up long enough for outcomes to occur).[16] A total of 9 items were extracted, and each item was scored as 1. The total score of NOS ranged from 0 to 9, and studies with a score of at least 6 were considered high quality.
2.4. Statistical analysis
We pooled the hazard ratios (HRs) (95% CIs) extracted from the studies using the Stata 13.0 software (StatCorp, College Station, TX). Statistical heterogeneity was assessed by calculating the I 2 statistic, and assessing the P value.[17,18] An I 2 value exceeding 50% and/or the P value less than .05 indicated the presence of heterogeneity, and a random-effects model was used. An observed HR < 1 suggested a more favorable prognosis in patients with aberrant miRNA, and an HR > 1 indicated a worse prognosis. Egger test was used to assess publication bias.
2.5. Ethical consideration
Ethical approval was not required for this study.
3. Results
3.1. Selection of studies
A flow diagram of the study selection process is shown in Figure 1. A total of 206 publications were identified in the initial search. After reviewing the titles and abstracts of these articles, we identified 15 articles evaluating the use of prognostic miRNA biomarkers in MM. We then carefully reviewed the full texts of these articles and excluded an additional 5 articles. In total, 10 articles (38 studies)[19,20,21,22,23,24,25,26,27,28] were eligible for inclusion in this meta-analysis.
Figure 1.
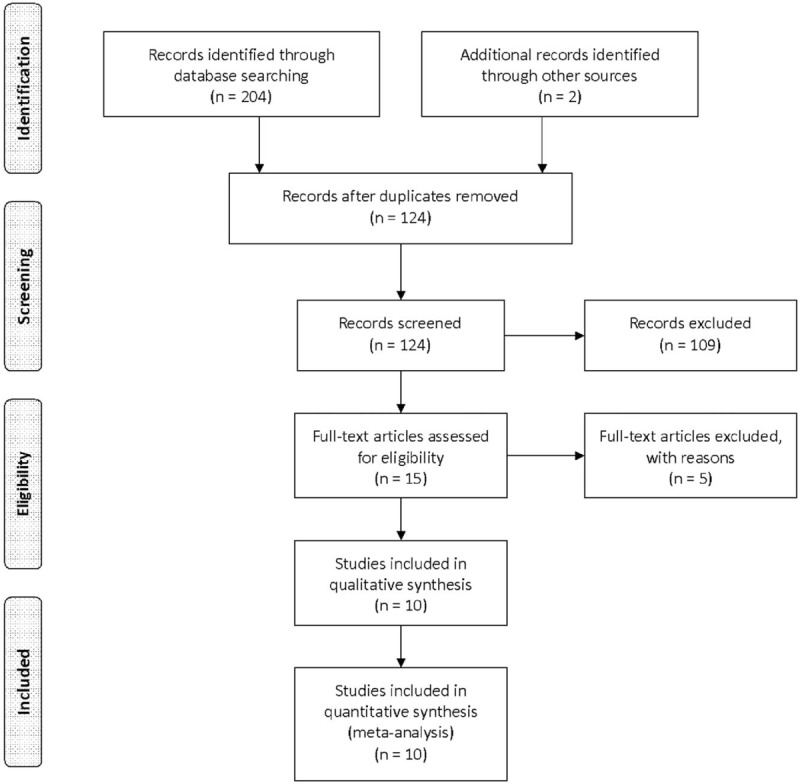
Flow diagram of the study selection procedure.
3.2. Characteristics of the included studies
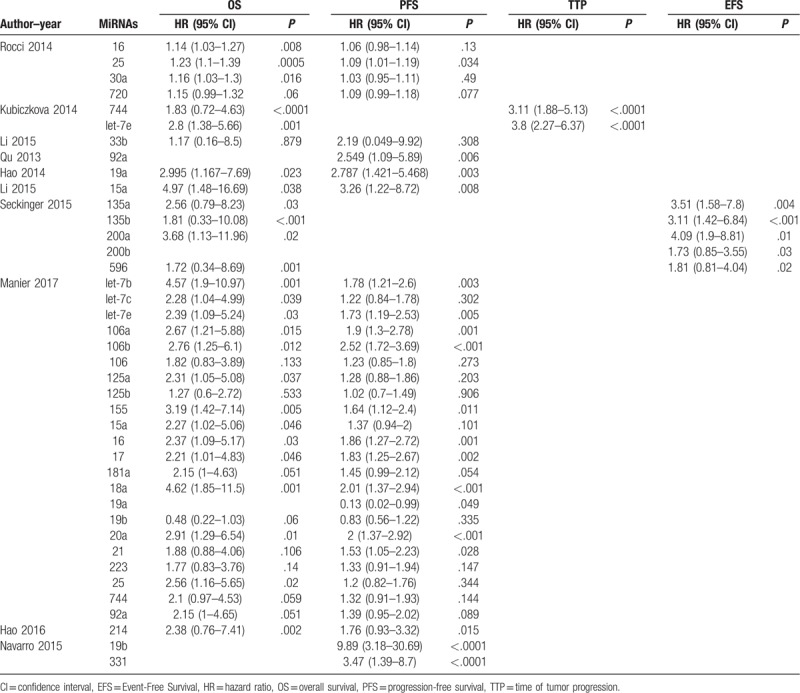
A total of 4810 MM patients were assessed in the 10 included articles, with a median sample size of 156 patients (range, 33–234 patients). These studies reported the prognostic values of 32 different miRNAs. The levels of miRNA expression were mainly detected in serum samples. Three studies used bone marrows, and 1 study used purified plasma cells. Six studies did not directly report HR data. Thus, we estimated the HRs using the methods described above (Table 1). In the included articles, increased expression of 7 miRNAs were associated with poor prognosis in MM. Among these miRNAs, 7 were reported by at least 2 studies (Table 2). Thus, we performed this meta-analysis to summarize the effect of these seven miRNAs.
Table 1.
Main characteristics of the eligible studies.
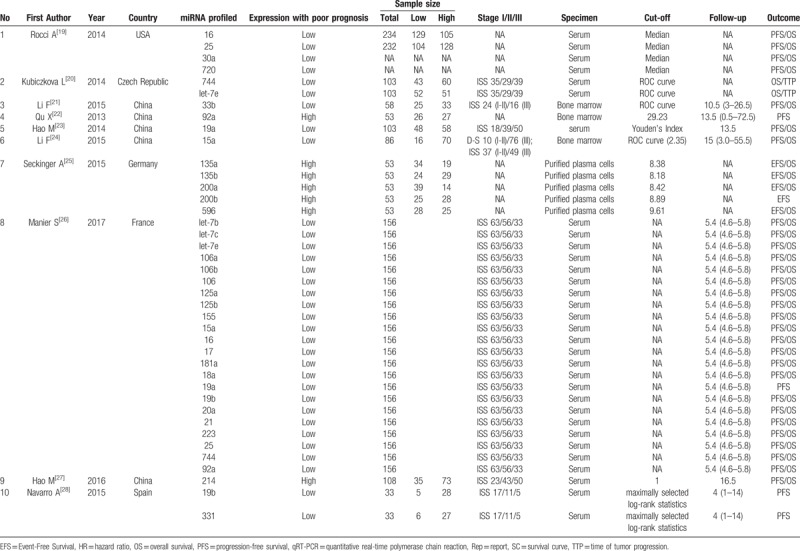
Table 2.
Descriptive characteristics and related data from included studies.
3.3. Quality assessment
The NOS scores of every study ranged from 7 to 9, with an average of 8.30. The detailed information of NOS scores is shown in Table 3.
Table 3.
Newcastle-Ottawa scale scores.
3.4. MiRNAs and MM prognosis
3.4.1. MiR-16
Two articles (n = 822) suggested that downregulation of miR-16 was associated with poor prognosis in patients with MM, both of which reported OS and progression-free survival (PFS) data[25,29] and calculated crude HR for miR-16. The observed interstudy heterogeneity for PFS (I 2 = 87.6%, P = .005) and OS (I 2 = 70.0%, P = .058) was both significant, and the Egger test results indicated the absence of significant publication bias (P = .035). The fixed-effects model revealed that miR-16 expression was inversely associated with PFS (HR: 1.08, 95% confidence interval (CI): 1.01–1.17) and OS (HR: 1.15, 95% CI: 1.04–1.28) in MM patients (Fig. 2).
Figure 2.
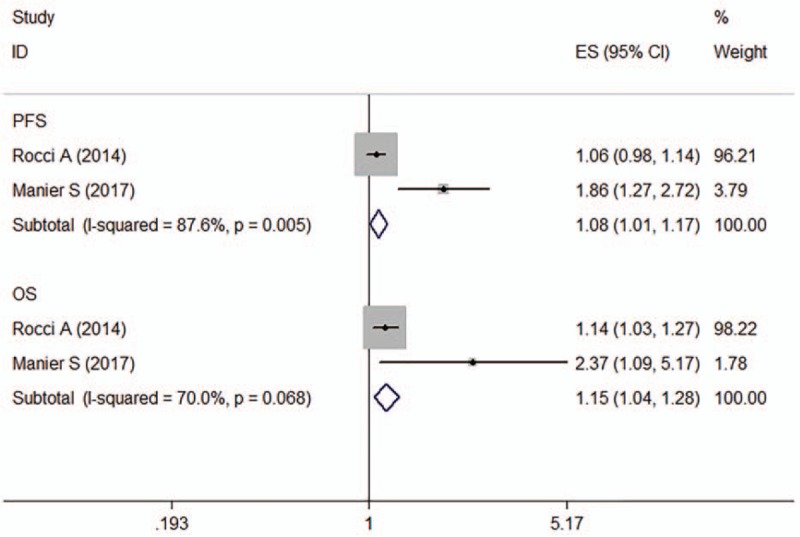
Forest plot of the HRs for the association between miR-16 and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
3.4.2. MiR-25
Two articles (n = 820) reported the effect of miR-25 on the prognosis of MM patients and OS and PFS data.[19,25] No significant heterogeneity was observed for PFS across studies (I 2 = 0.0%, P = .629). However, significant interstudy heterogeneity was observed for OS (I 2 = 69.0%, P = .073). The fixed-effects model revealed that miR-25 expression was inversely associated with PFS (HR: 1.09, 95% CI: 1.01–1.19) and OS (HR: 1.25, 95% CI: 1.11–1.40) in MM patients. There was no significant evidence of publication bias (Egger test, P = .229) (Fig. 3).
Figure 3.
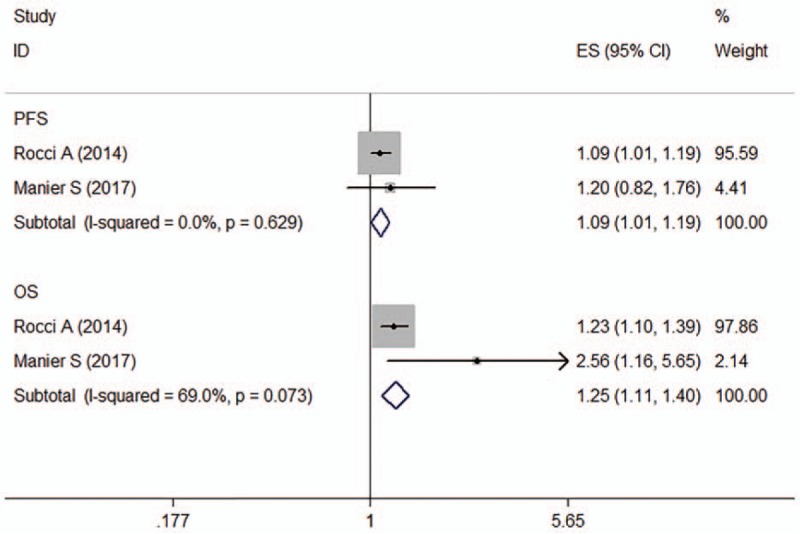
Forest plot of the HRs for the association between miR-25 and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
3.4.3. MiR-744
Two articles (4 studies, n = 259) assessed the association between miR-744 expression and prognosis in MM. Of these studies, 2 provided OS data,[20,25] 1 PFS data[25] and 1 TTP data.[20] For OS, no significant heterogeneity was observed across studies (I 2 = 0.0%, P = .823). The fixed-effects model revealed that decreased miR-744 expression was predictive of shorter OS (crude HR: 1.99, 95% CI: 1.10–3.59) (Fig. 4).
Figure 4.
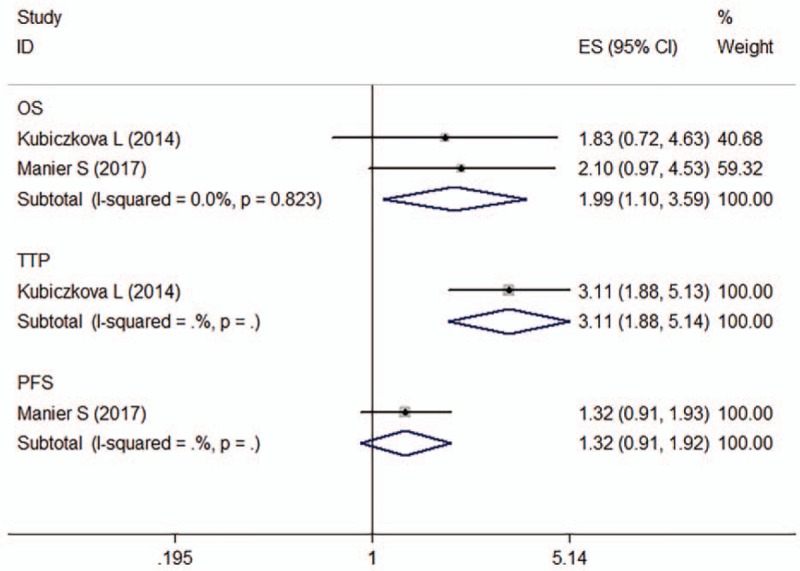
Forest plot of the HRs for the association between miR-744 and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival, TTP = time of tumor progression.
3.4.4. MiR-15a
Two articles evaluated the association between miR-15a expression and the prognosis of MM patients (n = 242), both of which reported OS and PFS data.[24,25] For PFS, the observed interstudy heterogeneity was significant (I 2 = 61.6%, P = .107). For OS, no significant interstudy heterogeneity was observed (I 2 = 10.6%, P = .290). The Egger test results indicated the absence of significant publication bias (P = .006). The fixed-effects model revealed that miR-15a expression was inversely associated with PFS (HR: 1.53, 95% CI: 1.08–2.18) and OS (HR: 2.88, 95% CI: 1.48–5.62) in MM patients (Fig. 5).
Figure 5.
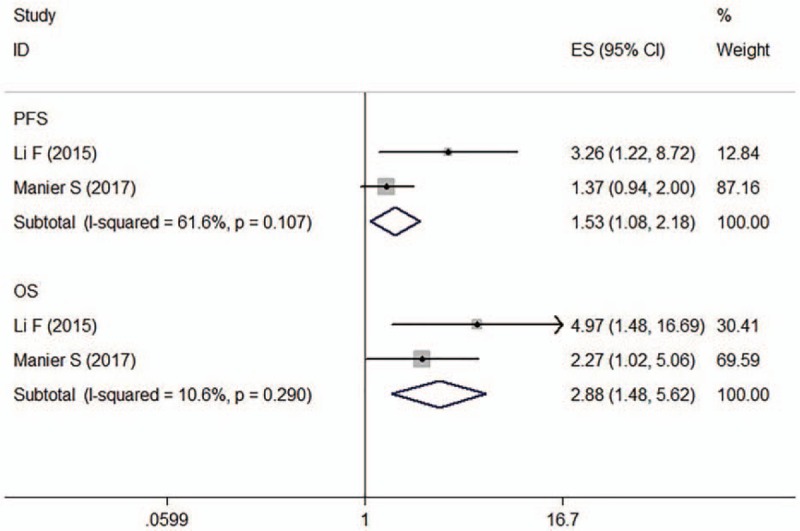
Forest plot of the HRs for the association between miR-15a and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
3.4.5. Let-7e
Two articles describing four studies reported lower let-7e expression to be a predictive factor for poor prognosis of patients with MM using univariate analyses (n = 259). One of them provided PFS data,[25] one TTP data,[20] and 2 OS data.[20,25] No significant heterogeneity was observed across studies (OS, I 2 = 0.00%, P = .769). The fixed-effects model revealed that let-7e expression was inversely associated with OS (HR: 2.61, 95% CI: 1.54–4.41) in MM patients (Fig. 6). The Egger test results indicated the absence of significant publication bias (P = .479).
Figure 6.
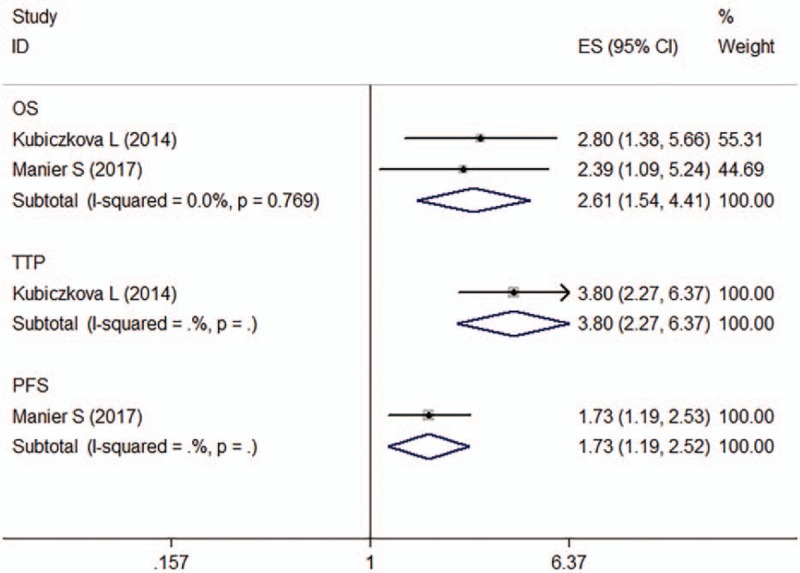
Forest plot of the HRs for the association between let-7e and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival, TTP = time of tumor progression.
3.4.6. MiR-92a
Two articles determined the association between miR-92a expression and prognosis of patients with MM (n = 209), of which one provided PFS data[30] and the other OS and DFS (disease-free survival) data.[25] For PFS, no significant heterogeneity was observed across studies (I 2 = 38.8%, P = .201). The fixed-effects model revealed that upregulation of miR-92a was predictive of shorter PFS (HR: 1.54, 95% CI: 1.09–2.17) (Fig. 7).
Figure 7.
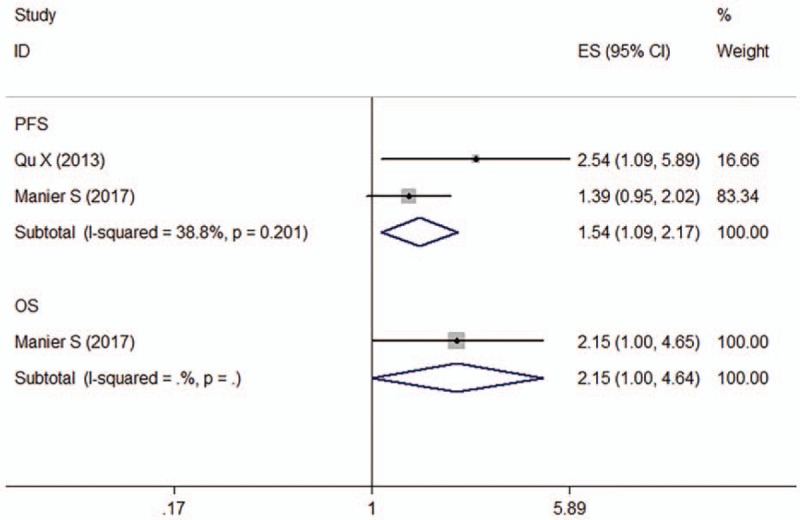
Forest plot of the HRs for the association between miR-92a and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
3.4.7. MiR-19b
Two articles determined the association between miR-19b expression and prognosis of patients with MM (n = 189), of which one provided PFS data[28] and the other OS and DFS data.[25] The observed interstudy heterogeneity for PFS was significant (I 2 = 93.9%, P = .000). The fixed-effects model revealed that miR-19b expression was not associated with PFS for MM patients (crude HR: 1.08, 95% CI: 0.75–1.56) (Fig. 8).
Figure 8.
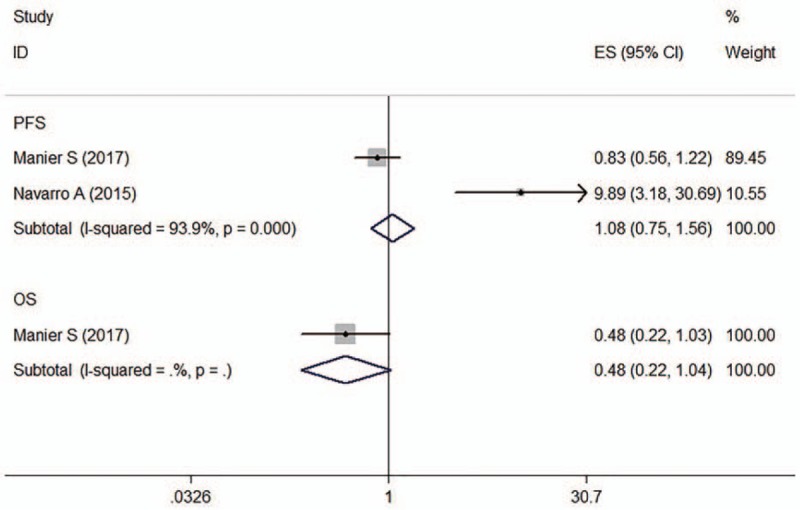
Forest plot of the HRs for the association between miR-19b and MM survival. HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
4. Discussion
A comprehensive systematic literature review was conducted to explore the utility of miRNA biomarkers that can be accurately and robustly evaluated in predicting the prognosis of patients with MM. Although various miRNAs were found to be associated with the prognosis in MM patients, most of miRNAs were assessed only in a single study. Seven miRNAs were evaluated in at least 2 studies. We, therefore, performed a meta-analysis of the effect of these 7 miRNAs on the survival of MM patients. The results of this study showed that lower expression of miR-15a, miR-16, miR-25, miR-744, and let-7e predicted worse OS in MM patients. Similarly, downregulation of miR-15a, miR-16, and miR-25 and upregulation of miR-92a were associated with shorter PFS.
MiR-15a and miR-16 are clustered at chromosomal location 13q14 and possess similar sequences. They are considered to have similar tumor suppressor functions and to be involved in the regulation of cell differentiation, proliferation, apoptosis, or angiogenesis in several types of human cancer, including MM.[31,32,33,34,35] Xu et al proved that miR-16 may serve as a potential diagnostic biomarker for MM.[36] Roccaro et al[37] identified that miR-15a/16–1 regulates the proliferation of MM cells in vitro and in vivo by inhibiting AKT serine/threonine protein kinase, ribosomal-protein-S6, MAP-kinases, and NF-kB activator MAP3KIP3. Several other studies also revealed that miR-15a/16–1 targets multiple genes that are related to cell cycle, apoptosis, and angiogenesis, such as BCL2, MCL1, CCND1, WNT3A, and VEGF.[38] MiR-15a/16 directly targets calcineurin-binding protein 1 (CABIN1) mRNA and negatively regulates its RNA and protein expression in MM cells. As a result, the downregulation of miR-15a and miR-16 promotes tumor proliferation in MM by increasing CABIN1 expression.[39]
MiR-25 is hosted by the minichromosome maintenance protein-7 gene and is transcribed as part of the mir-106b-25 polycistron.[40] MiR-25 has dual functionality, acting as either an oncogenic miRNA or a tumor suppressor. Previous studies have reported this miRNA to be downregulated in ovarian cancer[41] and upregulated in pediatric brain cancer, medulloblastomas, prostate cancer, hepatocellular cancer, gastric cancer, lung adenocarcinoma, and colorectal cancer.[42,43,44,45,46,47] Xiang et al pointed out that hsa-miR-25 is 1 of the key miRNA biomarkers that could be applied in the treatment of MM, indicating its potential function as an outcome predictor.[48]
MiR-92a, a known hypoxia-regulated miRNA, has been found to be upregulated in many cancers. The human myeloid leukemia cell line K562 secretes exosomes containing a large amount of miR-92a that enhances angiogenesis under normoxic and hypoxic conditions.[49] When it comes to hematology, miR-92a expression plays a crucial role in lymphocyte ontogeny. For instance, Husby et al identified that miR-92a is significantly differentially expressed in patients who died of mantle cell lymphoma (MCL).[50] Furthermore, miR-92a has been proven to be related to MM progression, and Qu et al identified that the effect of miR-92a on the progress of MM may involve the c-Jun pathway.[22]
MiR-744 lies in the 17p12 region, close to the TP53 gene (17p13). Deletions at chromosome 17p13.1 to 17p12 were previously found to be associated with poor prognosis[51] of MM patients. Meanwhile, low TP53 gene expression, which is highly correlated with loss of heterozygosity of the TP53 locus, was associated with shorter event-free survival and OS.[52] Levels of miR-744 and let-7e showed a significant positive correlation with thrombocyte count and albuminlevels and showed a significant negative correlation with C-reactive protein, creatinine, and beta-2microglobulinlevels.[20] Let-7 is a direct regulator of RAS expression in human cells,[53] which may explain the observation of let-7 downregulation in MM.
The results of the current study are confined by some restrictions. First, the number of studies available was limited. Second, marked heterogeneity observed in some of the analyses were likely identified due to differences in patient characteristics and assay methods, cut-off values for miRNA expression levels, follow-up durations, and HR extraction methods.
To summarize, miRNAs such as miR-16, miR-25, miR-744, miR-15a, miR-92a, and let-7e are closely related to the outcome of MM, and further studies are needed to understand the molecular mechanism underlying the effect of miRNAs in MM. Future integrated analyses of several RNA signatures will allow further characterization of biology and prognosis in relation to given therapies.
Author contributions
Conceptualization: Peipei Xu.
Data curation: Tian Xia.
Methodology: Yipeng Ling.
Software: Yipeng Ling.
Writing – original draft: Peipei Xu.
Writing – review & editing: Bing Chen.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, HR = hazard ratio, ISS = International Staging System, MCL = mantle cell lymphoma, MM = multiple myeloma, NHL = non-Hodgkin lymphoma, NOS = Newcastle-Ottawa Scale, OS = overall survival, PFS = progression free survival, R-ISS = Revised International Staging System, TTP = time to progress.
PX and TX contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (81570174), the Six Talent Peaks Project of Jiangsu Province (2018-WSN-136), the Jiangsu Provincial Medical Youth Talent (QNRC2016039), and the Technique Development Foundation of Nan Jing (JQX17057).
The authors have no conflicts of interests to disclose.
References
- [1]. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412–20. [DOI] [PubMed] [Google Scholar]
- [2]. Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol 2015;33:2863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6:857–66. [DOI] [PubMed] [Google Scholar]
- [4]. Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 2010;101:2087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Gu L, Li H, Chen L, et al. MicroRNAs as prognostic molecular signatures in renal cell carcinoma: a systematic review and meta-analysis. Oncotarget 2015;6:32545–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Frampton AE, Krell J, Jamieson NB, et al. microRNAs with prognostic significance in pancreatic ductal adenocarcinoma: A meta-analysis. Eur J Cancer 2015;51:1389–404. [DOI] [PubMed] [Google Scholar]
- [7]. Wang Z, Cai Q, Jiang Z, et al. Prognostic role of microRNA-21 in gastric cancer: a meta-analysis. Med Sci Monit 2014;20:1668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 2005;353:1793–801. [DOI] [PubMed] [Google Scholar]
- [9]. Craig VJ, Cogliatti SB, Imig J, et al. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood 2011;117:6227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Guo X, Guo L, Ji J, et al. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun 2010;398:1–6. [DOI] [PubMed] [Google Scholar]
- [11]. Li X, Zhang Y, Zhang H, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res 2011;9:824–33. [DOI] [PubMed] [Google Scholar]
- [12]. Yu S, Lu Z, Liu C, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res 2010;70:6015–25. [DOI] [PubMed] [Google Scholar]
- [13]. Manier S, Liu CJ, Avet-Loiseau H. Prognostic role of circulating exosomal miRNAs in multiple myeloma 2017;129:2429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Luo X, Gu J, Zhu R, et al. Integrative analysis of differential miRNA and functional study of miR-21 by seed-targeting inhibition in multiple myeloma cells in response to berberine. BMC Syst Biol 2014;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [16]. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Maxwell L, Santesso N, Tugwell PS, et al. Method guidelines for Cochrane Musculoskeletal Group systematic reviews. J Rheumatol 2006;33:2304–11. [PubMed] [Google Scholar]
- [18]. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [19]. Rocci A, Hofmeister CC, Geyer S, et al. Circulating miRNA markers show promise as new prognosticators for multiple myeloma. Leukemia 2014;28:1922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Kubiczkova L, Kryukov F, Slaby O, et al. Circulating serum microRNAs as novel diagnostic and prognostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. Haematologica 2014;99:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Li F, Hao M, Feng X, et al. Downregulated miR-33b is a novel predictor associated with disease progression and poor prognosis in multiple myeloma. Leuk Res 2015;39:793–9. [DOI] [PubMed] [Google Scholar]
- [22]. Qu X, Zhang S, Wu S, et al. Expression level of microRNA-92a and its clinical significance in multiple myeloma patients. Chin J Hematol 2013;34:332–6. [DOI] [PubMed] [Google Scholar]
- [23]. Hao M, Zang M, Wendlandt E, et al. Low serum miR-19a expression as a novel poor prognostic indicator in multiple myeloma. Int J Cancer 2015;136:1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Li F, Xu Y, Deng S, et al. MicroRNA-15a/16-1 cluster located at chromosome 13q14 is down-regulated but displays different expression pattern and prognostic significance in multiple myeloma. Oncotarget 2015;6:38270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Manier S, Liu CJ, Avet-Loiseau H, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood 2017;129:2429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Seckinger A, Meissner T, Moreaux J, et al. miRNAs in multiple myeloma – a survival relevant complex regulator of gene expression. Oncotarget 2015;6:39165–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Hao M, Zang M, Zhao L, et al. Serum high expression of miR-214 and miR-135b as novel predictor for myeloma bone disease development and prognosis. Oncotarget 2016;7:19589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Navarro A, Diaz T, Tovar N, et al. A serum microRNA signature associated with complete remission and progression after autologous stem-cell transplantation in patients with multiple myeloma. Oncotarget 2015;6:1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Qu X-y, Zhang S-s, Wu S, et al. Expression level of microRNA-92a and its clinical significance in multiple myeloma patients. Zhonghua Xue Ye Xue Za Zhi 2013;34:332–6. [DOI] [PubMed] [Google Scholar]
- [31]. Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002;99:15524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Huang E, Liu R, Chu Y. miRNA-15a/16: as tumor suppressors and more. Future Oncol 2015;11:2351–63. [DOI] [PubMed] [Google Scholar]
- [33]. Kang W, Tong JH, Lung RW, et al. Targeting of YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor function in gastric adenocarcinoma. Mol Cancer 2015;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Sun CY, She XM, Qin Y, et al. miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis 2013;34:426–35. [DOI] [PubMed] [Google Scholar]
- [35]. Tung YT, Huang PW, Chou YC, et al. Lung tumorigenesis induced by human vascular endothelial growth factor (hVEGF)-A165 overexpression in transgenic mice and amelioration of tumor formation by miR-16. Oncotarget 2015;6:10222–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Xu YN, Xiao CR, Huang YD, et al. Circulating Serum MicroRNA as Diagnostic Biomarkers for Multiple Myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25:471–5. [DOI] [PubMed] [Google Scholar]
- [37]. Roccaro AM, Sacco A, Thompson B, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood 2009;113:6669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 2010;17:215–20. [DOI] [PubMed] [Google Scholar]
- [39]. Zhang L, Zhou L, Shi M, et al. Downregulation of miRNA-15a and miRNA-16 promote tumor proliferation in multiple myeloma by increasing CABIN1 expression. Oncol Lett 2018;15:1287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Poliseno L, Salmena L, Riccardi L, et al. Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 2010;3:ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Meng X, Joosse SA, Muller V, et al. Diagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patients. Br J Cancer 2015;113:1358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Birks DK, Barton VN, Donson AM, et al. Survey of MicroRNA Expression in Pediatric Brain Tumors. Pediatr Blood Cancer 2011;56:211–6. [DOI] [PubMed] [Google Scholar]
- [43]. Dacic S, Kelly L, Shuai Y, et al. miRNA expression profiling of lung adenocarcinomas: correlation with mutational status. Mod Pathol 2010;23:1577–82. [DOI] [PubMed] [Google Scholar]
- [44]. Li X, Yang C, Wang X, et al. The expression of miR-25 is increased in colorectal cancer and is associated with patient prognosis. Med Oncol 2014;31:781. [DOI] [PubMed] [Google Scholar]
- [45]. Li Y, Tan W, Neo TWL, et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci 2009;100:1234–42. [DOI] [PubMed] [Google Scholar]
- [46]. Petrocca F, Visone R, Onelli MR, et al. E2F1-regulated microRNAs impair TGF beta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell 2008;13:272–86. [DOI] [PubMed] [Google Scholar]
- [47]. Poliseno L, Salmena L, Riccardi L, et al. Identification of the miR-106b similar to 25 MicroRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 2010;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Xiang T, Hu A-X, Sun P, et al. Identification of four potential predicting miRNA biomarkers for multiple myeloma from published datasets. PeerJ 2017;5:e2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Ohyashiki JH, Umezu T, Ohyashiki K. Exosomes promote bone marrow angiogenesis in hematologic neoplasia: the role of hypoxia. Curr Opin Hematol 2016;23:268–73. [DOI] [PubMed] [Google Scholar]
- [50]. Husby S, Ralfkiaer U, Garde C, et al. miR-18b overexpression identifies mantle cell lymphoma patients with poor outcome and improves the MIPI-B prognosticator. Blood 2015;125:2669–77. [DOI] [PubMed] [Google Scholar]
- [51]. Carrasco DR, Tonon G, Huang YS, et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell 2006;9:313–25. [DOI] [PubMed] [Google Scholar]
- [52]. Xiong W, Wu X, Starnes S, et al. An analysis of the clinical and biologic significance of TP53 loss and the identification of potential novel transcriptional targets of TP53 in multiple myeloma. Blood 2008;112:4235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 MicroRNA family. Cell 2005;120:635–47. [DOI] [PubMed] [Google Scholar]


