Abstract
Background and aims:
There is currently no consensus regarding the influence of tumor necrosis on the prognosis of gastrointestinal stromal tumors (GISTs). Therefore, we conducted a meta-analysis to determine the prognostic role of tumor necrosis in patients with GIST.
Methods:
PubMed, Embase, and Web of Science electronic databases were searched from their inception to March 2018. Studies reporting data on the relationship between tumor necrosis and GIST prognosis were eligible. The measure of the effect of interest was the odds ratios (ORs) with 95% confidence intervals (CIs). This study has been registered in the Prospero (number CRD42018096036).
Results:
In total, 18 studies including 2320 patients were identified. The total odds of tumor necrosis were associated with a poor GIST prognosis (OR = 5.54, 95% CI = 4.39–6.99). Subgroup analysis of different observed outcomes indicated that tumor necrosis was associated with a decreased disease-free survival (OR = 7.08, 95% CI = 4.78–10.49), recurrence-free survival (OR = 3.96, 95% CI = 2.48–6.32), and overall survival (OR = 4.29, 95% CI = 2.02–9.13). In addition, any tumor site, tumor size, follow-up time, ethnicity, different outcomes of GIST, and different degrees of positive staining of immunohistochemical markers subgroups showed a significantly increased risk of a poor prognosis.
Conclusions:
Tumor necrosis may likely predict a poorer prognosis for GIST. However, further well-designed prospective studies with large sample size are required in the future.
Keywords: gastrointestinal stromal tumor, prognosis, tumor necrosis
1. Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor that arises from the gastrointestinal (GI) tract,[1,2] probably originating from the interstitial cells of Cajal. Activating mutations of kinase inhibitor tyrosine (KIT) and less commonly platelet-derived growth factor receptor alpha (PDGFRA) are believed to be pivotal in the molecular pathogenesis of GIST.[3,4] One of the most predominant characteristics of GIST is its malignant potential varying from small lesions with benign behavior to aggressive sarcoma.[5] About 40% tumors in patients with GIST that were localized at the time of detection give rise to metastasis, and 10% to 20% of patients present with overt metastasis.[6,7] To estimate the malignant potential of GIST, several criteria have been proposed to predict the outcome of patients with GIST, including classifications from National Institutes of Health (NIH), the modified NIH, the Armed Forces Institute of Pathology.[8–11] However, even the prognosis of patients according to risk classification can vary. So far, there remains no consensus on the potential factors influencing the prognosis of patients with GIST, excepting for mitotic count, tumor size, tumor site, and tumor rupture, which are included in the existing prognostic criteria. The identification of independent and reproducible prognostic factors may affect therapeutic decisions and influence the performance of clinical work. Other factors such as genotype,[12] immune infiltrates,[13] positive surgical margins,[14] and tumor necrosis may play an important role in the prognosis.
Tumor necrosis, a distinct type of cell death, is usually associated with abnormal processes such as exposure to various toxins or teratogens, infections, trauma, and ischemia.[15] Tumor necrosis was shown to be an independent prognostic factor of soft tissue sarcoma early in 1984 by Costa et al[16] and Trojani et al[17] and was later included in the National Cancer Institute grading and the French Federation of Cancer Centers Sarcoma Group gradings. Trojani et al[17] showed that tumor differentiation, mitosis count, and tumor necrosis were necessary and sufficient to retain all the prognostic information in soft tissue sarcoma in a multivariate analysis. Costa et al[16] reported tumor necrosis to be the single best histopathologic parameter to predict the time to recurrence and the overall survival (OS) of patients with soft tissue sarcoma, as well as the clinical course after recurrence independent of patient age and sex and tumor location and size.
In recent years, studies on the possible prognostic factors for GIST reported that tumor necrosis might independently influence the recurrence or survival of patients with GIST.[18–21] However, other studies reported contradictory findings.[22,23] The discordance may be due to small sample sizes and different characteristics among studies. Since GIST is a soft tissue sarcoma,[1] the ability of tumor necrosis to predict the outcome of patients with GIST remained controversial as a result of the inconsistent results of published studies. Thus, tumor necrosis may be a significant prognostic factor for GIST. Therefore, we conducted a meta-analysis to clarify the relationship between tumor necrosis and GIST prognosis.
2. Methods
2.1. Search strategy and selection criteria
Two investigators independently performed a systematic search of the PubMed, Embase, and Web of Science databases (last updated on March 2018), using the following search terms: “gist,” “gists,” “gastrointestinal stromal tumor,” “gastrointestinal stromal tumors” combined with “necrosis.” The articles cited in selected articles were also examined to identify additional relevant studies. All studies were carefully evaluated to avoid duplicate data. We included published studies that reported the correlation between tumor necrosis and the prognosis of GIST. The criteria used for the study selection were as follows: participants (P): all patients were required to have morphology compatible with GIST and positive immunostaining for KIT (CD117) or PDGFRA (discovered on GIST-1 [DOG-1]). Recent research showed the PKCθ sensitivity is similar to CD117 and superior to DOG1 sensitivity.[24] Thus, the expression of PKCθ in CD117/DOG1 negative GISTs was added to our diagnostic criteria. Interventions (I) and comparisons (C): comparing the prognosis of GIST with necrosis versus GIST without necrosis. Outcomes (O): recurrence-free survival (RFS), disease-free survival (DFS), OS, and risk classification (high risk level) by the modified NIH. Study design (S): retrospective or prospective study. Enough data for the estimation of odds ratios (ORs) and 95% confidence intervals (CIs). We excluded articles if they were not published in English, did not include sufficient information to calculated ORs and 95% CIs and failed to report a prognosis.
2.2. Data extraction and qualitative assessment
Two investigators independently extracted the required data from all eligible studies. Discrepancies between the 2 investigators were resolved by discussion or consensus with a senior investigator. Descriptive and quantitative data were extracted from each study for the following: first author, year of study recruitment, nation, sample size, tumor site, tumor size, male to female ratio, mean patient ages, percentage of GIST with tumor necrosis, mean follow-up time, and outcomes of patients with tumor necrosis-positive GIST. The measure of the effect of interest was the OR with 95% CI, which were estimated according to the available data if they could not be directly acquired in the included articles. Qualitative assessment was performed for the included articles using the Newcastle–Ottawa Quality Assessment Scale for case–control studies.
2.3. Statistical analysis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) check list was used as a protocol and guideline for the meta-analysis. To evaluate the prognostic value of tumor necrosis, we extracted or calculated the ORs and corresponding 95% CI for the clinical outcomes observed in the eligible studies. Data on the predictive ability of tumor necrosis were combined across the eligible studies by inverse variance using ORs. A fixed-effects model was used to estimate the pooled ORs and 95% CIs. An OR >1 suggested a worse prognosis for GIST with tumor necrosis. Chi-square-based Q tests were used for checking the heterogeneity assumption, in which a P > .10 indicated a lack of heterogeneity among studies. The effect of heterogeneity was quantified using I2 tests. I2 values of <25%, about 50%, >75%, respectively, were considered “low,” “moderate,” and “high.” Subgroup analysis was performed for observed clinical outcomes, tumor size, tumor site, follow-up time, and patient ethnicity. Sensitivity analysis was conducted to determine whether exclusion of any studies affected the results. The effect of publication bias on the reported outcomes was assessed graphically by both Egger's and Begg's tests. A P < .05 was considered significant statistically. All P values in this study were 2-sided.
3. Results
3.1. Baseline study characteristics
The initial searches included 411 records. After further review, 63 articles were assessed for eligibility. Of these, 45 articles were excluded due to insufficient data. Among them, the full text was not available for 8 articles and 37 articles were excluded because they did not include necessary direct or indirect data. The screening process is shown in Figure 1. A total of 18 studies were included in the meta-analysis.[18–23,25–36]
Figure 1.
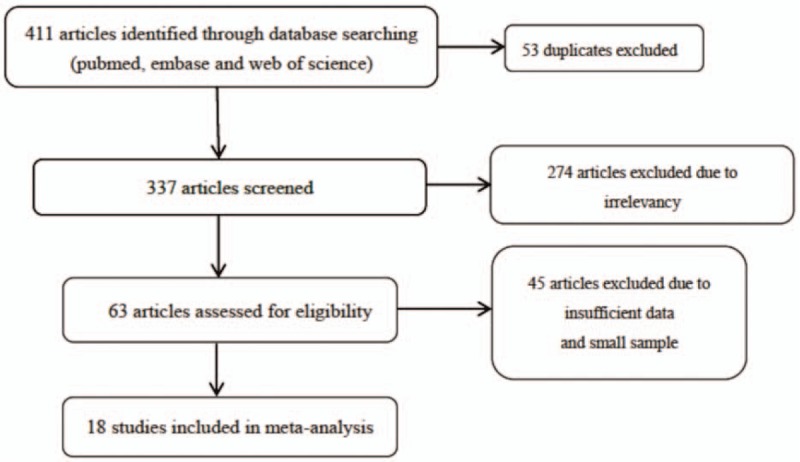
Flow chart of study selection.
The characteristics of included studies are shown in Table 1. In total, 2320 patients were included in the analysis; of these, 792 patients had GIST with tumor necrosis. All eligible articles were published between 1998 and 2017. The prevalence tumor necrosis positivity rate ranged between 15.0% and 76.9%. Among the clinical outcomes observed in patients with GIST, DFS was observed in 8 studies, RFS in 5 studies, and OS in 2 studies. Two studies described the outcomes using risk classification (high risk) by the modified NIH and 1 study used the biologic aggressiveness score as the prognostic criterion for GIST. For tumor sites, 3 articles focused on gastric, 4 on small intestine, 1 on the rectum, and 1 on GIST out of GI tract (extra GI). Among all the 18 studies, 4 were carried out in the USA, 6 in China, 2 in Korea, 2 in Japan, 1 in Switzerland, 1 in Italy, 1 in Singapore, and 1 in India. The mean follow-up time ranged from 24 months to 69 months. The mean tumor size was ≤5 cm, 5 to 10 cm, and >10 cm in 3, 8, and 2 studies. The quality of the studies assessed by the Newcastle–Ottawa Quality Assessment Scale ranged from 5 to 8, with scores of 5 in 2 articles, 6 in 5 articles, 7 in 7 articles, and 8 in 4 articles.
Table 1.
Main characteristics of the included studies.

3.2. Meta-analysis and subgroup analysis
In the pooled analysis of all 18 studies, the meta-analysis revealed that GIST with tumor necrosis had a significantly poorer prognosis than that in GIST without tumor necrosis (OR = 5.54, 95% CI = 4.39–6.99, P < .001) (Fig. 2). This finding indicated a lack of heterogeneity in the pooled analysis (I2 = 6.2%, Pheterogeneity = .38). Subgroup analysis was conducted to assess the influence of different outcomes observed in studies, patient ethnicity, follow-up time, tumor site, and tumor size, as shown in Table 2. DFS, RFS, and OS were reported in different studies. Moreover, (high) risk classification according to NIH 2008 classification was also used as an indicator for the prognosis of GIST. Studies describing DFS (OR = 7.08, 95% CI = 4.78–10.49, I2 = 0.0%), RFS (OR = 3.96, 95% CI = 2.48–6.32, I2 = 0.0%), OS (OR = 4.29, 95% CI = 2.02–9.13, I2 = 0.0%) indicated that tumor necrosis was significantly associated with a reduced DFS, RFS, and OS. Among them, tumor necrosis was most related to a reduced DFS, least to RFS, and OS was between them. Tumor necrosis was also related to a high risk according to the NIH 2008 classification (OR = 8.11, 95% CI = 5.04–13.06, I2 = 0.0%). Mean tumor size was evaluated by 13 articles. Studies where mean tumor size was ≤5 cm (OR = 9.28, 95% CI = 3.29–26.18, I2 = 0.0%), 5 to 10 cm (OR = 4.23, 95% CI = 2.78–6.43, I2 = 16.2%), or >10 cm (OR = 5.64, 95% CI = 2.32–13.75, I2 = 0.0%) indicated that tumor necrosis could predict a poor prognosis for GIST of any tumor size. However, for tumors ≤5 cm, the impact of necrosis on GIST prognosis was greater than that of tumors ranging from 5 cm to 10 cm and tumors >10 cm. Studies on GIST at any site (OR = 5.14, 95% CI = 3.66–7.22, I2 = 0.0%,), GI tract (OR = 6.48, 95% CI = 2.98–14.09, I2 = 50.6%), gastric (OR = 7.41, 95% CI = 4.67–11.77, I2 = 0.0%), small intestine (OR = 3.75, 95% CI = 2.07–6.79, I2 = 29.6%) indicated that tumor necrosis could predict a poor prognosis for GIST at any site. In addition, the risk of poor prognosis for gastric GIST with tumor necrosis was higher than that of GIST of small intestine. Due to the lack of reports on the rectum and extra GI, 2 studies were not included the subgroup analysis. Mean follow-up times ranging from 24 months to 36 months were found in 7 studies and >36 months in 3 studies. Analysis of follow-up times of 24 to 36 months (OR = 5.54, 95% CI = 3.84–7.97, I2 = 40.1%) and >36 months (OR = 6.14, 95% CI = 4.01–9.42, I2 = 28.7%) indicated a poorer outcome for GIST with a follow-up time >24 months; with increasing follow-up time, the impact of tumor necrosis on GIST also increased. Two ethnicities were included in the pooled analysis: 12 included Asian and Pacific Islands patients, while 6 studies included Caucasian patients. Tumor necrosis predicted a poor prognosis for GIST in both the Asian and Pacific (OR = 5.86, 95% CI = 4.54–7.57, I2 = 0.0%) and Caucasian (OR = 4.25, 95% CI = 2.43–7.42, I2 = 35.3%) groups. Subgroup analysis of these ethnicities revealed that people in Asian and Pacific Islanders who had GIST with tumor necrosis may have a worse outcome than that of Caucasians. Moreover, subgroup analyses on immunohistochemical staining showed a higher impact of tumor necrosis to prognosis of GIST in the groups where a higher rate of positive immunohistochemical staining was found for CD117 (>90%) (OR = 6.23, 95% CI = 4.22–9.19, I2 = 0.0%), CD34 (>50%) (OR = 6.86, 95% CI = 4.52–10.40, I2 = 0.0%), and S100 (>20%) (OR = 6.00, 95% CI = 3.27–15.15, I2 = 45%). Contrary results were found in subgroup of lower rate of immunohistochemical staining for smooth muscle actin (SMA) (≤30%) (OR = 6.82, 95% CI = 2.71–17.19, I2 = 33%).
Figure 2.

Pooled ORs on the association of tumor necrosis with GIST prognosis. GIST = gastrointestinal stromal tumor, OR = odds ratio.
Table 2.
Subgroup analysis.

3.3. Sensitivity analysis
To assess whether any 1 study had a dominating effect on the summary effect size or heterogeneity, each study was excluded and repeated analyses were conducted. The pooled ORs were not significantly influenced by the omission of any single study.
3.4. Publication bias
Egger's test revealed no evidence of publication bias (Fig. 3). The shape of the funnel plots showed no obvious asymmetries.
Figure 3.
Funnel pots by Egger's test.
4. Discussion
Due to the sensitivity of selective imatinib therapy and common tumor recurrence after complete surgical resection,[37,38] identifying the significant prognostic factors is important for individualized risk classification. The influence of tumor necrosis on the prognosis of GIST has been demonstrated in recent years. However, literature regarding the relationship between tumor necrosis and GIST prognosis is inconclusive. The present meta-analysis demonstrated that the presence of tumor necrosis, as part of either preoperative computed tomography (CT) or pathologic findings, predict a poorer prognosis for GIST regardless of tumor site, tumor size, follow-up time, or patient ethnicity. It may enable clinicians to generate more accurate schemes to determine individual imatinib therapy.
Subgroup analysis of different outcomes indicated that tumor necrosis could influence the DFS, OS, RFS, and the risk classification of GIST (by NIH 2008). Tumor necrosis was most related to DFS, RFS was least related, and OS was between them. Patients with GIST with tumor necrosis had an approximately 7-fold increased risk of disease progression, including disease metastasis, recurrence, and death, and 4-fold increased risk of recurrence compared with those in patients with GIST without tumor necrosis. In addition, subgroup analysis indicated that tumor necrosis was most related to the NIH 2008 risk classifications. Thus, tumor necrosis could be used as a potential factor to distinguish between high-risk GIST and non-high-risk GIST; however, this requires confirmation in future studies. In the subgroup meta-analysis on tumor size, the impact of tumor necrosis to GIST prognosis for tumors <5 cm was higher than that of tumors >5 cm, which means in GIST <5 cm, the prognostic role of tumor necrosis needs more attention. Liu et al[20] reported that tumor necrosis was associated with a larger tumor size (P < .01), a higher mitotic count, tumor rupture, and the presence of nuclear atypia. As larger tumor size, especially >10 cm, indicates aggressive behavior, the impact of tumor necrosis on the GIST prognosis may probably be weakened, which leads to increased awareness of tumor necrosis for tumors <5 cm. In the present study, no correlation between tumor necrosis and mitotic count, tumor rupture and nuclear atypia were identified due to the lack of corresponding information. Further studies are needed to clarify the relationship between tumor necrosis and other predictors. In subgroup analysis of tumor sites, GIST at any site with tumor necrosis had a poorer prognosis than that of tumor necrosis-negative GIST. However, compared to that in small intestine GIST, the impact of tumor necrosis in gastric GIST was greater, suggesting that tumor necrosis in gastric GIST may need more attention. Subgroup analysis also revealed that a longer follow-up time (>36 months) could increase the risk of a poorer prognosis in patients with tumor necrosis-positive GIST. In a study including 2459 patients, Joensuu et al[5] reported estimated 5 and 15-year RFS rates for GIST treated with surgery alone of 70.5% and 59.9%, respectively, indicating that a short follow-up time could lead to underestimates regarding the poor clinical outcome of patients with GIST. This could explain the result of this subgroup analysis. Subgroup analysis of patient ethnicity indicated an increased impact of tumor necrosis on the prognosis of GIST among Asian and Pacific Islander patients compared to that in Caucasians (ORs: 5.86 vs. 4.25). This difference may be caused by the inconsistent level of medical care service between the 2 regions. Moreover, we found a higher impact of tumor necrosis to the prognosis of GIST in the groups with higher rate of positive immunohistochemical staining of CD117, CD34, S100, and in the group with lower rate of SMA (+). Because of limited data, other important markers such as DOG1, protein kinase C-theta, and programmed death ligand (PDL)-1 were not analyzed in this meta-analysis. The reason for this observation remained unclear. In another research by Blakely et al,[39] expression of PDL-1 was associated with tumor necrosis, as well as tumor behavior and clinical outcomes of various tumor types, which may reveal a potential correlation between tumor necrosis and other immunohistochemical markers.
Tumor necrosis, characterized by the presence of dead cells in the form of anucleate “ghost cells” with preservation of the tissue architecture, has been established as a prognostic factor for a variety of malignancies. Sengupta et al[40] reported that tumor necrosis was retained as an independent predictor of outcome for clear cell and chromophobe renal cell carcinoma and suggested that it be incorporated into prognostic models for more accurate risk estimation. In the report by Hiraoka et al[41] including 348 patients with pancreatic duct carcinoma, histologic necrosis was a simple, accurate, and reproducible predictor of postoperative outcome. In another study on colorectal cancer based on 343 patients, tumor necrosis was associated with cancer-specific survival. However, the impact of tumor necrosis on colorectal cancer may be due to its close associations with the host systemic and local inflammatory responses.[42] In addition, a review by Gkogkou et al[43] also reported tumor necrosis to be an independent prognostic factor affecting therapeutic decisions in nonsmall cell lung carcinoma.
The mechanisms by which tumor necrosis results in a poor prognosis in GIST are still unclarified. One hypothesis is that rapid cell proliferation outgrowing the vasculature leads to hypoxic conditions in tumors, causing subsequent tumor cell death and promoting metastatic cascade.[44,45] The presence of necrosis histologically reflects intratumoral hypoxia, which is a common feature of human cancers.[41] Areas of hypoxic tumor tissue are resistant to treatment and associated with a poor clinical prognosis due to the capacity of hypoxia to drive genomic instability and alter DNA damage repair pathways.[46] Hypoxia induces a transcription program mediated by hypoxia-introducible factor-1α, which could promote aggressive tumor phenotypes.[47] In addition, tumor necrosis is directly associated with both an attenuation of local infiltration of inflammatory cells and the presence of systematic inflammatory response.[48] Inflammatory processes including the local accumulation of products of cyclooxygenase activity[49] and the local production of nitric oxide[50] could promote cell proliferation and death at the sites of inflammation, which are related to hypermethylation of the promoter regions in tumor-suppressor and proapoptotic genes.[51] Following the acquisition of genetic limitations in apoptotic pathways, the resultant increase in necrotic cell death leads to the release of cellular contents, which in turn promote cell growth and cancer progression.[52] Coagulative necrosis within the primary tumor may comprise the tumor vasculature, thereby facilitating the systematic dissemination of malignant cells.[53]
This study has several limitations. First, a limited number of studies were included in this meta-analysis due to the lack of relevant studies. Second, in other studies, tumor necrosis was categorized as absent; minimal (necrotic areas not exceeding 15% of the tumor); moderate (necrotic tissue 15%–50% of the tumor); and massive (necrosis over 50% of the tumor).[16] The definition of tumor necrosis was not clear for each of the included studies and the relationship between the degree of necrosis and GIST prognosis was not analyzed in this meta-analysis. Third, previous studies have showed a significant relation between prognosis of GIST and tumor size, tumor site, mitotic count, tumor rupture, microenvironment,[39] and some blood parameters such as combination of high neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, neutrophil-to-white blood cell ratio, and low lymphocyte-to-white blood cell ratio.[54] However, because of limited data (the included original articles only showed mean tumor size and mean mitotic count), the exact relationship between tumor necrosis and other predictors such as mitotic count and tumor size were not assessed in this study. Correlation between tumor necrosis and blood parameters was not assessed either. Further studies of large sample sizes are needed to identify whether tumor necrosis is an independent factor for a poor GIST prognosis. A fourth limitation was that we could not obtain information regarding the main confounders from most of the studies, especially the main known predictors for GIST such as mitosis, tumor size, and tumor site. We could only extract the adjustments for 6 of 18 studies as shown in Table 1. Therefore, this result should be considered with some caution according to potential confounding. Finally, the definitions of the oncological outcome were not consistent between the 18 articles. Therefore, we conducted subgroup analysis, which indicated that tumor necrosis was associated with decreased DFS, RFS, as well as OS. Besides these, the results of our study may help to define the prognostic role of necrosis in GIST and may be useful in clinical work, especially in clinical consultation.
In conclusion, the presence of tumor necrosis, as part of either preoperative CT or pathologic findings, could predict a significantly poorer prognosis in patients with GIST. In addition, the presence of tumor necrosis present in GIST could predict a poor DFS, OS, and RFS for GIST regardless of tumor site, tumor size, follow-up time, or patient ethnicity. In addition, the value of tumor necrosis in risk classification (predicting a high risk level) in the NIH 2008 classification may make it an important factor to distinguish between high risk GIST and non-high risk GIST.
Author contributions
Y.Z., M.Y., and L.X. conceived together the study. All authors contributed to the research and development process that resulted in this article. M.Y. and L.X.; Y.Z., X.W., W.Z., Y.C., R.Z., and Q.W.; and L.D. performed data extraction, analysis, and interpretation of data. M.Y. wrote the manuscript under the guidance of Y.Z. All of authors read the manuscript and approved the final manuscript.
Conceptualization: Mengshi Yi, Wen Zhuang, Yong Zhou.
Data curation: Mengshi Yi, Lin Xia, Wen Zhuang, Rui Zhao, Qianyi Wan, Liang Du.
Formal analysis: Mengshi Yi, Lin Xia, Yan Zhou, Wen Zhuang, Rui Zhao, Qianyi Wan, Liang Du, Yong Zhou.
Funding acquisition: Yong Zhou.
Investigation: Mengshi Yi, Xiaoting Wu, Wen Zhuang, Rui Zhao, Qianyi Wan, Yong Zhou.
Methodology: Mengshi Yi, Lin Xia, Yan Zhou, Xiaoting Wu, Wen Zhuang, Yi Chen, Rui Zhao, Yong Zhou.
Project administration: Mengshi Yi, Lin Xia, Yi Chen, Yong Zhou.
Resources: Mengshi Yi, Lin Xia, Rui Zhao, Qianyi Wan.
Software: Mengshi Yi, Qianyi Wan.
Supervision: Mengshi Yi, Rui Zhao, Qianyi Wan, Liang Du, Yong Zhou.
Validation: Mengshi Yi, Lin Xia, Yan Zhou, Wen Zhuang, Yi Chen, Rui Zhao, Liang Du, Yong Zhou.
Visualization: Mengshi Yi, Xiaoting Wu, Wen Zhuang, Yi Chen, Liang Du, Yong Zhou.
Writing – original draft: Mengshi Yi.
Writing – review & editing: Mengshi Yi, Yong Zhou.
Yong Zhou orcid: 0000-0002-0214-8151.
Footnotes
Abbreviations: BA = biologic aggressiveness, CI = confidence interval, CT = computed tomography, DFS = disease-free survival, GI = gastrointestinal, GIST = gastrointestinal stromal tumor, KIT = kinase inhibitor tyrosine, M/F = male/female ratio, NIH = National Institutes of Health, OR = odds ratio, OS = overall survival, PDGFRA = platelet-derived growth factor receptor alpha, PRISMA = The Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RFS = recurrence-free survival.
MY and LX are co-first authors.
This work was supported by Chinese Medical Board Grant on Evidence-Based Medicine, New York, USA (No. 98–680), National Natural Science Foundation of China (No. 30901427), and Sichuan Provincial Science and Technology Support Project (2016SZ0047).
The study protocol was approved by the ethics committee of West China Hospital, Sichuan University. The analysis did not involve interaction with human subjects or use personal identifying information. The methods were performed in accordance with the approved guidelines.
The authors have no conflicts of interest to disclose.
References
- [1].Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973–83. [DOI] [PubMed] [Google Scholar]
- [2].Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466–78. [DOI] [PubMed] [Google Scholar]
- [3].Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–80. [DOI] [PubMed] [Google Scholar]
- [4].Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708–10. [DOI] [PubMed] [Google Scholar]
- [5].Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265–74. [DOI] [PubMed] [Google Scholar]
- [6].Woodall CE, 3rd, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 2009;144:670–8. [DOI] [PubMed] [Google Scholar]
- [7].Emile JF, Brahimi S, Coindre JM, et al. Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs. Med Oncol 2012;29:1765–72. [DOI] [PubMed] [Google Scholar]
- [8].Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459–65. [DOI] [PubMed] [Google Scholar]
- [9].Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52–68. [DOI] [PubMed] [Google Scholar]
- [10].Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery 2007;141:748–56. [DOI] [PubMed] [Google Scholar]
- [12].Wozniak A, Rutkowski P, Schoffski P, et al. Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a European multicenter analysis based on ConticaGIST. Clin Cancer Res 2014;20:6105–16. [DOI] [PubMed] [Google Scholar]
- [13].Rusakiewicz S, Semeraro M, Sarabi M, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res 2013;73:3499–510. [DOI] [PubMed] [Google Scholar]
- [14].Park CK, Lee EJ, Kim M, et al. Prognostic stratification of high-risk gastrointestinal stromal tumors in the era of targeted therapy. Ann Surg 2008;247:1011–8. [DOI] [PubMed] [Google Scholar]
- [15].Proskuryakov SY, Gabai VL. Mechanisms of tumor cell necrosis. Curr Pharm Des 2010;16:56–68. [DOI] [PubMed] [Google Scholar]
- [16].Costa J, Wesley RA, Glatstein E, et al. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer 1984;53:530–41. [DOI] [PubMed] [Google Scholar]
- [17].Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37–42. [DOI] [PubMed] [Google Scholar]
- [18].Yoo C, Koh YW, Park YS, et al. Prognostic relevance of p53 overexpression in gastrointestinal stromal tumors of the small intestine: potential implication for adjuvant treatment with imatinib. Ann Surg Oncol 2015;22Suppl 3:S362–9. [DOI] [PubMed] [Google Scholar]
- [19].Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu X, Qiu H, Zhang P, et al. Prognostic role of tumor necrosis in patients undergoing curative resection for gastric gastrointestinal stromal tumor: a multicenter analysis of 740 cases in China. Cancer Med 2017;6:2796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goh BK, Chow PK, Yap WM, et al. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol 2008;15:2153–63. [DOI] [PubMed] [Google Scholar]
- [22].Bucher P, Taylor S, Villiger P, et al. Are there any prognostic factors for small intestinal stromal tumors? Am J Surg 2004;187:761–6. [DOI] [PubMed] [Google Scholar]
- [23].Yokoi K, Tanaka N, Shoji K, et al. A study of histopathological assessment criteria for assessing malignancy of gastrointestinal stromal tumor, from a clinical standpoint. J Gastroenterol 2005;40:467–73. [DOI] [PubMed] [Google Scholar]
- [24].Kovecsi A, Jung I, Szentirmay Z, et al. PKCtheta utility in diagnosing c-KIT/DOG-1 double negative gastrointestinal stromal tumors. Oncotarget 2017;8:55950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fujimoto Y, Nakanishi Y, Yoshimura K, et al. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 2003;6:39–48. [DOI] [PubMed] [Google Scholar]
- [26].Zhou C, Duan X, Zhang X, et al. Predictive features of CT for risk stratifications in patients with primary gastrointestinal stromal tumour. Eur Radiol 2016;26:3086–93. [DOI] [PubMed] [Google Scholar]
- [27].Yang TH, Hwang JI, Yang MS, et al. Gastrointestinal stromal tumors: computed tomographic features and prediction of malignant risk from computed tomographic imaging. J Chin Med Assoc 2007;70:367–73. [DOI] [PubMed] [Google Scholar]
- [28].Yan H, Marchettini P, Acherman YI, et al. Prognostic assessment of gastrointestinal stromal tumor. Am J Clin Oncol 2003;26:221–8. [DOI] [PubMed] [Google Scholar]
- [29].Vij M, Agrawal V, Kumar A, et al. Evaluation of biologic potential and risk stratification for predicting disease-free survival after resection of primary gastrointestinal stromal tumor: a multivariate clinicopathological study. Indian J Cancer 2015;52:351–7. [DOI] [PubMed] [Google Scholar]
- [30].Vasconcelos RN, Dolan SG, Barlow JM, et al. Impact of CT enterography on the diagnosis of small bowel gastrointestinal stromal tumors. Abdom Radiol 2017;42:1365–73. [DOI] [PubMed] [Google Scholar]
- [31].Reith JD, Goldblum JR, Lyles RH, et al. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol 2000;13:577–85. [DOI] [PubMed] [Google Scholar]
- [32].Qi Y, Zhao W, Wang Z, et al. Tumor sites and microscopic indicators are independent prognosis predictors of gastrointestinal stromal tumors. Tohoku J Exp Med 2014;233:65–72. [DOI] [PubMed] [Google Scholar]
- [33].Lv A, Li Z, Tian X, et al. SKP2 high expression, KIT exon 11 deletions, and gastrointestinal bleeding as predictors of poor prognosis in primary gastrointestinal stromal tumors. PloS One 2013;8:e62951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dong C, Jun-Hui C, Xiao-Jun Y, et al. Gastrointestinal stromal tumors of the rectum: clinical, pathologic, immunohistochemical characteristics and prognostic analysis. Scand J Gastroenterol 2007;42:1221–9. [DOI] [PubMed] [Google Scholar]
- [35].Chiappa A, Zbar AP, Innis M, et al. Prognostic factors affecting survival after surgical resection of gastrointestinal stromal tumours: a two-unit experience over 10 years. World J Surg Oncol 2006;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chang MS, Choe G, Kim WH, et al. Small intestinal stromal tumors: a clinicopathologic study of 31 tumors. Pathol Int 1998;48:341–7. [DOI] [PubMed] [Google Scholar]
- [37].Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Valsangkar N, Sehdev A, Misra S, et al. Current management of gastrointestinal stromal tumors: surgery, current biomarkers, mutations, and therapy. Surgery 2015;158:1149–64. [DOI] [PubMed] [Google Scholar]
- [39].Blakely AM, Matoso A, Patil PA, et al. Role of immune microenvironment in gastrointestinal stromal tumours. Histopathology 2018;72:405–13. [DOI] [PubMed] [Google Scholar]
- [40].Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer 2005;104:511–20. [DOI] [PubMed] [Google Scholar]
- [41].Hiraoka N, Ino Y, Sekine S, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer 2010;103:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Richards CH, Roxburgh CS, Anderson JH, et al. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg 2012;99:287–94. [DOI] [PubMed] [Google Scholar]
- [43].Gkogkou C, Frangia K, Saif MW, et al. Necrosis and apoptotic index as prognostic factors in non-small cell lung carcinoma: a review. SpringerPlus 2014;3:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci 1999;24:68–72. [DOI] [PubMed] [Google Scholar]
- [45].Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14:198–206. [DOI] [PubMed] [Google Scholar]
- [46].Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008;8:180–92. [DOI] [PubMed] [Google Scholar]
- [47].Harris AL. Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38–47. [DOI] [PubMed] [Google Scholar]
- [48].Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol 2004;4:641–8. [DOI] [PubMed] [Google Scholar]
- [49].Sonoshita M, Takaku K, Sasaki N, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 2001;7:1048–51. [DOI] [PubMed] [Google Scholar]
- [50].Rieder G, Hofmann JA, Hatz RA, et al. Up-regulation of inducible nitric oxide synthase in Helicobacter pylori-associated gastritis may represent an increased risk factor to develop gastric carcinoma of the intestinal type. Int J Med Microbiol 2003;293:403–12. [DOI] [PubMed] [Google Scholar]
- [51].Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 2003;349:2042–54. [DOI] [PubMed] [Google Scholar]
- [52].Pekarek LA, Starr BA, Toledano AY, et al. Inhibition of tumor growth by elimination of granulocytes. J Exp Med 1995;181:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sabo E, Boltenko A, Sova Y, et al. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res 2001;7:533–7. [PubMed] [Google Scholar]
- [54].Feng F, Tian Y, Liu S, et al. Combination of PLR, MLR, MWR, and tumor size could significantly increase the prognostic value for gastrointestinal stromal tumors. Medicine (Baltimore) 2016;95:e3248. [DOI] [PMC free article] [PubMed] [Google Scholar]


