Abstract
Background:
The aim of this study was to systematically evaluate the prognostic role of microvessel density (MVD) in patients with glioma through performing a meta-analysis.
Methods:
Web of Science, EMBASE, PubMed, Cochrane Library, and China National Knowledge Infrastructure were searched for potentially relevant literature. The study characteristics and relevant data were extracted. Hazard ratios (HRs) with 95% confidence intervals (CIs) were pooled to estimate the prognostic role of MVD in patients with glioma.
Results:
Nine studies with 536 patients were included. The pooled HR of higher MVD for overall survival (OS) was 1.64 (95% CI, 1.07–2.50) in patients with glioma. Subgroup analyses were also performed. The pooled HRs of higher MVD in studies from East Asia studies examining high-grade gliomas and studies using anti-CD105 antibodies were 1.99 (95% CI, 1.04–3.80), 1.60 (95% CI, 1.09–2.34) and 2.99 (95% CI, 1.50–5.99), respectively. No significant publication bias was found (P = .592), but significant between-study heterogeneity was observed (I2 = 80.5%, P <.001) in the meta-analysis.
Conclusion:
Our results suggested that higher MVD was associated with worse OS in patients with glioma. The findings may assist future research on antiangiogenic therapy and help predict prognosis in glioma. However, due to the limited number of studies, more well-designed studies are warranted to further verify our results.
Keywords: glioma, microvessel density, prognosis, survival
1. Introduction
Glioma is the most common type of primary intracranial tumor.[1] Gliomas are generally classified into 4 grades (I, II, III, and IV) according to the World Health Organization classification of tumors of the central nervous system.[2] Glioblastoma (grade IV), a common type of glioma, accounts for about 80% of all primary malignant central nervous system tumors.[3,4] Current standard therapies for glioblastoma include maximal safe resection, temozolomide chemotherapy, and radiotherapy.[5] In spite of that, the median overall survival (OS) of patients with newly diagnosed glioblastoma is still only 12 to 18 months.[3,5] Several prognostic factors for gliomas have been suggested, such as age, Karnofsky Performance Status scale at diagnosis, histology, and molecular makers.[2,3,6] However, it is worthwhile to explore new markers for the prognosis and management of gliomas.
Angiogenesis is a process to form new blood vessels from preexisting vasculature and was first reported to be related to tumor metastasis by Weidner et al.[7] Microvessel density (MVD), evaluated by microscopy after microvessel staining, is a common approach to assess intratumoral angiogenesis.[7] Currently, antibodies for staining endothelial cells of microvessel mainly include those against platelet/endothelial cell adhesion molecule CD31, pan-endothelial marker CD34, homodimeric transmembrane protein CD105, and von Willebrand Factor.[8] It has been proved that MVD is correlated with the prognosis in patients with various malignancies, including breast cancer,[9] colorectal cancer,[10] non-small cell lung cancer,[11] and so on.
In recent years, many researchers have investigated the prognostic role of MVD in patients with glioma. However, the results were inconsistent. Some studies found that MVD was related to poorer survival in glioma,[12–14] but other studies did not reach this conclusion.[15,16] Due to the controversy, we aimed to systematically evaluate the prognostic role of MVD in patients with glioma through performing a meta-analysis.
2. Methods
2.1. Search strategy
Since this is a meta-analysis, ethical approval was not necessary. We followed the developed guidelines for systematic reviews and meta-analyses in performing our study.[17] Web of Science, EMBASE, PubMed, Cochrane Library and China National Knowledge Infrastructure were searched for potentially relevant literature (last search ran on April 1, 2018). The following keywords were used: “glioma” and (“microvessel density” or “microvascular density” or “MVD”) and (“prognosis” or “outcome” or “survival” or “mortality”). Reference lists of relevant studies were also screened for additional literature. Languages were restricted to Chinese and English.
2.2. Study selection
Two authors (CF and JZ) independently performed the study selection process, and disagreements were resolved by consensus. Titles and abstracts of citations were screened first. Then potentially eligible studies were assessed in full text. Studies were considered eligible provided they met all of the following inclusion criteria:
-
(1)
the patients were diagnosed with any grade of glioma by histopathological examination;
-
(2)
the MVD of the tumor was measured under microscope;
-
(3)
patients were followed up for survival outcomes;
-
(4)
enough data was reported to estimate the prognostic role of MVD in patients with glioma.
Unrelated articles, case reports, reviews, letters, conference abstracts, and studies without enough data were excluded. If multiple studies were performed at the same center and the patients overlapped, the study with the largest sample size was included.
2.3. Data extraction
Relevant data of the eligible studies were extracted independently by 2 researchers (JZ and ZL), with any disagreements being discussed. The primary data was hazard ratio (HR) for OS with 95% confidence interval (CI) or the data that could be used to calculate the HR and 95% CI. Estimates calculated from multivariate analyses were extracted over those calculated from univariate analyses. The characteristics of the studies and patients were also extracted, including first author, publication year, country, the number of patients, sex of patients, mean or median age of patients, tumor subtype, type of antibody, and cut-off value of MVD.
3. Statistical analysis
The log HR and variance were calculated from the HR and 95% CI and were used for aggregation. Forest plots were constructed to estimate the pooled prognostic role of MVD in patients with glioma. The pooled HR was considered significant if the P value was less than .05 and the 95% CI did not overlap 1. The between-study heterogeneity was assessed, with I2 >50% or P <.10 indicating significant heterogeneity.[18] Random effect models were used in pooling the studies no matter whether heterogeneity exited since some heterogeneity among studies was expected due to differences in study and patient characteristics across studies.[19] If heterogeneity was significant, sensitivity analysis was performed to assess the contribution of each study to heterogeneity by excluding individual studies 1 at a time. Subgroup analyses were also performed according to patient source, tumor grade, type of antibody, and cut-off value of MVD. Publication bias was assessed by Begg test, with P >.05 implying no significant publication bias. All the above mentioned statistical analyses were performed by STATA 11.0 (STATA Corporation, College Station, TX).
4. Results
4.1. Literature research
The initial literature search retrieved 557 citations. After removing duplicates, 440 studies were screened by titles and abstracts. Then 403 studies were excluded according to the predefined inclusion and exclusion criteria. The rest 37 studies were assessed in full text and 27 were further excluded due to unrelated, lacking enough data or other reasons. One study [20] examined the HR for progression-free survival other than OS, so it was also excluded. Eventually, 9 articles [12–16,21–24] met the inclusion criteria and were included. The study selection process was shown in Figure 1.
Figure 1.
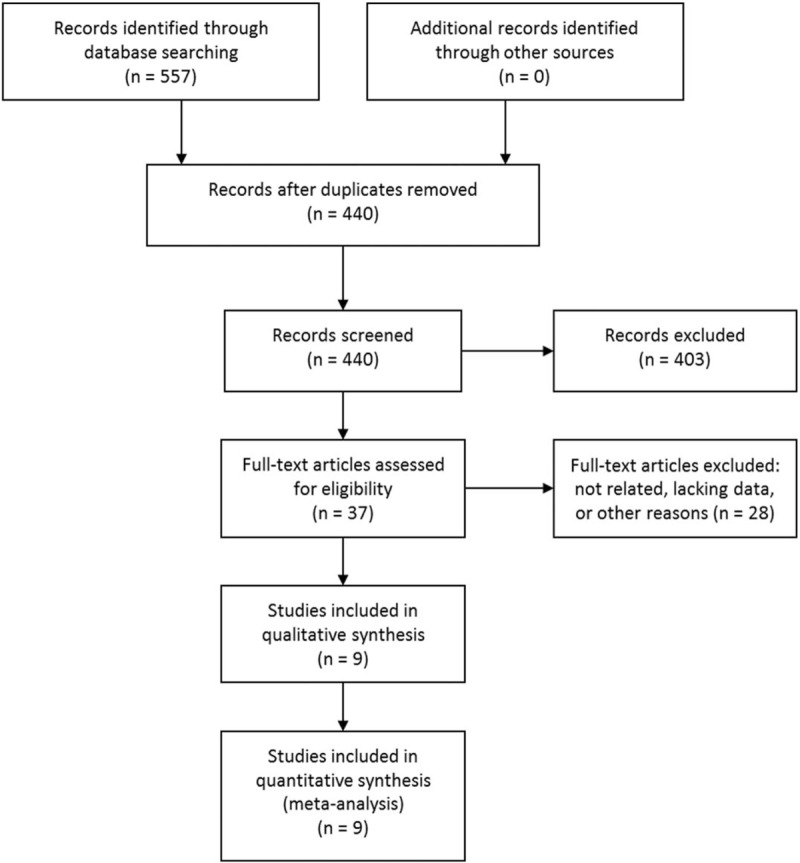
Selection process of studies.
4.2. Study characteristics
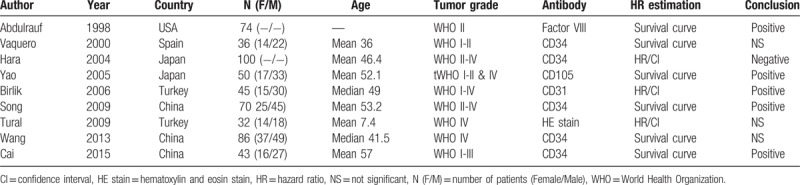
The basic characteristics of the 9 included studies were shown in Table 1. The studies were conducted in 5 different countries. A total of 536 patients were included. The grades of glioma varied among the studies. Some studies included low-grade gliomas, some included high-grade gliomas, and others included both low and high-grade gliomas. The antibodies to identify micro-vessels included CD31, CD34, CD105, and factor VIII. One study used hematoxylin and eosin stain.[15] Three studies reported HRs with 95% CI from multivariate analyses, and the HRs were calculated from survival curves in the rest 6 studies. The conclusions were positive in 5 studies, negative in 1 study and not statistically significant in 3 studies.
Table 1.
Characteristics of the included studies.
4.3. Overall analysis
In the 9 studies, 10 data sets were extracted and pooled together. The pooled HR of higher MVD for OS was 1.64 (95% CI, 1.07–2.50) (Fig. 2). Significant between-study heterogeneity was observed (I2 = 80.5%, P <.001). In performing sensitivity analysis, after excluding 1 study at a time, the heterogeneities were still above 70%. After excluding the study by Birlik et al,[13] the heterogeneity shrink to the lowest value of 72.8% and the pooled HR remained statistically significant (1.79, 95% CI, 1.07–2.98).
Figure 2.
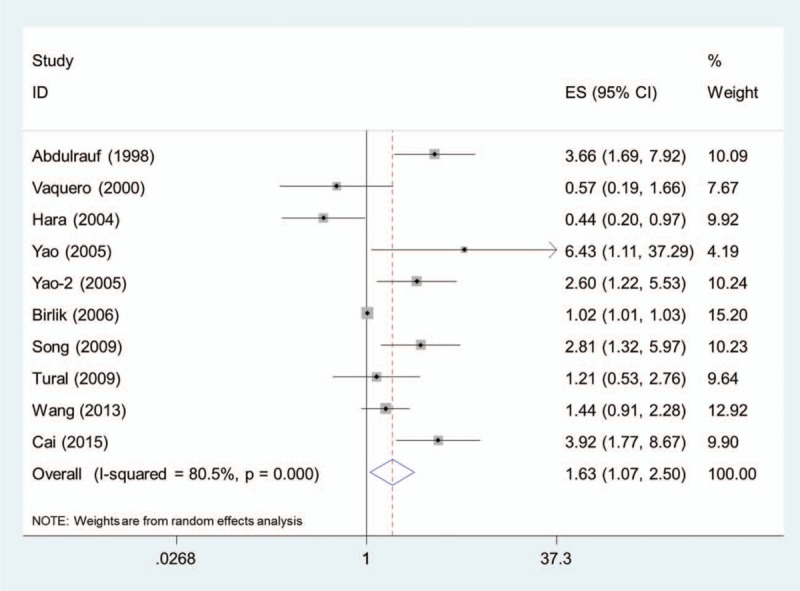
Pooled HR of higher MVD for overall survival in patients with glioma. HR = hazard ratio, MVD = microvessel density.
4.4. Subgroup analysis
4.4.1. Patient source
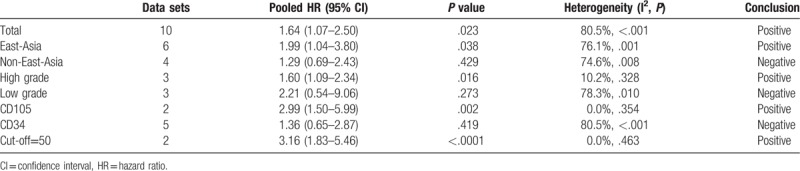
Among the 10 data sets, 6 were from China and Japan (East-Asia group)[12,14,16,23,24] and the rest were from Turkey, Spain and the USA (non-East-Asia group).[13,15,21,22] The pooled HR of higher MVD for OS was 1.99 (95% CI, 1.04–3.80) in the East-Asia group. In the non-East-Asia group, the pooled HR of higher MVD for OS was 1.29 (95% CI, 0.69–2.43).
4.4.2. Tumor grade
Three studies examined medulloblastoma or glioblastoma (high-grade group)[12,15,16] and 3 studies examined low-grade gliomas (low-grade group).[12,21,22] In the high-grade group, the pooled HR of higher MVD for OS was 1.60 (95% CI, 1.09–2.34). The pooled HR of higher MVD for OS was 2.21 (95% CI, 0.54–9.06) in the low-grade group.
4.4.3. Type of antibody
Two data sets used antibodies for CD105 (CD105 group)[12] and 5 studies used antibodies for CD34 (CD34 group).[14,16,22–24] The pooled HR of higher MVD for OS was 2.99 (95% CI, 1.50–5.99) in the CD105 group. In the CD34 group, the pooled HR of higher MVD for OS was 1.36 (95% CI, 0.65–2.87).
4.4.4. Cut-off value of MVD
One study used the cut-off value of 50 per 200 × field[14] and another study used 52 per 200 × field.[12] The pooled HR of the 2 studies of higher MVD for OS was 3.16 (95% CI, 1.83–5.46).
All the meta-analyses results were summarized in Table 2.
Table 2.
Summary of meta-analysis results.
4.5. Publication bias
No significant publication bias was found in the meta-analysis (P =.592). The Begg plot of publication bias of the 9 studies was shown in Figure 3.
Figure 3.
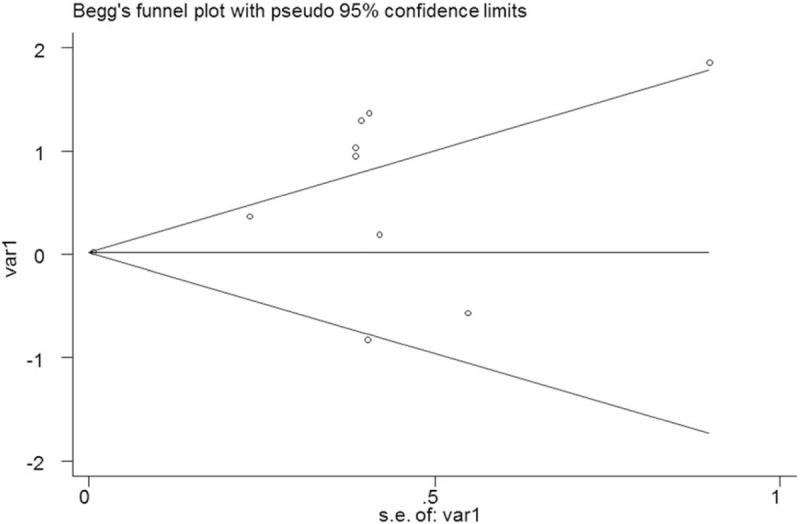
The Begg's publication bias plot of the 9 included studies.
5. Discussion
This study aimed to evaluate the prognostic role of MVD in patients with glioma. We performed a meta-analysis to summarize the existing evidence, and 9 studies were included. To our best knowledge, this is the first meta-analysis on this topic. Our results suggested that higher MVD was associated with poorer OS in patients with glioma.
Subgroup analyses were performed to further explore the role of MVD in patients with glioma. In East Asia countries, higher MVD was found to be associated with worse OS in patients with glioma. However, in other countries, the association was not significant, suggesting that the prognostic role of MVD may differ among different ethnicities. As to different tumor grades, the association was also different. For high-grade gliomas, higher MVD was found to be associated with worse OS. But the association was not significant in low grades gliomas. These results may imply different prognostic role of MVD in different grades of gliomas, but the results may also be due to the limited number of studies. In the low-grade group, there were only 3 studies and 2 of them suggested that higher MVD predicted worse survival.[12,21] Besides, in the studies that included patients in different grades of gliomas, the results indicated that higher MVD was related to poorer OS.[13,14,24] In the studies that used antibodies for CD105, the relationship between MVD and OS was significant. But the relationship was not significant after pooling the results together in the studies that used antibodies for CD34. In a meta-analysis examining the prognostic role of MVD in non-small cell lung cancer, the authors found that the anti-CD105 group had the highest pooled HR for OS.[25] Moreover, Yao et al compared the utilities of anti-CD105 antibody and anti-CD31 antibody for the prediction of prognosis in astrocytic tumors.[12] They found that anti-CD105 antibody may be a better marker than anti-CD31 antibody in evaluation of angiogenesis and prediction of prognosis in astrocytic tumors. Thus, more studies are warranted for the standardization of angiogenesis assessment. We also pooled the HRs of the studies using the cut-off value of 50 per 200 × field, and the pooled HR was 3.16 (95% CI, 1.83–5.46). As a rule of thumb, a prognostic factor with an HR >2 is of good practical use.[26] Based on the above findings, anti-CD105 antibody and the cut-off value of 50 microvessels per 200 × field may be suggested in the prognosis of glioma for MVD. However, due to the limited number of studies in this meta-analysis and in the subgroups, more studies are needed to verify the findings and to explore the role of MVD among different ethnicities and different grades of gliomas.
Angiogenesis is driven by many molecular pathways through redundant networks and the major mediator is vascular endothelial growth factor (VEGF).[27] In recent years, a number of preclinical and clinical data indicate that anti-VEGF therapies are effective in the treatment of cancer, and several VEGF-targeting drugs that have received US Food and Drug Administration approval.[27] For example, bevacizumab was approved for first-line therapy in combination with chemotherapy in patients with non-small cell lung cancer,[28] breast cancer,[29] and colorectal cancer.[30] In addition, sorafenib was approved for treatment of advanced renal cell and hepatocellular carcinoma,[31,32] and sunitinib was approved for use in patients with progressive gastrointestinal stromal tumors and advanced renal cell carcinoma.[33,34] Gliomas are highly angiogenic tumors and anti-VEGF therapies have also become a promising therapeutic strategy in glioma patients.[35] Our results suggested that glioma patients with higher MVD had worse OS, which supports future research on antiangiogenic therapy in glioma. Current antiangiogenic therapy in glioma mainly includes VEGF ligand sequestration, tyrosine kinase inhibitors targeting VEGF receptor, inhibitors of alternate proangiogenic signaling pathways and inhibitors of endothelial cell migration.[27] Apart from the impact on therapeutic strategy exploration, our research also provides a marker to predict the prognosis of patients with glioma. However, the best antibody and cut-off value should be further agreed upon.
The prognostic value of MVD has also been studied in other intracranial tumors. Barresi et al demonstrated that higher density of microvessels positive for CD105 showed inverse significant correlation with OS and recurrence-free survival in meningiomas.[36] Some researchers also found MVD correlated with survival in the primary central nervous system lymphomas.[37,38]
However, as we all know, gliomas are a heterogeneous group of tumors, which include various subtypes, such as astrocytic tumor, oligodendroglial tumor, pilocytic astrocytomas, ependymal tumor, and so on. Therefore, more studies are needed to evaluate the role of MVD in different types of gliomas. Besides, previous studies have found that urokinase-type plasminogen activator also played an important role in glioma prognosis,[39] which might be a possible confounding factor of MVD.
Significant between-study heterogeneity was observed in our meta-analysis. Sensitivity analysis did not identify any study that contributed greatly to heterogeneity, and the heterogeneity stayed significant after excluding 1 study at a time. During the subgroup analyses, we noticed that the heterogeneity dropped sharply to 10.2% in the high-grade group and even disappear in the CD105 group and among the studies using the cut-off value of 50 per 200 × field. Therefore, potential sources of heterogeneity may come from different tumor grades, different antibodies, different cut-off values of MVD and even different ethnicities.
There were several limitations in our meta-analysis. First and foremost, the number of included studies was limited in the meta-analysis, as well as in the subgroups. Therefore, caution should be applied as to the results, and much more studies are needed. Moreover, the characteristics of the studies and patients varied. For example, the gender, age, tumor grade, antibodies, and cut-off values of MVD differed between the studies. Besides, publication bias should not be completely excluded although no significant publication bias was found in our meta-analysis since it was a major concern for all meta-analyses.
In conclusion, our results suggested that higher MVD was associated with worse OS in patients with glioma. The findings may assist future research on antiangiogenic therapy and help predict prognosis in glioma. However, due to the limited study number and heterogeneity among the studies, more well-designed studies are warranted to further verify our results.
Acknowledgments
We would like to thank the reviewers for their constructive comments.
Author contributions
Conceptualization: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Min He, Jianguo Xu.
Data curation: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Min He, Ting Du, Yanlin Song, Yimeng Fan.
Formal analysis: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Yanlin Song, Yimeng Fan.
Investigation: Jing Zhang.
Methodology: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Min He, Tianyi Kang, Ting Du.
Software: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Min He, Ting Du.
Supervision: Jianguo Xu.
Validation: Jing Zhang, Tianyi Kang, Yanlin Song.
Writing – original draft: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Min He, Yanlin Song, Yimeng Fan.
Writing – review & editing: Chaofeng Fan, Jing Zhang, Zhiyong Liu, Min He, Tianyi Kang, Ting Du, Yanlin Song, Yimeng Fan, Jianguo Xu.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, MVD = microvessel density, OS = overall survival, VEGF = vascular endothelial growth factor.
CF, JZ, ZL, and MH contributed equally to this work.
The authors declare no conflict of interests.
References
- [1].Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol 2012;14suppl 5:v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- [3].Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev 2017;40:1–4. [DOI] [PubMed] [Google Scholar]
- [4].Ostrom QT, Gittleman H, Stetson L, et al. Epidemiology of gliomas. Cancer Treat Res 2015;163:1–4. [DOI] [PubMed] [Google Scholar]
- [5].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [6].Wiencke JK, Koestler DC, Salas LA, et al. Immunomethylomic approach to explore the blood neutrophil lymphocyte ratio (NLR) in glioma survival. Clin Epigenetics 2017;9:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. New Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- [8].Ma G, Zhang J, Jiang H, et al. Microvessel density as a prognostic factor in esophageal squamous cell cancer patients: a meta-analysis. Medicine 2017;96: e7600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uzzan B, Nicolas P, Cucherat M, et al. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res 2004;64:2941–55. [DOI] [PubMed] [Google Scholar]
- [10].Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006;94:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fontanini G, Bigini D, Vignati S, et al. Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol 1995;177:57–63. [DOI] [PubMed] [Google Scholar]
- [12].Yao Y, Kubota T, Takeuchi H, et al. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathol Off J Jpn Soc Neuropathol 2005;25:201–6. [DOI] [PubMed] [Google Scholar]
- [13].Birlik B, Canda S, Ozer E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl Neurobiol 2006;32:532–8. [DOI] [PubMed] [Google Scholar]
- [14].Cai H, Xue Y, Liu W, et al. Overexpression of roundabout4 predicts poor prognosis of primary glioma patients via correlating with microvessel density. J Neurooncol 2015;123:161–9. [DOI] [PubMed] [Google Scholar]
- [15].Tural S, Gercek A, Konya D, et al. Microvessel density and vascular endothelial growth factor expression as predictors of childrens’ survival from cerebellar medulloblastoma. J Clin Neurosci Off J Neurosurg Soc Australasia 2009;16:1199–202. [DOI] [PubMed] [Google Scholar]
- [16].Wang SY, Ke YQ, Lu GH, et al. Vasculogenic mimicry is a prognostic factor for postoperative survival in patients with glioblastoma. J Neurooncol 2013;112:339–45. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Specogna AV, Turin TC, Patten SB, et al. Factors associated with early deterioration after spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One 2014;9: e96743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bartels U, Hawkins C, Jing M, et al. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg 2006;104suppl 5:314–20. [DOI] [PubMed] [Google Scholar]
- [21].Abdulrauf SI, Edvardsen K, Ho KL, et al. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosurg 1998;88:513–20. [DOI] [PubMed] [Google Scholar]
- [22].Vaquero J, Zurita M, Coca S, et al. Prognostic significance of clinical and angiogenesis-related factors in low-grade oligodendrogliomas. Surg Neurol 2000;54:229–34. [DOI] [PubMed] [Google Scholar]
- [23].Hara A, Okayasu I. Cyclooxygenase-2 and inducible nitric oxide synthase expression in human astrocytic gliomas: correlation with angiogenesis and prognostic significance. Acta Neuropathol 2004;108:43–8. [DOI] [PubMed] [Google Scholar]
- [24].Song S, Wan Z, Chen H. Expression of integrin b1, fibronectin and CD 34 in glioma and their relationship with prognosis. Shandong Medical Journal 2009;49:17–9. [Google Scholar]
- [25].Zhang J, Ma X, Li Y, et al. Microvessel density as a prognostic factor in non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med 2016;9:17676–89. [Google Scholar]
- [26].Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia 2001;6:375–92. [DOI] [PubMed] [Google Scholar]
- [27].Chi AS, Sorensen AG, Jain RK, et al. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist 2009;14:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. New Engl J Med 2006;355:2542–50. [DOI] [PubMed] [Google Scholar]
- [29].Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. New Engl J Med 2007;357:2666–76. [DOI] [PubMed] [Google Scholar]
- [30].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- [31].Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- [32].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [33].Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet (Lond, Engl) 2006;368:1329–38. [DOI] [PubMed] [Google Scholar]
- [34].Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- [35].Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci 2007;8:610–22. [DOI] [PubMed] [Google Scholar]
- [36].Barresi V, Cerasoli S, Vitarelli E, et al. Density of microvessels positive for CD105 (endoglin) is related to prognosis in meningiomas. Acta Neuropathol 2007;114:147–56. [DOI] [PubMed] [Google Scholar]
- [37].Takeuchi H, Matsuda K, Kitai R, et al. Angiogenesis in primary central nervous system lymphoma (PCNSL). J Neurooncol 2007;84:141–5. [DOI] [PubMed] [Google Scholar]
- [38].Sugita Y, Takase Y, Mori D, et al. (CD 105) is expressed on endothelial cells in the primary central nervous system lymphomas and correlates with survival. J Neurooncol 2007;82:249–56. [DOI] [PubMed] [Google Scholar]
- [39].Hsu DW, Efird JT, Hedley-Whyte ET. Prognostic role of urokinase-type plasminogen activator in human gliomas. Am J Pathol 1995;147:114–23. [PMC free article] [PubMed] [Google Scholar]


