Abstract
With further research into the molecular mechanisms and roles linking immune suppression and restraint of (pre)malignancies, immunotherapies have revolutionized clinical strategies in the treatment of cancer. However, nearly 70% of patients who received immune checkpoint therapeutics showed no response. Complementary and/or synergistic effects may occur when extracellular checkpoint antibody blockades combine with small molecules targeting intracellular signal pathways up/downstream of immune checkpoints or regulating the innate and adaptive immune response. After radiolabeling with radionuclides, small molecules can also be used for estimating treatment efficacy of immune checkpoint blockades. This review not only highlights some significant intracellular pathways and immune-related targets such as the kynurenine pathway, purinergic signaling, the kinase signaling axis, chemokines, etc., but also summarizes some attractive and potentially immunosuppression-related small molecule agents, which may be synergistic with extracellular immune checkpoint blockade. In addition, opportunities for small molecule-based theranostics in cancer immunology will be discussed.
Keywords: small molecules, theranostic agents, cancer immunology, molecular imaging, targeted therapy
Introduction
Cancer is still one of the leading causes of morbidity and mortality worldwide. In 2018, 18 million new cancer cases and 9 million cancer-related deaths occurred 1. Recent immuno-oncology therapies have seen significant success by remolding the immune system of patients to treat multiple cancers 2-6.
The mechanism of cancer immunotherapy is based on the blockade of tumor-mediated inhibition of immune responses rather than direct targeting of tumor cells. “Immune checkpoints” means the stimulation or inhibition of receptor-ligand signal axes between tumor cells and immune cells including T cells, dendritic cells (DCs), and macrophages in the tumor microenvironment 7, 8. It wasn't until 1992 when the first immunotherapy drug - PROLEUKIN® (aldesleukin) was approved by the US Food and Drug Administration (FDA), which opened a new era of immunotherapy. Various immune checkpoint-directed antibodies such as anti-cytotoxic-T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death 1 (anti-PD-1), anti-programmed cell death ligand 1 (anti-PD-L1), and anti-CD19 have shown to affect various cancers and are approved by the US FDA 9. In addition, other new and promising drugs for targets such as T-cell immunoglobulin mucin 3 (TIM3), tumor necrosis factor receptor superfamily member 4 (TNFRSF4), and lymphocyte-activation gene 3 (LAG-3) are being investigated in clinical trials, such as NCT02817633 and NCT01303705 (Table 1) 10.
Table 1.
Representative drugs approved by the US FDA and other checkpoint inhibitors
| (Generic/Brand name) | Target | Mainly indication (Approved time) | Status |
|---|---|---|---|
| Aldesleukin | IL-2 receptor | Metastatic Melanoma (1998.1) Metastatic Renal Cell Carcinoma (mRCC) (1992.5) |
Approved |
| Ipilimumab/ Yervoy |
CTLA-4 | Metastatic Colorectal Cancer (mCC) (2018.7) Advanced Renal Cell Carcinoma (aRCC) (2018.4) Metastatic Melanoma (2017.7) Late-Stage Melanoma (2011.5) |
Approved |
| Nivolumab/ Opdivo | PD-1 | Hepatocellular Carcinoma (HCC) (2017.9) Metastatic Urothelial Carcinoma (mUC) (2017.2) Head and Neck Cancer (HNC) (2016.11) Hodgkin Lymphoma (HL) (2016.5) mRCC (2015.11) Advanced Melanoma (2014.12) |
Approved |
| Pembrolizumab/Keytruda | PD-1 | mSCLC (2019.6); Squamous Cell HNC (2019.6); aRCC (2019.4); NSCLC (2019.4); Advanced or Metastatic Merkel Cell Carcinoma (a/mMCC) (2018.12); HCC (2018.11); mNSCLC (2018.10) Primary Mediastinal Large B-Cell Lymphoma (PMBCL) (2018.6) Metastatic Cervical Cancer (mCC) (2018.6) Advanced or Metastatic Gastric Cancer (2017.9) Advanced or Metastatic UC (2017.5); HL (2017.3); mNSCLC (2016.10) Advanced Melanoma (2014.9) |
Approved |
| Cemiplimab/ Libtayo | PD-1 | Squamous Cell Carcinoma (2018.9) | Approved |
| toripalimab | PD-1 | Advanced or Metastatic Melanoma | Phase 2 |
| Atezolizumab/ Tecentriq |
PD-L1 | Extensive-Stage SCLC (2019.3) Metastatic Triple-Negative Breast Cancer (mTNBC) (2019.3.) Metastatic Non-Squamous NSCLC (2018.12) Advanced Bladder Cancer (2017.4) Metastatic Lung Cancer (2016.10) Urothelial Carcinoma(2016.5) |
Approved |
| Avelumab/ Bavencio | PD-L1 | Advanced Renal Cell Carcinoma (2019.5) Urothelial Carcinoma (2017.5) Merkel Cell Carcinoma (2017.3) |
Approved |
| Durvalumab/ Imfinzi |
PD-L1 | Non-Small Cell Lung Cancer (2018.2) Urothelial Carcinoma (2017.3) |
Approved |
| MEDI4736 | PD-L1 | NSCLC | Phase 3a |
| Avelumab | PD-L1 | Ovarian Cancer | Phase 2 |
| Tisagenlecleucel/Kymriah | CD19 | Large B-Cell Lymphoma (2018.5) Acute Lymphoblastic Leukemia (2017.8) |
Approved |
| Axicabtagene ciloleucel/ Yescarta |
CD19 | Large B-Cell Lymphoma (2017.10) | Approved |
| Cyclophosphamide | CD19 | Lymphocytic Leukemia | Phase 1 |
| Sym023 | TIM-3 | Metastatic Cancer; Solid Tumor; Lymphoma | Phase 1 |
| TSR-022 | TIM-3 | Advanced or Metastatic Solid Tumors | Phase 1 |
| MEDI6469 | TNFRSF4 | Head and Neck Cancer; Progressive Metastatic Prostate Cancer | Phase 1 |
| KHK4083 | TNFRSF4 | B-Cell Non-Hodgkin Lymphoma | Phase 2 |
| PF-04518600 | TNFRSF4 | Metastatic Renal Cell Cancer | Phase 2 |
| Sym022 | LAG-3 | Advanced Solid Tumor Malignancies or Lymphomas | Phase 1 |
| BMS 986016 | LAG-3 | Gliosarcoma and Recurrent Brain Neoplasm | Phase 1 |
However, new problems have arisen in the course of immunotherapy: 1) nearly 70% of patients who received immune checkpoint therapeutics showed no response or only showed a short-term beneficial effect with recurrence soon afterwards 8; 2) immune checkpoint blockade increases the activity of the immune system and can result in immune-related adverse events such as myocarditis, vasculitis, heart failure, dermatitis, endocrine dysfunction, and even death 11-13; 3) there are primary, adaptive, and acquired resistances to cancer immunotherapies 14; and 4) the disadvantages of antibodies include their long half-life (even multiple weeks) and persistent side effects once injected into the body. Therefore, it is necessary to identify novel immune-related oncology molecular targets and small molecule drugs to expand the treatment range of tumors and/or subtypes of patients, limit adverse events and reduce resistances of immunotherapy.
Recently, combination therapies are widely considered as the most promising oncology treatment strategy. Exploiting intracellular immune-related signal pathways to improve the effect of tumor treatment is an important transition. Intracellular pathways downstream of checkpoint blockade such as the kynurenine pathway, purinergic signaling, and the kinase pathway axis have been explored, and small-molecule-mediated therapeutic agents are being developed, which may show complementary and/or synergistic effects when combined with extracellular checkpoint antibody blockades. Small-molecule drugs possess some advantages over antibodies: 1) the ability go across cellular membranes and other physiological barriers and reach intracellular targets; 2) oral bioavailability; 3) various dosage forms and excellent pharmacokinetic characteristics such as good tumor penetration, efficient delivery into brain tissues, appropriate half-life, and intracellular targets 15; 4) lower manufacturing costs; and 5) diversified strategies for combined therapy by passing into the cytoplasm and interacting with multiple intracellular targets 16, 17. Importantly, kinase-targeted small molecule inhibitors have been established, which are clinically effective and possess appropriate selectivity to avoid or manage clinical toxicities.
This review summarizes the recent use of small-molecule drugs in tumor immunotherapies and immunodiagnostics in (pre)clinical trials, and provides thoughts regarding their future utility both as therapeutic agents and diagnostic tracers. Several comprehensive reviews on small molecules in cancer immunotherapy have been published previously 17-19, which highlighted the small molecule-based immune mechanism, therapeutic compound structures or imaging application in immunity. This review provides a recent update on not only the immune mechanism and therapeutic compounds but also small molecule-based diagnostic radiotracers. Broad applications of small molecules as theranostic agents in cancer immunology are presented and discussed.
Immune-related targets in tumor microenvironment
Clinical investigators reported that the combination of checkpoint inhibitors with other targeted agents provide multiple points of opportunity for cancer treatments. The various cell types and targets/signal pathways involved in cancer immunity supply prosperous potential targets of intervention for small-molecule-mediated agents, including receptors, extracellular enzymes, and intracellular signal transduction pathways.
In general, the tumor microenvironment is quite complex. It is made up of tumor cells, multiple immune cells, lymphovascular cells, and extracellular matrix 20, 21. The interactions between different tumor variants and diverse immune cells either annihilate tumors by activating immune responses or boost immune tolerance and eventually result in tumor proliferation and/or metastasis (Figure 1) 20, 22.
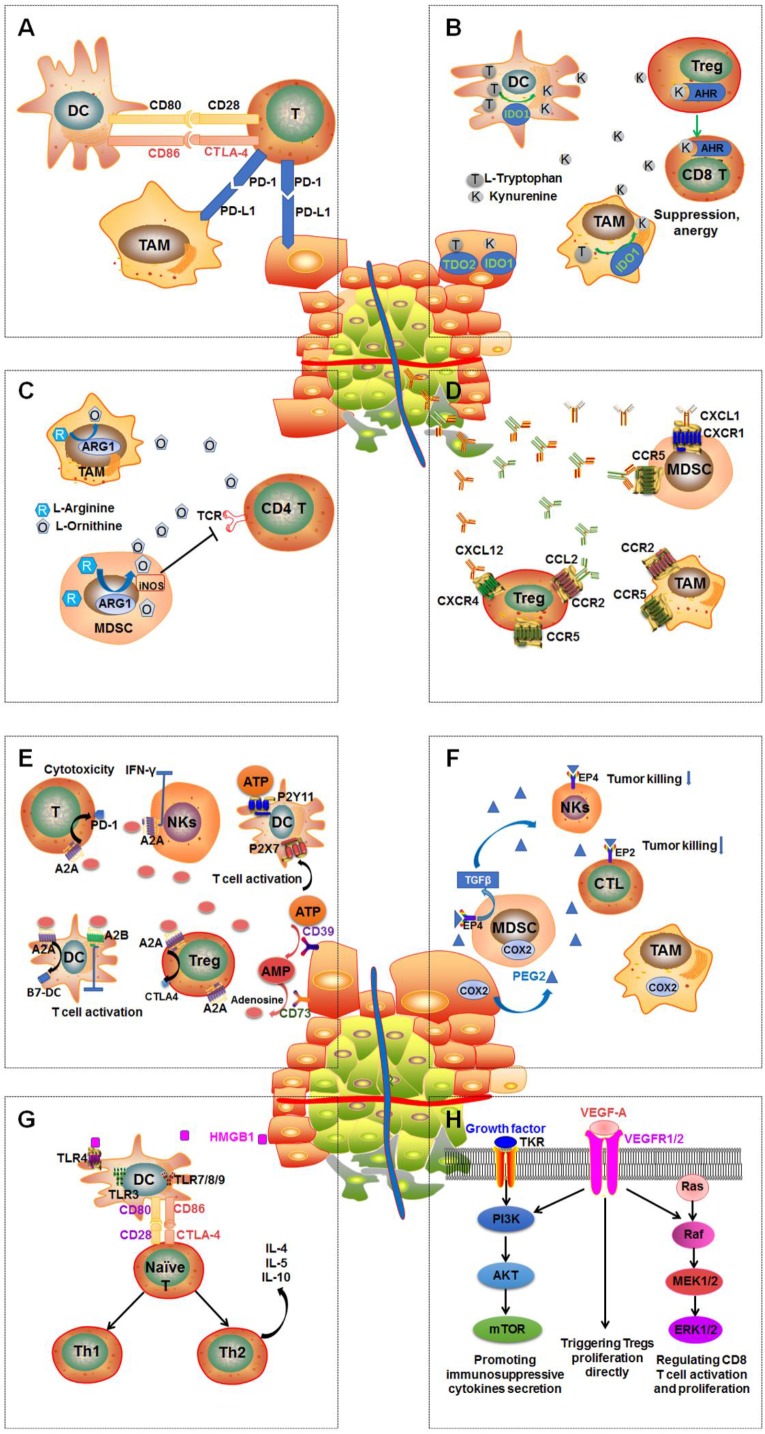
Figure 1.
Multiple immunosuppressive mechanisms coexist in tumor microenvironment. A: PD-1regulates T-cell activation through binding to its ligand PD-L1. CTLA-4 can stop potentially autoreactive T cells at the initial stage of naive T-cell activation. B: Overexpression of DCs and TAMs or IDO and tryptophan 2,3‑dioxygenase (TDO2) in tumor leads to extracellular tryptophan depletion and tryptophan production, subsequently causing defective antigen presentation of DCs and Tregs activations and effector T cell function suppression. C: Upregulation of arginase 1 (ARG1) in tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) results in arginine the depletion, leading to MDSC-mediated immune suppression and impaired CD4+ T cell function. D: Chemokines CXCL1, CXCL12, CCL2, and CCL5 are secreted by the tumor then spread to the vasculature leading to the recruit and activity of immunosuppressive MDSCs, TAMs and Tregs through interacting with their receptors: CXCR1, CXCR4, CCR2 and CCR5. E: ATP is dephosphorylated to adenosine by CD39 and CD73. Extracellular adenosine interacts with their receptors A2AR and A2BR overexpressed on Tregs, DCs, and T cells to regulate immunosuppressive functions through boosting upregulation of CTLA-4, PD-1 and B7 proteins. F: COX2 is overexpressed on tumor cells TAMs and MDSCs to stimulate PGE2 production and then enhance the tumor proliferation and immunosuppressive function of TAMs and MDSCs. In addition, enhancing TGF-β production by MDSCs can inhibit the function of NKs. G: Secretion of the high-mobility-group box 1 (HMGB1) protein by dying tumor cells can stimulate the expression of CD80 and CD86 on DCs by binding to TLR4 overexpressed by DCs, which contributes to the differentiation if naïve and/or activated T cells into T helper 1 (Th1) and T helper 2 (Th2). H: Activation of PI3K-AKT-mTOR pathway is able to boost expression of immunosuppressive cytokines, chemokines, and checkpoint ligands and recruit regulatory immune cell subsets such as MDSCs and Tregs into tumor. RAS-RAF-MEK-ERK1/2 pathway plays a critical role in CD8 T cell activation, proliferation, and survival by regulating the production of IL-2. The activation of VEGF-A/VEGFR axis enhances PD-1 expression on CD8+ T cells leading to the exhaustion of anti-tumor immune cells. In addition, VEGF-A/VEGFR could enhance the pathway of PI3K-AKT-mTOR and RAS-RAF-MEK-ERK1/2.
CTLA-4, PD-1 and PD-L1 are considered the most prominent immune pathway checkpoints 23. CTLA-4 and PD-1 are mainly overexpressed by T cells and increase the tolerance of immune cells. PD-L1 is mainly expressed on tumor cells, which binds to PD-1 on immune cells to induce immune suppression. The activation of the PD-1/PD-L1 signal axis also can inhibit proliferation and survival of effector T cells, and secretions of interferon gamma (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α) 24, 25. CTLA-4 is upregulated on activated T cells. CTLA-4 can resist CD28 activity via binding to CD80 and CD86 with a much higher affinity than that of CD28, subsequently inducing inhibition of T-cell activation. In addition, activated CD8+ T cells also overexpress CTLA-4, which counteracts the activity of helper T cells downstream and boosts the regulatory T cells (Tregs) immunosuppressive activity. Therefore, CTLA-4 plays a significant role in the early development of immune tolerance. CTLA-4 inhibitors are able to stimulate activated T-cell activation, subsequently showing antitumor immune response 26.
Tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) are also able to cause the suppression of immune effectors 27. M2 macrophages present anti-inflammatory and pro-tumorigenic effects such as promoting tumor neovasculature formation, invasion and metastasis. In addition, MDSCs can secrete transforming growth factor beta (TGF-β) and IL-10 to produce direct immunosuppressive effects on T effector cells or induce Tregs generation. Spleen MDSCs are able to downregulate the cell adhesion molecule L-selectin on CD4+ and CD8+ T cells, resulting in a decrease in the activation and homing of CD8+ cells in lymph nodes 28. Cancer-associated fibroblasts (CAFs) can be stimulated by TGF-β and fibroblast growth factor (FGF), thereby promoting tumorigenesis, lymphatic vascularization, and metastasis 29. The function of MDSCs, DCs, macrophages and TAMs can be regulated by indoleamine 2,3-dioxygenase 1 (IDO1), chemokines (CXCRs), arginase 1 (ARG1), or toll-like receptors (TLRs) 17.
Reprogramming of energy metabolism of cancer cells is very important in immunosuppression. Intratumoral hypoxia induces upregulation of hypoxia inducible factor-1α (HIF-1α) through regulating ATP-binding cassette (ABC) transporters. On one hand, accumulated ATP stimulates antitumor immune response via the P2 purinergic receptors (P2XRs or P2YRs) mainly expressed on macrophages, DCs, CD4+ T cells, and CD8+ T cells. On the other hand, accumulated ATP can be further degraded to adenosine by the catalysis of ectonucleotidases CD39 and CD73 mainly overexpressed on tumor cells, B cells and Tregs 30. Extracellular adenosine mediates immunosuppression by interacting with four subtypes of adenosine receptors, A1R (presented on neutrophils and immature DCs), A2AR (presented on most immune cells and platelets), A2BR (presented on tumor cell macrophages, DCs, and mast cells), and A3R (presented on neutrophils and mast cells) 31. In addition, intracellular cyclic AMP (one downstream signaling molecule of ATP) of MDSCs, TAMs, Tregs and tumor cells, is also associated with immunosuppression by COX2 (overexpressed on tumor cells, MDSCs, TAMs, and Tregs), EP2 receptor (presented on cytotoxic T lymphocytes (CTLs) and Tregs), and EP4 receptor (mainly expressed on DCs, natural killer cells (NKs), TH1, and TH17 cells) to regulate MDSCs, Tregs, NKs, and tumor cells 17.
Intracellular signal transduction pathways which are involved in immune resistance have been thoroughly explored. Various kinase signal pathways are the key regulatory factors in the immune system 32. Activin-like kinase 5 (ALK5) can diminish TGF-β signaling leading to activation of CD8+ T cells, stimulation of NKs, and generation of CTLs 33. Phosphatidylinositol-3-OH kinase (PI3Kδ) is able to attenuate Tregs function, causing activation of effector T cells 34. Colony stimulating factor 1 (CSF1) stimulates M1 to M2 polarization, and then boosts tumor proliferation and survival 35. Small-molecule drugs, such as vemurafenib and dabrafenib (v-Raf murine sarcoma viral oncogene homolog B1 [BRAF] inhibitors), cobimetinib and trametinib (mitogen-activated extracellular signal-regulated kinase [MEK] inhibitors), and sorafenib, and pazopanib (vascular endothelial growth factor [VEGF] inhibitors) have been approved by the US FDA for the treatment of multiple cancers 36.
Small molecules as immunotheranostics
Current strategies of immunotherapy aim to reverse immune resistance either by promoting the recognition of tumor-associated antigens or by modulating signals of T cell co-receptors through biological modalities. Multiple clinical trials suggested that small molecule-based approaches of targeted multiple immune-related targets mentioned above show complementary and/or synergistic effect with immune checkpoint inhibitors, which can further promote the response rates of patients and improve survival rates. Nearly 25% of immunotherapy clinical trials combine small molecules as partners for immune checkpoint blockades 37. Therefore, it is necessary to summarize the latest developments of small-molecule-mediated targeting agents as immunotherapies in cancer, and to offer considerations about their utility both as mono-agents and/or in combination with other anti-cancer drugs.
PD-1/PD-L1 immune checkpoint
PD-1 is overexpressed by multiple immune cells such as activated T cells, B cells, NKs, DCs, and TAMs, and it is a critical regulator protein for immune inhibition in the innate and adaptive immune systems 38. PD-L1 is overexpressed on various solid tumors and hematological malignancies 39, 40. The PD-1/PD-L1 signal axis inhibits the T cell functions by creating a “molecular shield” in the tumor microenvironment. The interactions of PD-1 and PD-L1 may promote T cell exhaustion, or induce the CD4+ and CD8+ T cell apoptosis, or enhance immunosuppression of intratumoral Tregs. To date, multiple PD-1/PD-L1 inhibitor antibodies have been effective in some advanced cancer types; however, a remarkable proportion of patients remain resistant to these antibody-based immunotherapies. In order to further expand the response rates of patients to immunotherapies, various small molecule drugs are being explored (Figure 2).
Figure 2.
Small-molecule inhibitors of PD-1/PD-L1.
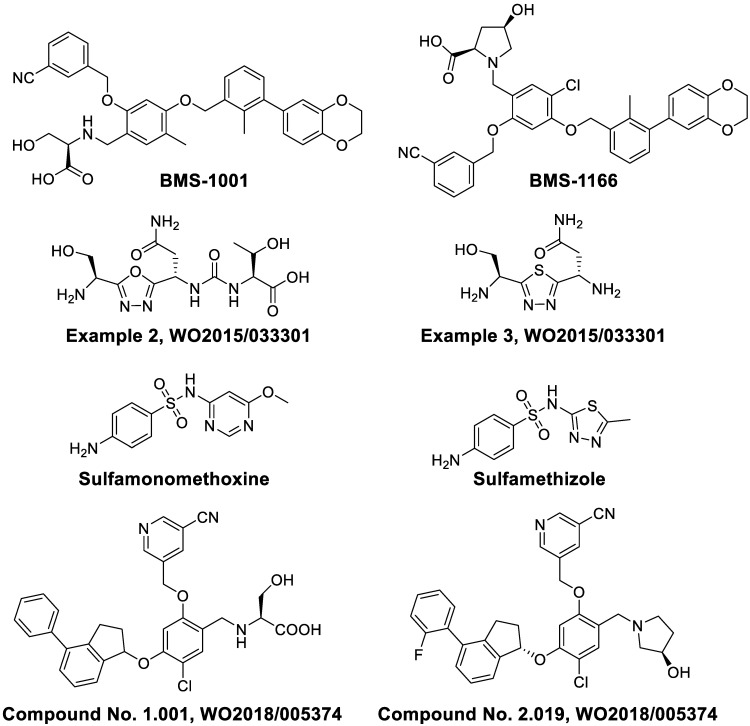
Researchers at Bristol-Myers Squibb (BMS) first synthesized multiple biaryl drugs as PD-1/PD-L1 and CD80/PD-L1 interaction small molecule inhibitors (WO2015/034820). The interaction mechanism may induce the dimerization of PD-L1, subsequently occluding the PD-1 interaction surface 41. The best lead compounds are BMS-1001 and BMS-1166 which can induce dimerization of PD-L1 to exert therapeutic activities 42. BMS-1001 and BMS-1166 can completely restore anti-CD3-mediated T cell activation in nuclear factor of activated T cells (NFAT) luciferase reporter-transfected Jurkat T cells 43. In addition, scientists from Aurigene Discovery Technologies Limited synthesized two compounds with 1,3,4-oxadiazole and 1,3,4-thiadiazole scaffolds (Figure 2, Examples 2 and 3, respectively), which are able to inhibit the PD-1 signaling pathway (WO2011/082400 A3). Sharpe and colleagues synthesized and tested small-molecule PD-1 modulators sulfamonomethoxine and sulfamethizole and their derivatives, which both inhibited the expression of PD-1 in transgenic mouse T cells (WO2011/082400 A3). Most recently, the company Curis Inc. reported compounds CA-170 and CA-327, which not only bind to PD-L1 but also antagonize VISTA or TIM3 binding respectively 44. They can boost T cell proliferation and cytokine secretion. CA-170 is being evaluated in a trial of clinical phase I in humans with advanced solid tumors or lymphomas (NCT02812875). However, their structures have not been disclosed yet.
In 2018, a patent (WO2018/005374 A1) reported by ChemoCentryx Inc. described immunomodulatory inhibitors (Compound No. 1.001 and Compound No. 2.019) that are able to inhibit the PD-1 pathway as shown by ELISA platform-based biochemical interaction assay using human PD-L1.
Amino acid catabolism
The metabolism of amino acids plays an important role in regulating the innate immune response when diseases occur. Particularly the catabolism of tryptophan and arginine can regulate the immune responses to T cell proliferation and activation. The metabolic pathway of tryptophan catabolized to kynurenine is an essential regulator in maintaining the immunosuppressive microenvironment in many types of cancers. Indoleamine-2,3-dioxygenase (IDO) and TRP-2,3-dioxygenase (TDO) are the key and rate-limiting enzymes in establishing and maintaining immune privilege in tumor immune escape 45. Recruitment of ARG1-expressing MDSCs at a tumor site results in the depletion of L-arginine, which causes reduced proliferation of T-cells and NKs and inhibition of the antitumor immune response. Recruitment of ARG1-overexpressed MDSCs at a tumor site causes L-arginine depletion, which decreases the proliferation of T-cells and NKs and inhibition of the antitumor immune response.
Indoleamine-pyrrole 2,3-dioxygenase (IDO)
The IDO family consists of IDO isozymes (IDO1 and IDO2) and tryptophan 2,3‑dioxygenase (TDO2), catalyzing tryptophan to N-formylkynurenine and subsequently to kynurenine and other metabolites. IDOs are overexpressed in macrophages, DCs and various tumor types and contribute to immunosuppression, leading to poor prognosis. The IDO pathway can diminish immune antigen recognition by inducing differentiation and hyper-activation of Tregs and inhibiting immune responses of effector T cells and decreasing DC function. The interaction of kynurenine with aryl hydrocarbon receptor has been verified as a pivotal pathway in immunosuppression functions.
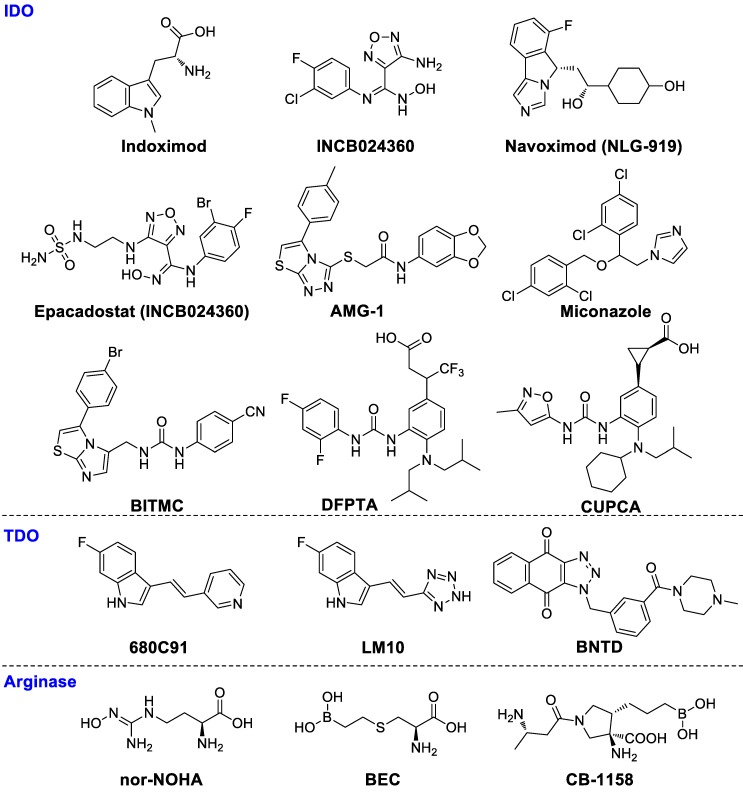
D-1MT, or indoximod (Figure 3), the D-enantiomer of 1-methyl tryptophan, has been investigated in combination with pembrolizumab in a clinical phase II trial for patients with metastatic melanoma (NCT02073123). Indoximod in combination with a taxane compound is also being investigated in a phase II clinical study for treating patients with metastatic breast cancer (NCT01792050). INCB024360 (Figure 3), or epacadostat, a high selective human IDO1 antagonist, boosts effector T cells and NKs growth, enhances IFN-γ production, increases the amounts of CD86high DCs and diminishes Tregs conversion 46. Although phase III clinical trials (NCT02752074) showed that epacadostat in combination with pembrolizumab for the treatment of melanoma did not reveal superior outcome compared to pembrolizumab alone, the failed trial may have several caveats: 1) it is uncertain as to whether the target was adequately inhibited; 2) a mechanistic rationale for the combination needs to be tested further. Nevertheless, regarding immune-related toxic effects, epacadostat in combination with pembrolizumab therapy seemed to be well tolerated compared with other immunotherapy combinations such as ipilimumab plus pembrolizumab 47. Better rationalized compounds and trial designs will be significant in the future to accurately evaluate medical impact. IDO1 antagonist navoximod (NLG-919/GDC-0919) has also entered a clinical phase I trial for the therapy of advanced solid tumors (NCT02471846). The combination of navoximod and atezolizumab showed acceptable tolerability, safety, and pharmacokinetics for patients with advanced cancer. However, there was no clear evidence of benefit from adding navoximod to atezolizumab although activity was observed. In a CT26 colon carcinoma model with high IDO1 activity, PF-06840003 can reduce over 80% intratumoral kynurenine levels and inhibited tumor growth both in monotherapy and, with an increased efficacy, in combination with a humanized anti-PD-L1 antibody avelumab 48. AMG-1 49, miconazole 50, imidazothiazole derivative BITMC 51, and a 2-aminophenylurea derivatives DFPTA and CUPCA (Figure 3) are some promising lead compounds 52.
Figure 3.
Chemical structures of IDO, TDO, and arginase inhibitors.
Tryptophan-2,3-dioxygenase (TDO)
TDO is significantly overexpressed in glioma, lung, breast and colorectal cancer, which is closely related to malignant progression and poor survival 53-58. In glioma, the higher tumor TDO expression is negatively correlated with CD8+ immune cell infiltration 53. Salter et al. first reported a new TDO inhibitor 680C91 (Figure 3), which could effectively inhibit TDO activity, but the solubility and bioavailability were poor 59. LM10 is a potent TDO inhibitor with higher solubility and better bioavailability as compared to 680C91. The plasma concentration of LM10 is about 330 times over the 680C91 after seven days of oral administration of 160 mg/kg/day. LM10 demonstrated convincing anti-tumor activity in a preclinical assay showing approximately 57% inhibition of mice with tumor progression compared to normal drinking water (P < 0.001) 54. Wu and coworkers synthesized a highly potent TDO inhibitor BNTD, but further evaluations should be done to verify its therapeutic effects in vivo 60. In a word, TDO inhibitors as novel cancer treatments need further investigations.
Arginase
The arginine catabolism pathway is a promising approach to reversing immune suppression in the tumor microenvironment 61. MDSC and TAMs both express ARG1, which can decompose the amino acid arginine into ornithine and urea. The consumption of extracellular arginine leads to the CD3ζ chain depletion of T-cell receptors (TCRs), subsequently causing tolerance of T cell responses to antigens. The concentration of ARG1 in MDSCs is elevated in breast cancer and renal cell carcinoma 62, 63. ARG1 inhibition has been shown to prevent lung carcinoma proliferation in mice 64. Small molecule arginase inhibitors including nor-NOHA 65, 66, BEC 67 and CB-1158 are under evaluation.
Chemokines and chemokine receptors
Chemokines are the pivotal mediators of cancer related chronic inflammation, which modify expression in malignancies, and mediate leukocyte activation and recruitment, angiogenesis, and the proliferation and metastasis of cancer cells. More importantly, the appropriate recruitment of immune cells is orchestrated by the temporal and spatial expression of chemokines and chemokine receptors 68.
CXCR family
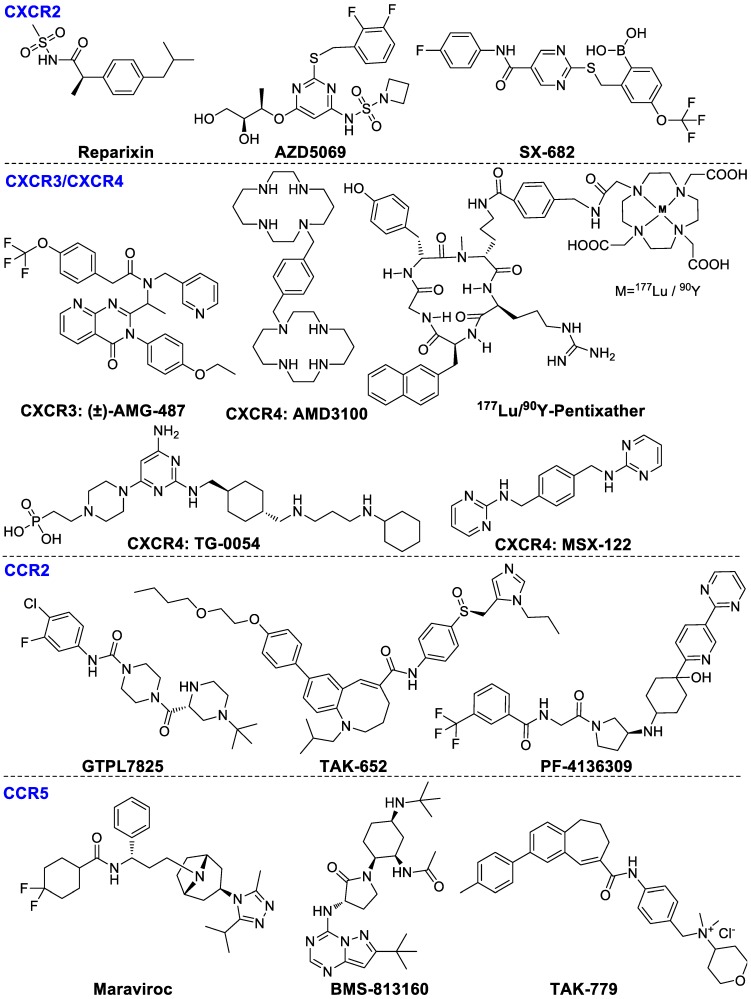
Chemokines, secreted proteins, are critical for lymphoid system development and homing, retention, infiltration and activation of T cells to tumors 69. Chemokines are divided into four main subgroups: CC, XC, CXC, and CX3C. Chemokines are mainly found on the surface of immunocytes, tumor cells and stromal cells. The main immunological function of CXCR2 is to regulate trafficking of neutrophils from the bone marrow to inflammation sites 70. In addition, CXCR2 also modulates MDSCs migrations and mediates local immunosuppression 71. CXCR2-targeted reparixin and PF-04136309 have been investigated in clinical studies for the therapy of breast cancer and pancreatic neoplasms patients respectively 17. The combination AZD5069 (CXCR2 antagonist) with durvalumab (ant-PD-L1) is being investigated in phase Ib/II trials in patients with advanced solid malignancies and metastatic pancreatic ductal adenocarcinoma 72. SX-682 73, a dual CXCR1/2 antagonist, in combination with pembrolizumab is also being investigated in clinical phase I for metastatic melanoma therapy (NCT03161431). CXCR3 is mainly overexpressed by effector CD8+ T cells, NKs and TH1 cells. The ligands of CXCR3 are CXC-chemokine ligand 9 (CXCL9) and CXCL10, whose elevated levels are related with enhanced amounts of tumor-infiltrating CD8+ T cells, subsequently decreased cancer metastasis and improved survival rates of colon cancer and ovarian cancer patients. CXCR3-targeted AMG487 remarkably decreased metastasis and enhanced host anti-tumor immunity in a 4T1 mammary tumor model 74. The CXCR4-CXCL12 signaling pathway mediates Tregs homing to the bone marrow 75 and plasmacytoid precursor dendritic cells transitioning into tumors 76, regulating metastasis and vascularization of the tumor 77. CXCR4 antagonist plerixafor (AMD3100) combining with anti-PD-1 induced T-cell rapid accumulation among cancer cells and acted synergistically with α-PD-L1 to significantly decrease tumor volume 78. In addition, CXCR4-targeted endoradiotherapy with 177Lu- or 90Y-pentixather were well-tolerated and exerted anti-myeloma activity even at patients with advanced stage multiple myeloma. Nevertheless, further assessment of toxicity studies and prospectively designed clinical trials is highly warranted 79. Pentixather also can be developed as different diagnostic agents when it is radiolabeled with shorter half-life radionuclides such as 68Ga 80, 81. Other CXCR4 inhibitors such as TG‑0054 and MSX-122 are being investigated in clinical studies currently 82, 83. The ligand structures of CXCR family are summarized in Figure 4.
Figure 4.
Ligands of chemokine receptors CXCR2, CXCR3, CXCR4, CCR2, and CCR5.
CCR family
The CCR2-CCL2 pathway axis is able to induce macrophage migration into the tumor microenvironment and stimulate tumor proliferation and invasion 84. Various CCR2 antagonists have been investigated in clinical studies including GTPL7825, TAK-652 and PF-04136309. PF-4136309 can enhance antitumor effects of the immune system and inhibit tumor proliferation and invasion in patients with pancreatic cancer 85.
CCR5 is primarily expressed by lymphocytes, macrophages and metastatic tumor cells. The upregulation of CCR5 on CD8+ T cells, TH1 cells, monocytes and macrophages promotes Tregs infiltration and stimulates progenitor cells differentiation into TAMs and MDSCs 86. Maraviroc was shown to block CCR5 and inhibit tumor metastases in colorectal cancer patients in a clinical phase I evaluation (NCT01736813). In addition, BMS-813160, a dual CCR2/5 antagonist, in combination with nivolumab has been developed for the therapy of patients with colorectal and pancreatic cancers (NCT03184870). TAK-779 is able to block migration of tumor-associated Tregs consequently inhibiting tumor growth specifically in pancreatic adenocarcinoma 87. The ligand structures of CCR family are summarized in Figure 4.
Purinergic signaling
Hypoxia activates tumor cells to release the pro-inflammatory adenosine triphosphate (ATP), which is subsequently dephosphorylated to immunosuppressive adenosine by CD39 and CD73. The biological actions of adenosine and ATP depend on the activation of purinergic receptors such as P2Xs, P2Ys, CD39, CD73 and adenosine receptors, which are significantly overexpressed on tumor cells and infiltrating immune cells 88.
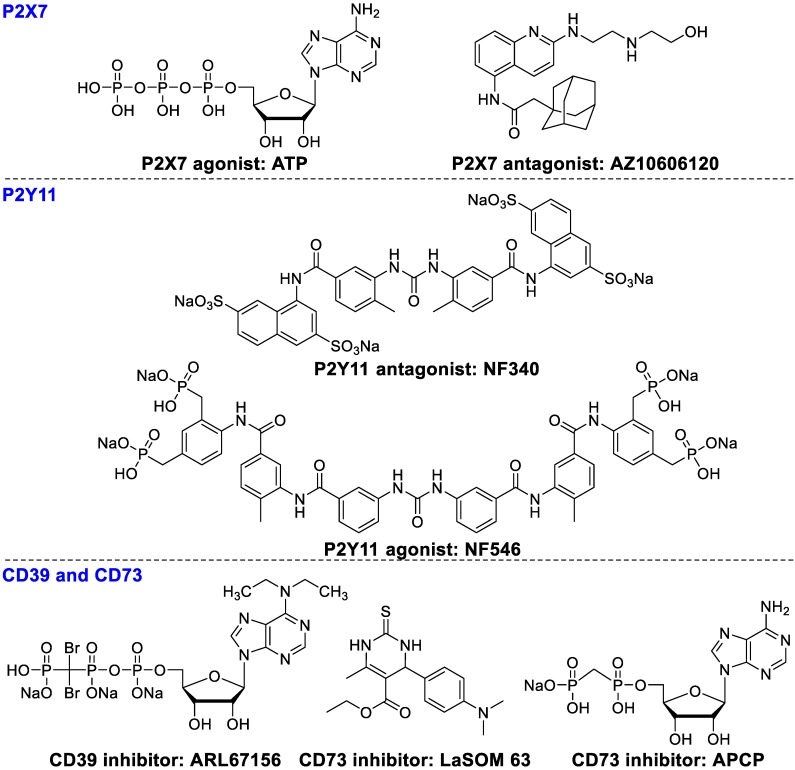
P2 family
P2Xs (ion channel receptors) and P2Ys (G protein-coupled receptors) are overexpressed on various immune cells in the tumor microenvironment. High levels of extracellular ATP can stimulate P2X expression in macrophages and DCs, inducing IL-1β secretion, subsequently enhancing the cytotoxicity of CD8+ T cells. Extracellular ATP can also induce apoptosis of Tregs and diminish immunosuppressive activity 89. However, some studies have shown the opposite results, where overexpression of P2Xs promotes tumor growth and survival in vivo . AZ10606120 (Figure 5), an antagonist of P2X7, significantly inhibited tumor growth. These contradictory data may result from a slow upgrading of extracellular ATP levels, which causes differences in acute apoptotic response. P2Y11 receptors moderate ATP-induced semi-maturation of monocyte-derived dendritic cells and mediate dendritic cell-based immunotherapy 90. P2Y11 antagonist NF546 stimulated thrombospondin-1 and interleukin 8 (IL-8) release and inhibited lipopolysaccharide-stimulated IL-12 secretion, whereas agonist NF340 reversed these effects 91.
Figure 5.
Ligands of P2X7. P2Y11, CD39, and CD73.
CD39 and CD73
CD39 and CD73 play a pivotal role in tumor immunosuppression through converting ATP and ADP to AMP and then to adenosine, resulting in immunosuppression and subsequently the onset and progression of tumor growth 92-94. CD39 is overexpressed on endothelial cells, leukocytes, and B cells 95. CD39 modulates immune and tumor cells to promote tumor growth by catalyzing extracellular ATP or ADP to AMP 96, 97. Subsequently, AMP is hydrolyzed by CD73 into adenosine, which is responsible for immunosuppressive and anti-inflammatory functions of Tregs 93, 98. In addition, T-cell subsets Thpp cells also overexpress CD73 and suppress the CD4+ or CD8+ T cell proliferation in the presence of exogenous AMP 99. ARL67156 (Figure 5) inhibits the activity of CD39 and partially overwhelms hyporesponsiveness of T cell in some patients with follicular lymphoma 100. LaSOM 63 is able to inhibit the activity of Ecto-5' Nucleotidase/CD73 subsequently causing glioma cell apoptosis 101. APCP, a selective CD73 inhibitor, inhibited tumor proliferation and enhanced efficacy of adoptive T cell therapy 102.
Adenosine A2A receptor (A2AR) and adenosine A2B receptor (A2BR)
After ATP is dephosphorylated to adenosine by CD39 and CD73, the accumulated extracellular adenosine interacts with receptors A1R, A2AR, A2BR and A3R which regulate immunosuppressive functions 31, 103. Cyclic AMP (cAMP) is a downstream signaling molecule of adenosine receptors, which is stimulated by A2AR and A2BR, thereby enhancing immunosuppressive functions 104-106.
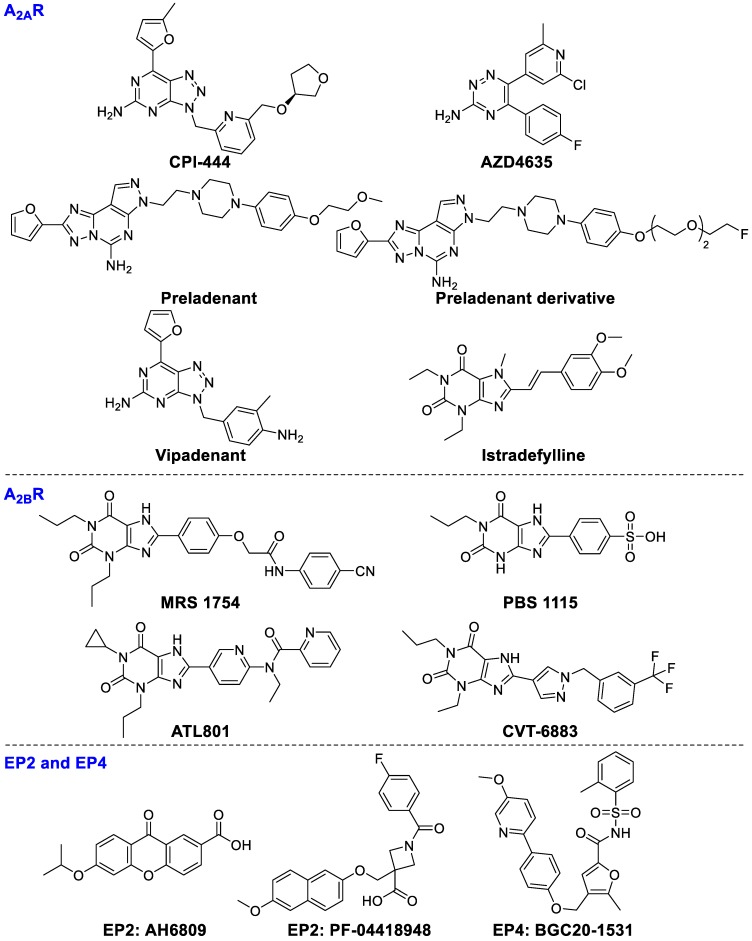
A2AR is mainly expressed on lymphocytes, NKs, DCs, and T cells. Activation of A2AR on T cells markedly inhibits TCR-mediated cytotoxicity and cytokine production, and restrains proliferation of T cells 107. On the other hand, A2AR activation can boost Tregs expansion which ultimately enhances immunosuppressive activity 108. CPI-444, a selective A2AR inhibitor, was used as a mono-drug or combined with atezolizumab (anti-PD-L1 antibody) for the therapies of patients with advanced non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), melanoma, and triple negative breast cancer (TNBC) (NCT02655822). The combination of CPI-444 and anti-PD-1 led to a synergistic inhibition of tumor growth (eliminating tumors in 90% of treated mice) and prolonged survival time compared to either agent alone 109. Based on the promising results, Phase 1b clinical study has been initiated (NCT02655822). Co-targeting A2AR (PBF‑509, structure not disclosed) with durvalumab is being evaluated in patients with NSCLC (NCT02403193). AZD4635 as a mono-agent or combined with durvalumab (ant-PD-L1) is being investigated for the therapy of patients with advanced solid malignancies, NSCLC, metastatic castrate-resistant prostate carcinoma (mCRPC), and colorectal carcinoma (CC) (NCT02740985), but it has not been completed until now. A2AR antagonist preladenant (SCH58261) could enhance NKs activity in mice with B16 melanoma metastasis 110. A fluorinated polyethylene glycol (PEG) derivative of preladenant is confirmed as a promising immunotherapeutic agent 111. Vipadenant and istradefylline are being evaluated in phase II and III studies in Parkinson's disease, which may be promising for treating cancer patients 112.
A2BR receptor is the least sensitive of the four adenosine receptors for the requirement of adenosine concentrations to achieve physiological functions. Expression of A2BR is enormously increased under hypoxic conditions. Activation of A2BR mainly promotes M1 macrophage to M2 macrophage switching, subsequently inhibiting the antitumor T cell activities and promoting angiogenesis in tumors. Under hypoxia, the A2BR overexpression on mature DCs can polarize DCs to a Th2-stimulating phenotype. MRS1754, an A2BR inhibitor, can enhance the secretion of IL-12p70 and TNF-α and increase the production of Th1 cytokine IFN-c in an mDCs-T-cell co-culture system 113. PBS1115, an A2BR-selective antagonist, increases the accumulation of tumor-infiltrating MDSCs in vivo 114. ATL801 not only inhibited the growth of 4T1 breast and MB49 bladder tumors but also reduced the metastasis of breast cancer cells, though it significantly increased the concentration of IFN-γ and chemokine CXCL10 115. CVT-6883, a potent selective A2BR antagonist, has entered into clinical trials to treat pulmonary inflammation and injury 116. Therefore, CVT-6883 is a very promising candidate drug for tumor immunotherapy.
Elevation of cyclic AMP (EP2 and EP4)
The expression of inducible cyclooxygenase (COX2) is correlated with lower survival rates of patients. The metabolites of COX2 are associated with immune tolerance of tumors through stimulating prostaglandin E2 (PGE2) generation to boost tumor proliferation and migration. COX2 upregulation leads to sustained high concentrations of PGE2 117. PGE2 activates its receptors EP2 and EP4 leading to elevation of cAMP levels 118, consequently promoting various immune-suppressive cells activity including Tregs, TAMs, and MDSCs 119-121. AH6809, an EP2 receptor antagonist, can diminish Tregs-mediated immune tolerance 122. PF‑04418948 (EP2 antagonist) 123 and BGC20‑1531 (EP4 antagonist) 124 have been studied in clinical trials for various non-oncology candidates, which may soon expand to anticancer therapy. The chemical structures of ligands are presented in Figure 6.
Figure 6.
Ligands of A2AR, A2BR, EP2, and EP4.
Toll-like receptor (TLR) and stimulator of interferon genes protein (STING)
TLRs and STING are regarded as crucial components of the innate immune sensing of tumors. The activation of TLRs and STING in the innate immune system can enhance the secretion of pro-inflammatory cytokines and T-cell recruitment factors subsequently modulate innate immunity, which is able to resist tumor-induced immunosuppression and shows a synergistic effect with present cancer therapies 18.
TLR
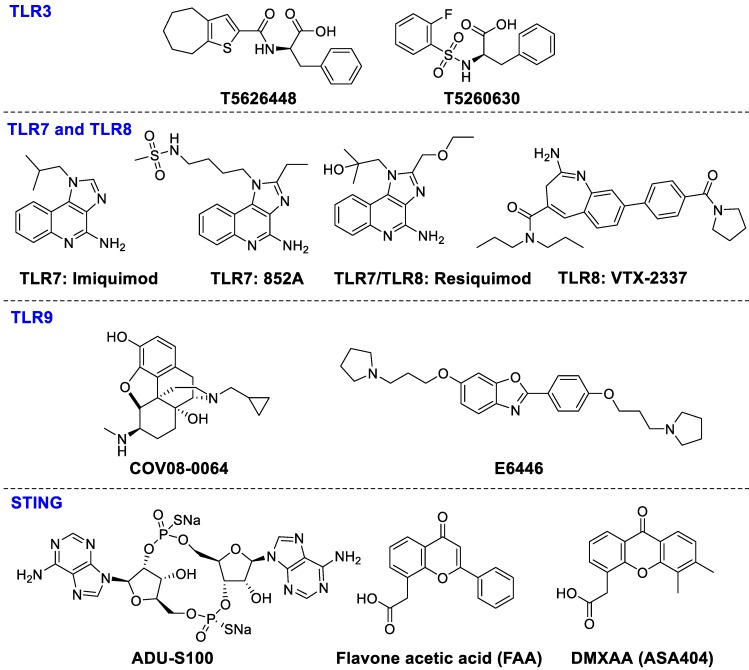
The TLR family is a critical member of the innate immune system 125. TLRs are primarily expressed by DCs, B cells, neutrophils, monocytes and macrophages, along with the gastrointestinal tract and lungs which are exposed to the external environment. TLRs take part in recognizing pathogen-associated and damage-associated molecular patterns 126. The TLR superfamily contains 13 members; TLR3, TLR7, TLR8 and TLR9 are distributed in the endosomal compartment and others in the cytoplasm. TLR agonists are being evaluated in (pre)clinical studies.
The agonists of TLR3, TLR7, TLR8 and TLR9 make up the majority of preclinical and clinical trials of TLRs. TLR3 signaling is stimulated by dsRNA subsequently causing secretion of pro-inflammatory cytokines and type I interferons such as IL-1, and IL-6, TNF-R to stimulate immune cell activation and recruitment during inflammation or viral infection 127. A series of TLR3-targeted inhibitors, such as T5626448 and T5260630, were evaluated in vitro 128. TLR7 and TLR8 are the key targets in the recognition of single-stranded RNA in certain cell types, such as pDC 129. Imiquimod significantly enhanced CD8+ T cell accumulation in spleen and draining lymph nodes after administration of DC vaccination 130, 131. Resiquimod, stimulating TLR7 and TLR8, is able to activate immune responses effectively against viral infections and tumors. Resiquimod is in clinical phase II studies for the therapies of viral skin lesions and skin cancer 132. 852A (TLR7 agonist) and VTX-2337 (TLR8 agonist) have been investigated in phase I for treating subjects with advanced solid tumors and lymphoma 133, 134. TLR9 has high affinity for unmethylated and CpG-rich DNA, which is an endosomal receptor for dsDNA in the extracellular compartment. TLR9 agonists COV08-0064 and E6446, can inhibit TLR9-mediated sterile inflammation in acute liver injury and acute pancreatitis models and restrain responses of deleterious inflammation in rodent malaria, respectively 135, 136. Although several trials with TLR modulators are underway, more investigation should be done to achieve more clinical benefits. The chemical structures of TLR ligands are summarized in Figure 7.
Figure 7.
Ligands of TLR3, TLR7, TLR8, TLR9, and STING.
STING
Transmembrane protein 173 (TMEM173), is expressed in T cells, DCs, and macrophages as well as in various epithelial and endothelial cells. STING activation results in the secretion of cytokines, interferons, and T-cell recruitment factors subsequently modulating innate immunity 137, 138.
STING signals can be activated by cyclic dinucleotides such as cyclic di-GMP and cGAMP, which can induce the expression of interferon-β 139. Recently, ADU-S100 ((R, R)-S2-CDA) is being evaluated in a clinical phase I study for treating advanced/metastatic solid tumors and lymphomas (NCT02675439). MK-1454 (structure undisclosed) is being studied for treating advanced/metastatic solid tumors or lymphoma in a clinical phase I study (NCT03010176). Flavone acetic acid (FAA) and DMXAA (ASA404) both failed in clinical trials for the therapy of advanced cancer 140. Despite some compounds being in clinical trials for antitumor therapy, administration methods and combinations with other drugs need to be further investigated. The chemical structures of STING ligands are presented in Figure 7.
Kinase
Kinase signaling pathways can drive many hallmark phenotypes of tumor biology such as metabolism, proliferation, and metastasis. Tumor cells can exert a considerable impact on the microenvironment to inhibit anti-tumor immune responses and escape the pivotal phylactic mechanism. The application of kinase inhibitors can directly inhibit tumor cells, reduce their immunosuppressive influences, and shift the local immunosuppression toward a proinflammatory state, subsequently boosting the activity of the immune activators. Therefore, the combination of kinase inhibitors such as PI3K, MAPK, BRAF, and MEK1/2 inhibitors with immune checkpoint inhibitors is a significant opportunistic proposition to increase the utility of immune modulation in oncology. Various pathways involving kinase inhibitors need to be elucidated to optimize their application in this setting 141.
PI3K-AKT-mTOR
Recently, inhibiting the PI3K-AKT-mTOR signal pathway has been evaluated to promote the production of immunosuppressive cytokines 142, 143, the tumor infiltration of MDSCs and Tregs, thereby inhibiting proliferation, migration and survival of tumor cells 144, 145. Thus, it is understandable that PI3K-AKT-mTOR inhibitors plus checkpoint blockade would be effective 146. PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ from the PI3K family are well studied in anti-tumor immunotherapy. PI3Kγ and PI3Kδ are primarily expressed by B and T cells and myeloid lineage cells 17.
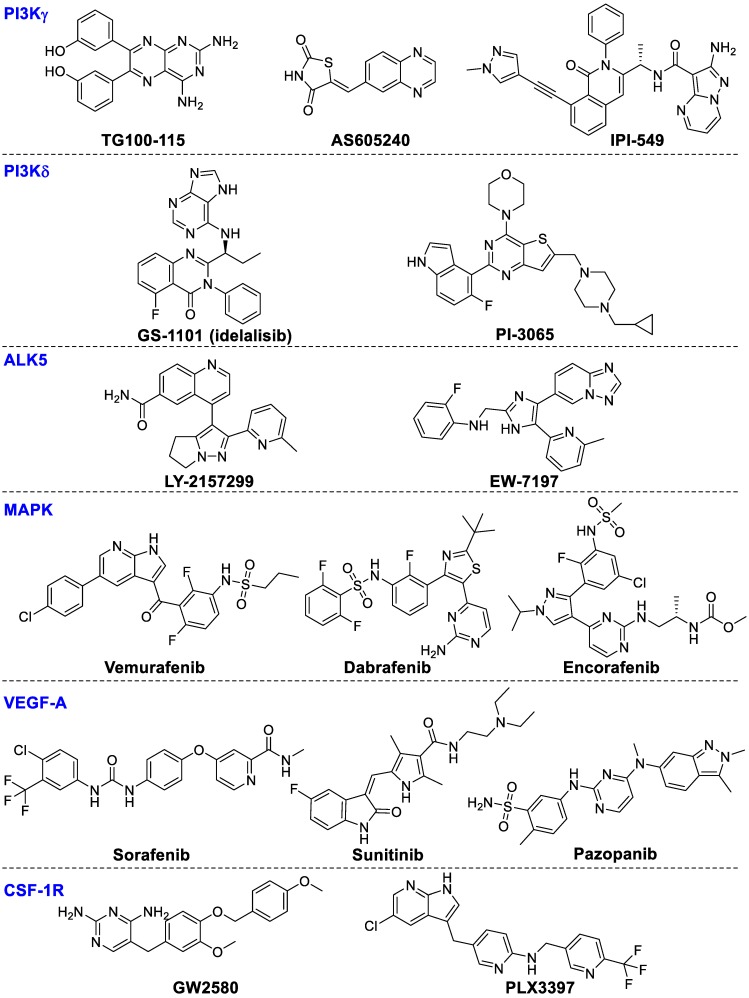
PI3Kγ
PI3Kγ mainly regulates the innate immune response of myeloid cells by regulating integrin α4β1-dependent macrophage chemotaxis into tumors and suppressing the proliferation and metastasis of tumors 147. TG100-115 and AS605240 could inhibit inflammation, angiogenesis and tumor proliferation in lung carcinoma but without directly affecting tumor cells 147. Infinity (IPI-549) could powerfully inhibit PI3K-γ-mediated neutrophil migration and is presently in clinical phase I studies for the therapy of advanced solid tumors 148. In addition, the combination of IPI-549 with anti-PD-1 treatment enhanced gene expression of anti-tumor immunity and inhibited gene expression of immune-suppressors, thereby hampering tumor growth in primary tumors from human papilloma virus (HPV)+ head and neck squamous cell carcinoma 149. These results suggest that PI3Kγ inhibition can synergize with T-cell-targeted immunotherapy to promote anti-tumor immune response.
PI3Kδ
PI3Kδ is mainly involved in modulating B-cell proliferation and differentiation. GS‑1101 (idelalisib), a PI3Kδ selective inhibitor, has been approved for treating chronic lymphocytic leukemia. Recently, preclinical results suggest PI3Kδ inhibition in Tregs results in boosting anti-tumor T-cell function and restricting tumor proliferation 34. Idelalisib in combination with pembrolizumab is being investigated for the therapy of chronic lymphocytic leukemia and non-Hodgkin lymphomas (NCT02332980). PI‑3065, a selective PI3Kδ inhibitor, can inhibit tumor proliferation and metastasis in 4T1 breast cancer models. The likely mechanism of PI‑3065's immune regulatory effect may result from enhancing the anti-tumor immune effect though inhibiting Tregs and MDSC function. These results showed PI3K-AKT-mTOR signaling pathways are important targets for the regulation of innate immunity. The chemical structures of the PI3Kγ or PI3Kδ inhibitors are summarized in Figure 8.
Figure 8.
Chemical structures of kinase inhibitors.
Activin receptor-like kinase 5 (ALK5)
TGF-β binds to ALK5 and TGF-β receptor type 2 to mediate phosphorylation of SMAD2 and SMAD3. Recently, LY-2157299, a selective ALK5 inhibitor, was able to block TGF-β signaling and inhibit tumor progression in preclinical models. LY-2157299 has entered a Phase I clinical study to evaluate antitumor activity in glioma patients 150. EW-7197 was reported to enhance activation of cytotoxic T lymphocytes thereby inhibiting tumor growth in melanoma-bearing mice 33.
Mitogen-activated protein kinase (MAPK)
The MAPK signal axis is activated by various mechanisms, and is a very important target for pathway targeting therapies especially for melanoma metastasis treatment 151, 152. Among the MAPK signaling cascades, the RAS-RAF-MEK-ERK1/2 pathway is very important for CD8 T cell activation, proliferation, and survival, subsequently regulating tumor proliferation and survival 153, 154. Inhibiting the MAPK signaling axis by MEK and B-Raf inhibitors has been an effective therapy for patients with metastatic tumors bearing B-Raf mutations 155. The approved B-Raf inhibitors include vemurafenib and dabrafenib, while encorafenib is being evaluated in multiple phase III trials. In addition, various MEK inhibitors also have been approved such as cobimetinib and trametinib, while binimetinib is presently being studied in various clinical phase III studies. Combinations of MEK inhibitor trametinib with checkpoint inhibitors were more effective than any single drug 156. Clinical evaluation of such combination strategies is underway. It is possible to expand these combinational therapeutic strategies towards other cancer types beyond melanoma.
Vascular endothelial growth factor A (VEGF-A)
VEGF-A, a proangiogenic factor produced by malignancies, can enhance the expression of PD-1 on CD8+ T cells through an overexpressed VEGF receptor causing the exhaustion of cytotoxic immune cells, which could be reversed by anti-angiogenic agents targeting VEGF-A-VEGFR 157. Some small-molecule VEGF inhibitors including sorafenib, sunitinib and pazopanib have been approved for renal cell cancer. VEGF inhibition enhanced the amounts of tumor-infiltrated effector T-cells and reduced Tregs accumulation in the tumor microenvironment in patients with primary and metastatic renal cell carcinoma 158. VEGF inhibitors in combination with anti-PD-1 or anti-PD-L1 showed positive treatment benefits in patients with renal cell carcinoma or clear-cell metastatic renal cell carcinoma (NCT01472081).
CSF-1 receptor (CSF-1R)
CSF-1R signaling is important for recruitment and function of distinct tumor-infiltrating myeloid cells subsets, including TAMs and MDSCs 159. CSF-1R inhibitor GW2580 combined with an anti-VEGFR-2 antibody synergistically inhibits tumor proliferation and hampers tumor angiogenesis 159. PLX3397, a selective CSF-1R inhibitor, is being investigated in clinical trials alone or combined with paclitaxel or checkpoint immunotherapies, or radiation therapy for treating patients with breast cancer, metastatic pancreatic cancer, glioblastoma, and other cancers 17, 160.
Small molecules for imaging cancer immunotherapy
With the increase in development of personalized-medicine approaches, discoveries of novel immune mechanisms and more selective-targeted drugs approvals, clinicians and researchers need novel methods for exploring the interaction and relationships between tumor cells, the immune system, and immunotherapy agents. It is essential to diagnose whether the patient expresses the related targets before drug administration 161. In addition, it is critical to track the dynamic changes of the targets in immune cells and in tumor cells, to guide clinicians when to switch one drug to another or to determine if a patient no longer needs to receive costly therapy 19. In this way, imaging will provide guidance for physicians to make better decisions on therapeutic regimens and patient follow-up. Several comprehensive reviews on molecular imaging in immunotherapy have been published previously 19, 161-169.
Antibodies can be quickly radiolabeled for imaging via conjugation to a contrast agent or radionuclide. In addition, radiolabeled antibodies keep their naturally high specificity and binding affinity toward their cognate antigens. However, the slow clearance rate and relatively poor penetration into target tissues are drawbacks of radiolabeled-antibody tracers 19. Clinicians must often wait several days before the background signal clears from blood circulation and various non-target tissues. Conversely, radiolabeled small molecules tracers not only possess excellent pharmacokinetic characteristics but also can go across cellular membranes and other physiological barriers and reach intracellular targets. In addition, the manufacturing costs of small molecules tracers are lower than radiolabeled antibodies.
PD-1/PD-L1 immune checkpoint
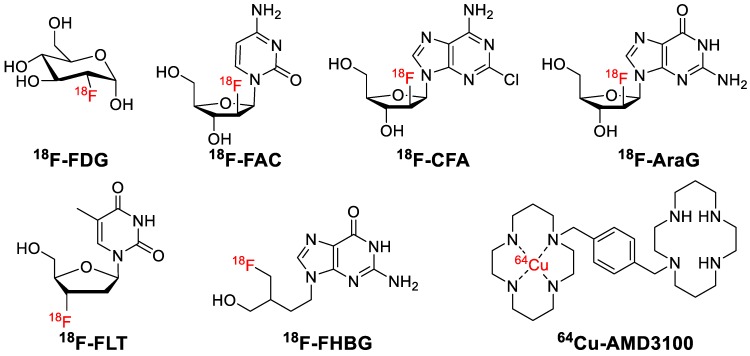
Early detection of therapy response is very pivotal for patients undergoing anti-cancer immunotherapy. 18F-labeled fluorodeoxyglucose (18F-FDG) (Figure 9) is the most widely used PET probe in nuclear medicine, which is being tested in immunotherapy settings. Chen and co-workers reported that the uptake of 18F-FDG has a positive correlation with the expression of both PD-1 and PD-L1 in patients with bladder tumors 170. In addition, Ferdinand and colleagues showed that 18F-FDG PET can reliably identify cancer patients who will most benefit from PD-1-therapy as early as two weeks after therapy initiation in stage IV melanoma (Figure 10A) 171. However, 18F-FDG used as an immuno-imaging PET tracer needs to be further evaluated in additional clinical trials. It is also expected that the results from 18F-FDG can be compared with other specific PD-L1 binding peptide probes, such as 64Cu-WL12 172.
Figure 9.
Small molecule-based PET probes for immuno-oncology imaging
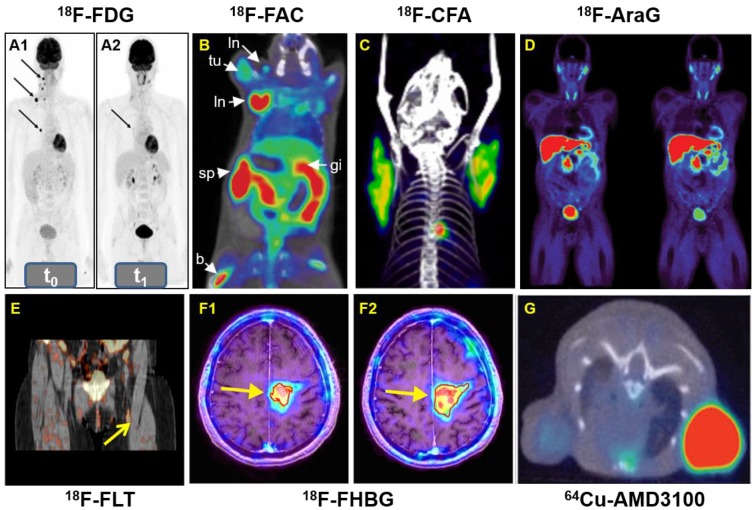
Figure 10.
A: Whole-body 18F-FDG-PET examination at two time points: before PD-1-therapy start (t0, base-line) (A1) and two weeks (t1, study examination) (A2). B: 18F-FAC was used to evaluate lymphoid organs and immune activation, tu: tumor; ln: lymph node; sp: spleen; gi: gastrointestinal tract; b: bone. C: Detection of immune responses after immunotherapy in glioblastoma using 18F-CFA. D: Pharmacokinetics of 18F-AraG in a healthy human volunteer to prepare to visualize activated T cells in acute graft-versus-host disease. E: Early identification of antigen-specific immune responses in vivo by18F-FLT. F: Reporter gene imaging of targeted T-cell immunotherapy in recurrent glioma by 18F-FHBG, F1: Pre-immunotherapy using CD8+ cytotoxic T lymphocytes (CTLs), F2: Post-immunotherapy using CD8+ CTLs. G: 64Cu-AMD3100-PET/CT for imaging of CXCR4 expression in subcutaneous brain tumor xenografts.
Metabolism of T Cell
PET probes targeting vital metabolic pathways, such as glucose metabolism and nucleotide synthesis and metabolism can potentially be used to monitor the efficacy of immunotherapy involved in innate and adaptive immunity 165.
18F-FAC and 18F-CFA
In 2008, Radu and co-workers synthesized 1-(2'-deoxy-2'-[18F]fluoroarabinofuranosyl) cytosine (18F-FAC) to map the deoxyribonucleotide salvage pathway 173. 18F-FAC was able to visualize lymphoid organs and was adequately sensitive to localize immune activation in an antitumor immunity mouse model. Additionally, early changes of lymphoid mass in systemic autoimmunity was detected by 18F-FAC (Figure 10B), which allowed for the real-time evaluation of immunosuppressive therapy. All the results confirmed 18F-FAC can be used for monitoring the process of immune response. However, the clinical application was limited by its rapid catabolism. Kim and colleagues developed an analogue of 18F-FAC, 18F-Clofarabine (18F-CFA), which accumulates in tissues with high dCK expression such as hematopoietic bone marrow and secondary lymphoid organs (Figure 10C) 174. Further studies proved that 18F-CFA might be a promising tracer to image the host antitumor immune response against intracranial tumors 175.
18F-AraG
9-(β-D-Arabinofuranosyl)guanine (AraG) is an analog of guanosine that has a demonstrable effectiveness for the therapy of T-cell lymphoblastic disease such as recurrent T-cell lymphoblastic leukemia and T-cell lymphoblastic lymphoma. AraG is triphosphorylated by multiple kinases to AraGTP, which preferentially distributes in malignant T-cells. 18F-AraG was synthesized and radiolabeled by Ronald et al in 2011, which showed favorable pharmacokinetic properties in healthy humans. PET imaging of 18F-AraG may also provide essential information for the early diagnosis of activated T cells in acute graft-versus-host disease (Figure 10D) 176.
18F-FLT
Fluorothymidine (FLT), a nucleoside analog, can be quickly absorbed by nucleoside transporters expressed on proliferating cells and then is phosphorylated by the S-phase specific thymidine kinase 1 (TK1), subsequently trapping it within the cells 177. The uptake level of 18F-FLT in lymph nodes correlates to the level of antigen-specific IgG antibodies and antigen-specific proliferation of T cells in the blood of patients with metastatic melanoma who received dendritic cell vaccine therapy (Figure 10E) 178.
Reporter genes of targeted T cells
Reporter gene imaging of engineered T cells is possible when the T cells are transfected with a PET reporter gene which encodes a protein. Reporter gene imaging plays an important role in visualizing their targeting/trafficking, proliferation/expansion, and retention/death using highly sensitive reporter systems, which would provide useful theranostic information 179.
Herpes simplex virus type 1 thymidine kinase (HSV-TK) is a viral nucleoside kinase which is coded by the HSV-TK gene (HSV-tk), whose substrates are thymidine or non-natural nucleosides 180. Preclinical results showed that 18F-labeled 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (18F-FHBG) was more sensitive than 14C-labeled 1-(2'-deoxy-2'-fluoro-β-D-arabinofuranosyl)-5-iodouracil (FIAU) (14C-FIAU) in the HSV1-sr39tk system 181. In 2017, Khun and co-workers reported immunotherapy using CD8+ cytotoxic T lymphocytes engineered to express both HSV1-TK and IL-13 zetakine chimeric antigen receptor (CAR), which is a promising therapy strategy for patients with recurrent glioma (Figure 10F) 179. Although 18F-FHBG PET imaging was safe and facilitated the longitudinal imaging of T cells stably transfected with a PET reporter gene in patients, problems with specificity and viral gene editing in humans may limit their primary application to ex vivo immune cell manipulation.
CXCR4-based immune cells
CXCR4 plays a pivotal role in recruiting immune cells and homing stem cells and progenitor cells 182-184. CXCR4 is overexpressed on multiple human tumor types including esophageal, prostate, ovarian, and renal cell carcinoma, boosting tumor proliferation and metastasis 185, 186. Jacobson and co-workers synthesized CXCR4-specific tracer 64Cu-AMD3100, which showed accumulation in CXCR4-expressing organs and tissues 187. Then Nimmagadda and colleagues reported that 64Cu-AMD3100 possessed optimized pharmacokinetics and can be applied to decipher graded levels of CXCR4 expression in subcutaneous brain tumor xenografts (Figure 10G) 188. 64Cu-AMD3100 is a promising PET tracer for diagnosis of CXCR4 expression.
Conclusion and perspective
Despite cancer immunotherapy having achieved clinical successes in the past decade, only about 30% of patients have benefited from immunotherapies. There are still many challenges for immuno-theranostics in cancer: 1) additional immune regulatory mechanisms to expand patients' response rates to immunotherapy need to be explored; 2) special immune-competent animal models are required, including transplantable, spontaneous, carcinogen-induced, or genetically engineered humanized malignancies 189; 3) immunotherapeutic effects may also be obstructed by external conditions such as certain bacterial or viral infections, which will modify the immune system 190, 191; 4) reducing or avoiding toxicities caused by general systemic immune activation and developing safer and more effective drugs is essential 192; and 5) more advanced imaging techniques and better characteristic probes need to be developed in order to achieve earlier diagnosis and offer more biological information about multiple cancers 19.
The combination of small-molecule drugs with biologic checkpoint inhibitors is an effective strategy to increase response rates of patients and the efficacy of immunotherapy. It is critical to develop promising imaging technologies and probes to monitor target expression, estimate therapeutic efficacy and potential toxic reactions, and identify who will benefit from immunotherapies. In order to achieve better cancer immune theranostic effect, 1) both intracellular signal pathways down/upstream of immune checkpoints and related therapeutic agents need to be explored; 2) new imaging techniques such as quantum-inspired imaging need to be developed to provide clearer images; 3) mathematic modeling to increasingly derive guiding principles for imaging design and application needs to be optimized; 4) radiomics enabling data to be extracted and applied to improve cancer diagnostic, prognostic, and predictive accuracy should be employed; and 5) more immunoimaging agents need to be developed to keep pace with drug development 19, 193, 194. In addition, immunotherapies can be extended to autoimmune diseases such as graft-versus-host disease 176 and rheumatoid arthritis 195, multiple sclerosis 196 and neurodegenerative diseases 197, 198. Furthermore, artificial intelligence and machine learning will likely improve the efficiency of drug screening and help physicians make better treatment plans.
Acknowledgments
This work was supported by the USC Research Center for Liver Diseases Pilot Funding (NIH Grant No. P30 DK048522), the Whittier Foundation for Translational Research, the USC Department of Radiology, and the China Scholarship Council (CSC) program (No. 201806310056).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn M-J. et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med. 2019;7:347–57. doi: 10.1016/S2213-2600(18)30500-9. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–15. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 5.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ. et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich MJ. Immunotherapy 2.0: Improving the response to checkpoint inhibitors. JAMA. 2019;321:131–3. doi: 10.1001/jama.2018.18306. [DOI] [PubMed] [Google Scholar]
- 7.Allison JP, Krummel M. The Yin and Yang of T cell costimulation. Science. 1995;270:932. doi: 10.1126/science.270.5238.932. [DOI] [PubMed] [Google Scholar]
- 8.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–68. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Yan H, Qiu W, Koehne de Gonzalez AK, Wei JS, Tu M, Xi CH. et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019;442:333–40. doi: 10.1016/j.canlet.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovas Res. 2019;115:854–68. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–3. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 13.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng B, Yuan WE, Su J, Liu Y, Chen J. Recent advances in small molecule based cancer immunotherapy. Eur J Med Chem. 2018;157:582–98. doi: 10.1016/j.ejmech.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–27. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 17.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14:603–22. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 18.Bayard R. Huck LK, and Klaus Urbahns. Small molecules drive big improvements in immuno-oncology therapies. Angew Chem Int Ed. 2018;57:4412–28. doi: 10.1002/anie.201707816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer AT, Gambhir SS. The immunoimaging toolbox. J Nucl Med. 2018;59:1174–82. doi: 10.2967/jnumed.116.185967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Ozpiskin OM, Zhang L, Li JJ. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics. 2019;9:1215–31. doi: 10.7150/thno.32648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis. 2012;29:381–95. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 23.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–47. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 24.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–86. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 27.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J. et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madar S, Goldstein I, Rotter V. 'Cancer associated fibroblasts' - more than meets the eye. Trends Mol Med. 2013;19:447–53. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–24. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 31.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–92. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson FM, Gray NS. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 2018;17:353–77. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 33.Yoon JH, Jung SM, Park SH, Kato M, Yamashita T, Lee IK. et al. Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Mol Med. 2013;5:1720–39. doi: 10.1002/emmm.201302524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL. et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–39. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Plieth J, Elmhirst E. PD-1/PD-L1 combination therapies. EvaluatePharma. 2017; May, 2017. https://info.evaluategroup.com/PD1-EPV.html.
- 38.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi L, Chen S, Yang L, Y L. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:1–6. doi: 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY, Zhang PF. et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics. 2019;9:4678–87. doi: 10.7150/thno.36276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zak KM, Grudnik P, Guzik K, Zieba BJ, Musielak B, Dömling A. et al. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1) Oncotarget. 2016;7:30323–35. doi: 10.18632/oncotarget.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soremekun OS, Olotu FA, Agoni C, Soliman MES. Recruiting monomer for dimer formation: resolving the antagonistic mechanisms of novel immune check point inhibitors against Programmed Death Ligand-1 in cancer immunotherapy. Mol Simulat. 2019;45:777–89. [Google Scholar]
- 43.Skalniak L, Zak KM, Guzik K, Magiera K, Musielak B, Pachota M. et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget. 2017;8:72167–81. doi: 10.18632/oncotarget.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr WG, Chisholm JD. The next generation of immunotherapy for cancer: small molecules could make big waves. J Immunol. 2019;202:11–9. doi: 10.4049/jimmunol.1800991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platten M, Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2014;5:1–7. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K. et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–30. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 47.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41–8. doi: 10.1007/s00281-018-0702-0. [DOI] [PubMed] [Google Scholar]
- 48.Gomes B, Driessens G, Bartlett D, Cai D, Cauwenberghs S, Crosignani S. et al. Characterization of the selective indoleamine 2,3-dioxygenase-1 (IDO1) catalytic inhibitor EOS200271/PF-06840003 supports IDO1 as a critical resistance mechanism to PD-L1 blockade therapy. Mol Cancer Ther. 2018;17:2530–42. doi: 10.1158/1535-7163.MCT-17-1104. [DOI] [PubMed] [Google Scholar]
- 49.Meininger D, Zalameda L, Liu Y, Stepan LP, Borges L, McCarter JD. et al. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta. 2011;1814:1947–54. doi: 10.1016/j.bbapap.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Rohrig UF, Majjigapu SR, Chambon M, Bron S, Pilotte L, Colau D. et al. Detailed analysis and follow-up studies of a high-throughput screening for indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors. Eur J Med Chem. 2014;84:284–301. doi: 10.1016/j.ejmech.2014.06.078. [DOI] [PubMed] [Google Scholar]
- 51.Tojo S, Kohno T, Tanaka T, Kamioka S, Ota Y, Ishii T. et al. Crystal structures and structure-activity relationships of imidazothiazole derivatives as IDO1 inhibitors. ACS Med Chem Lett. 2014;5:1119–23. doi: 10.1021/ml500247w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinmann H. Cancer immunotherapy: selected targets and small-molecule modulators. ChemMedChem. 2016;11:450–66. doi: 10.1002/cmdc.201500566. [DOI] [PubMed] [Google Scholar]
- 53.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 54.Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R. et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109:2497–502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D'Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS. et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–64. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS. et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–98. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen IC, Lee KH, Hsu YH, Wang WR, Chen CM, Cheng YW. Expression pattern and clinicopathological relevance of the indoleamine 2,3-Dioxygenase 1/tryptophan 2,3-dioxygenase protein in colorectal cancer. Dis Markers. 2016;2016:8169724. doi: 10.1155/2016/8169724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu CP, Song YL, Zhu ZM, Huang B, Xiao YQ, Luo DY. Targeting TDO in cancer immunotherapy. Med Oncol. 2017;34:73. doi: 10.1007/s12032-017-0933-2. [DOI] [PubMed] [Google Scholar]
- 59.Salter M, Hazelwood R, Pogson CI. et al. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem Pharmacol. 1995;49:1435–42. doi: 10.1016/0006-2952(95)00006-l. [DOI] [PubMed] [Google Scholar]
- 60.Wu JS, Lin SY, Liao FY, Hsiao WC, Lee LC, Peng YH. et al. Identification of substituted naphthotriazolediones as novel tryptophan 2,3-dioxygenase (TDO) inhibitors through structure-based virtual screening. J Med Chem. 2015;58:7807–19. doi: 10.1021/acs.jmedchem.5b00921. [DOI] [PubMed] [Google Scholar]
- 61.Arlauckas SP, Garren SB, Garris CS, Kohler RH, Oh J, Pittet MJ. et al. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics. 2018;8:5842–54. doi: 10.7150/thno.26888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boniface J, Mao Y, Schmidt-Mende J, Kiessling R, Poschke I. Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology. 2012;1:1305–12. doi: 10.4161/onci.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R. et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulo C. Rodriguez, David G. Quiceno, Jovanny Zabaleta, Blair Ortiz, Arnold H. Zea, Maria B. Piazuelo, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 65.Havlinova Z, Hroch M, Nagy A, Sispera L, Holecek M, Chladek J. Single- and multiple-dose pharmacokinetics of arginase inhibitor Nω-hydroxy-nor-L-arginine, and its effect on plasma amino acids concentrations in Wistar rats. Gen Physiol Biophys. 2014;33:189–98. doi: 10.4149/gpb_2013078. [DOI] [PubMed] [Google Scholar]
- 66.Havlinova Z, Babicova A, Hroch M, Chladek J. Comparative pharmacokinetics of Nω-hydroxy-nor-L-arginine, an arginase inhibitor, after single-dose intravenous, intraperitoneal and intratracheal administration to brown Norway rats. Xenobiotica. 2013;43:886–94. doi: 10.3109/00498254.2013.780672. [DOI] [PubMed] [Google Scholar]
- 67.Noel N. Kim, J. David Cox, Ricky F. Baggio, Frances A. Emig, Sanjay K. Mistry, Sandy L. Harper, et al. Probing erectile function: S-(2-boronoethyl)-L-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum. Biochemistry. 2001;40:2678–88. doi: 10.1021/bi002317h. [DOI] [PubMed] [Google Scholar]
- 68.Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol. 2019;10:1–10. doi: 10.3389/fimmu.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–72. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Highfill S, Cui Y, Giles A, Smith J, Zhang H, Morse E. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicholls DJ, Wiley K, Dainty I, MacIntosh F, Phillips C, Gaw A. et al. Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther. 2015;353:340–50. doi: 10.1124/jpet.114.221358. [DOI] [PubMed] [Google Scholar]
- 73.Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–32. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci. 2009;1173:310–7. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 75.Zou L, Barnett B, Safah H, LaRussa V, Hogan M, Mottram P. et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–5. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 76.Zou W, Machelon V, Hermin A, Borvak J, Nome F, Isaeva T. et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 77.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan M. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 78.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lapa C, Herrmann K, Schirbel A, Hanscheid H, Luckerath K, Schottelius M. et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed multiple myeloma. Theranostics. 2017;7:1589–97. doi: 10.7150/thno.19050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haug AR, Leisser A, Wadsak W, Mitterhauser M, Pfaff S, Kropf S. et al. Prospective non-invasive evaluation of CXCR4 expression for the diagnosis of MALT lymphoma using [68Ga]Ga-Pentixafor-PET/MRI. Theranostics. 2019;9:3653–8. doi: 10.7150/thno.31032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lapa C, Schreder M, Schirbel A, Samnick S, Kortum KM, Herrmann K. et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - Comparison to [18F]FDG and laboratory values. Theranostics. 2017;7:205–12. doi: 10.7150/thno.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3:47–75. doi: 10.7150/thno.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peled A, Abraham M, Avivi I, Rowe JM, Beider K, Wald H. et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin Cancer Res. 2014;20:469–79. doi: 10.1158/1078-0432.CCR-13-1302. [DOI] [PubMed] [Google Scholar]
- 84.Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011;30:125–40. doi: 10.1007/s10555-011-9280-5. [DOI] [PubMed] [Google Scholar]
- 85.Ziranu P, Atzori F, Puzzoni M, Demurtas L, Astara G, Scartozzi M. Effective combinatorial immunotherapy for castration-resistant prostate cancer: new future chance? Transl Cancer Res. 2017;6:S1014–7. [Google Scholar]
- 86.Weitzenfeld P, Ben-Baruch A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 2014;352:36–53. doi: 10.1016/j.canlet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Tan M, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE. et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–55. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6:1–9. doi: 10.1186/s40425-018-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 90.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic dells. J Immunol. 2001;166:7172–7. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 91.Meis S, Hamacher A, Hongwiset D, Marzian C, Wiese M, Eckstein N. et al. NF546 [4,4'-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)-carbonylimino))-bis(1,3-xylene-α,α'-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J Pharmacol Exp Ther. 2009;332:238–47. doi: 10.1124/jpet.109.157750. [DOI] [PubMed] [Google Scholar]
- 92.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–7. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–67. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–44. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kansas G, Wood G, Tedder T. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991;146:2235–44. [PubMed] [Google Scholar]
- 96.Bono MR, Fernandez D, Flores-Santibanez F, Rosemblatt M, Sauma D. CD73 and CD39 ectonucleotidases in T cell differentiation: beyond immunosuppression. FEBS Lett. 2015;589:3454–60. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 97.Takenaka MC, Robson S, Quintana FJ. Regulation of the T cell response by CD39. Trends Immunol. 2016;37:427–39. doi: 10.1016/j.it.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D. et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–43. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-ddenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 100.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O. et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–66. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Figueiró F, Mendes FB, Corbelini PF, Janarelli F, Jandrey EH, Russowsky D. et al. A monastrol-derived compound, LaSOM 63, inhibits ecto-5'Nucleotidase/CD73 activity and induces apoptotic cell death of glioma cell lines. Anticancer Res. 2014;34:1837–42. [PubMed] [Google Scholar]
- 102.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ. et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–82. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–24. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 104.A Ohta, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;41:916–20. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 105.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK. et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vecchio EA, Tan CY, Gregory KJ, Christopoulos A, White PJ, May LT. Ligand-independent adenosine A2B receptor constitutive activity as a promoter of prostate cancer cell proliferation. J Pharmacol Exp Ther. 2016;357:36–44. doi: 10.1124/jpet.115.230003. [DOI] [PubMed] [Google Scholar]
- 107.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:1–12. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Willingham S, Ho P, Leone R, Choy C, Powell J, McCaffery I. et al. Adenosine A2A receptor antagonist, CPI-444, blocks adenosine-mediated T cell suppression and exhibits anti-tumor activity alone and in combination with anti-PD-1 and anti-PD-L1. Ann Oncol. 2016;27:vi359–78. [Google Scholar]
- 110.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C. et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110:14711–6. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan G, Jankins TC, Patrick CG Jr, Philbrook P, Sears O, Hatfield S. et al. Fluorinated adenosine A2A receptor antagonists inspired by preladenant as potential cancer immunotherapeutics. Int J Med Chem. 2017;2017:4852537. doi: 10.1155/2017/4852537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allard D, Turcotte M, Stagg J. Targeting A2 adenosine receptors in cancer. Immunol Cell Biol. 2017;95:333–9. doi: 10.1038/icb.2017.8. [DOI] [PubMed] [Google Scholar]
- 113.Yang M, Ma C, Liu S, Shao Q, Gao W, Song B. et al. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2010;88:165–71. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 114.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets ─ what are the challenges? Nat Rev Drug Discov. 2013;12:265–86. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kalla RV, Zablocki J. Progress in the discovery of selective, high affinity A2B adenosine receptor antagonists as clinical candidates. Purinerg Signal. 2009;5:21–9. doi: 10.1007/s11302-008-9119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–91. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 118.Duan B, Davis R, Sadat EL, Collins J, Sternweis PC, Yuan D. et al. Distinct roles of adenylyl cyclase VII in regulating the immune responses in mice. J Immunol. 2010;185:335–44. doi: 10.4049/jimmunol.0903474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K. et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis. 2011;32:1333–9. doi: 10.1093/carcin/bgr128. [DOI] [PubMed] [Google Scholar]
- 120.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–54. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 121.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT. et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285:27571–80. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Birrell MA, Nials AT. At last, a truly selective EP2 receptor antagonist. Br J Pharmacol. 2011;164:1845–6. doi: 10.1111/j.1476-5381.2011.01494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Antonova M, Wienecke T, Maubach K, Thomas E, Olesen J, Ashina M. The pharmacological effect of BGC20-1531, a novel prostanoid EP4 receptor antagonist, in the prostaglandin E2 human model of headache. J Headache Pain. 2011;12:551–9. doi: 10.1007/s10194-011-0358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 126.Junt T, Barchet W. Translating nucleic acid-sensing pathways into therapies. Nature Rev Immunol. 2015;15:529–44. doi: 10.1038/nri3875. [DOI] [PubMed] [Google Scholar]
- 127.Barton G, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 128.Cheng K, Wang X, Yin H. Small-molecule inhibitors of the TLR3/dsRNA complex. J Am Chem Soc. 2011;133:3764–7. doi: 10.1021/ja111312h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 130.Guha M. Anticancer TLR agonists on the ropes. Nat Rev Drug Discov. 2012;11:503–5. doi: 10.1038/nrd3775. [DOI] [PubMed] [Google Scholar]
- 131.Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol. 2004;50:722–33. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 132.Meyer T, Surber C, French L, Stockfleth E. Resiquimod, a topical drug for viral skin lesions and skin cancer. Expert Opin Inv drugs. 2013;22:149–59. doi: 10.1517/13543784.2013.749236. [DOI] [PubMed] [Google Scholar]
- 133.Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S. et al. First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin Cancer Res. 2007;13:7119–25. doi: 10.1158/1078-0432.CCR-07-1443. [DOI] [PubMed] [Google Scholar]
- 134.Northfelt DW, Ramanathan RK, Cohen PA, Von Hoff DD, Weiss GJ, Dietsch GN. et al. A phase I dose-finding study of the novel Toll-like receptor 8 agonist VTX-2337 in adult subjects with advanced solid tumors or lymphoma. Clin Cancer Res. 2014;20:3683–91. doi: 10.1158/1078-0432.CCR-14-0392. [DOI] [PubMed] [Google Scholar]
- 135.Hoque R, Farooq A, Malik A, Trawick BN, Berberich DW, McClurg JP. et al. A novel small-molecule enantiomeric analogue of traditional (-)-morphinans has specific TLR9 antagonist properties and reduces sterile inflammation-induced organ damage. J Immunol. 2013;190:4297–304. doi: 10.4049/jimmunol.1202184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Franklin BS, Ishizaka ST, Lamphier M, Gusovsky F, Hansen H, Rose J. et al. Therapeutical targeting of nucleic acid-sensing Toll-like receptors prevents experimental cerebral malaria. Proc Natl Acad Sci U S A. 2011;108:3689–94. doi: 10.1073/pnas.1015406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015;36:250–6. doi: 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15:760–70. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vargas R, Lizon B, Apetoh L. Rationale for stimulator of interferon genes-targeted cancer immunotherapy. Eur J Cancer. 2017;75:86–97. doi: 10.1016/j.ejca.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gross S, Rahal R, Stransky N, Lengauer C, Hoeflich KP. Targeting cancer with kinase inhibitors. J Clin Invest. 2015;125:1780–9. doi: 10.1172/JCI76094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H. et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011;1:158–69. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O'Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 145.Xue G, Zippelius A, Wicki A, Mandala M, Tang F, Massi D, Integrated Akt/PKB signaling in immunomodulation and its potential role in cancer immunotherapy. J Natl Cancer Inst; 2015. p. 107. [DOI] [PubMed] [Google Scholar]
- 146.Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–99. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- 147.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG. et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–27. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Evans CA, Liu T, Lescarbeau A, Nair SJ, Grenier L, Pradeilles JA. et al. Discovery of a selective phosphoinositide-3-Kinase (PI3K)-gamma inhibitor (IPI-549) as an immuno-oncology clinical candidate. ACS Med Chem Lett. 2016;7:862–7. doi: 10.1021/acsmedchemlett.6b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S. et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539:437–42. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J. et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21:553–60. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD. et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Panka DJ, Atkins MB, Mier JW. Targeting the mitogen-activated protein kinase pathway in the treatment of malignant melanoma. Clin Cancer Res. 2006;12:2371s–5s. doi: 10.1158/1078-0432.CCR-05-2539. [DOI] [PubMed] [Google Scholar]
- 153.Cheng Y, Zhang G, Li G. Targeting MAPK pathway in melanoma therapy. Cancer Metastasis Rev. 2013;32:567–84. doi: 10.1007/s10555-013-9433-9. [DOI] [PubMed] [Google Scholar]
- 154.D'Souza WN, Chang CF, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–29. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Menzies AM, Long GV. Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. Clin Cancer Res. 2014;20:2035–43. doi: 10.1158/1078-0432.CCR-13-2054. [DOI] [PubMed] [Google Scholar]
- 156.Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T. et al. The BRAF and MEK inhibitors Dabrafenib and Trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639–51. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 157.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–48. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Desar IM, Jacobs JH, Hulsbergen-vandeKaa CA, Oyen WJ, Mulders PF, van der Graaf WT. et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int J Cancer. 2011;129:507–12. doi: 10.1002/ijc.25674. [DOI] [PubMed] [Google Scholar]
- 159.Priceman S, Sung J, Shaposhnik Z, Burton J, Collado A, Moughon D. et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L. et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2013;74:153–61. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ehlerding EB, England CG, McNeel DG, Cai W. Molecular imaging of immunotherapy targets in cancer. J Nucl Med. 2016;57:1487–92. doi: 10.2967/jnumed.116.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weissleder R, Schwaiger M, Gambhir S, Hricak H. Imaging approaches to optimize molecular therapies. Sci Transl Med. 2016;8:355ps16. doi: 10.1126/scitranslmed.aaf3936. [DOI] [PubMed] [Google Scholar]
- 163.Liu Z, Li Z. Molecular imaging in tracking tumor-specific cytotoxic T lymphocytes (CTLs) Theranostics. 2014;4:990–1001. doi: 10.7150/thno.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vaz SC, Capacho AS, Oliveira FP, Gil N, Teixeira Barros C, Parreira A. et al. Radiopharmacology and molecular imaging of PD-L1 expression in cancer. Clin Trans Imag. 2018;6:429–39. [Google Scholar]
- 165.Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W. Noninvasive PET imaging of T cells. Trends in Cancer. 2018;4:359–73. doi: 10.1016/j.trecan.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Du Y, Jin Y, Sun W, Fang J, Zheng J, Tian J. Advances in molecular imaging of immune checkpoint targets in malignancies: current and future prospect. Eur Radiol. 2019;29:4294–302. doi: 10.1007/s00330-018-5814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: current approaches and future directions. Radiology. 2019;290:9–22. doi: 10.1148/radiol.2018181349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Helfen A, Roth J, Ng T, Eisenblaetter M. In vivo imaging of pro- and antitumoral cellular components of the tumor microenvironment. J Nucl Med. 2018;59:183–8. doi: 10.2967/jnumed.117.198952. [DOI] [PubMed] [Google Scholar]
- 169.Broos K, Lecocq Q, Raes G, Devoogdt N, Keyaerts M, Breckpot K. Noninvasive imaging of the PD-1:PD-L1 immune checkpoint: Embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics. 2018;8:3559–70. doi: 10.7150/thno.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen R, Zhou X, Liu J, Huang G. Relationship between the expression of PD-1/PD-L1 and 18F-FDG uptake in bladder cancer. Eur J Nucl Med Mol Imaging. 2019;46:848–54. doi: 10.1007/s00259-018-4208-8. [DOI] [PubMed] [Google Scholar]
- 171.Seith F, Forschner A, Schmidt H, Pfannenberg C, Guckel B, Nikolaou K. et al. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging. 2018;45:95–101. doi: 10.1007/s00259-017-3813-2. [DOI] [PubMed] [Google Scholar]
- 172.Chatterjee S, Lesniak WG, Miller MS, Lisok A, Sikorska E, Wharram B. et al. Rapid PD-L1 detection in tumors with PET using a highly specific peptide. Biochem Biophys Res Commun. 2017;483:258–63. doi: 10.1016/j.bbrc.2016.12.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Radu CG, Shu CJ, Nair-Gill E, Shelly SM, Barrio JR, Satyamurthy N. et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2'-deoxycytidine analog. Nat Med. 2008;14:783–8. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim W, Le TM, Wei L, Poddar S, Bazzy J, Wang X. et al. [18F]CFA as a clinically translatable probe for PET imaging of deoxycytidine kinase activity. Proc Natl Acad Sci U S A. 2016;113:4027–32. doi: 10.1073/pnas.1524212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Antonios J, Soto H, Everson R, Moughon D, Wang A, Orpilla J. et al. Detection of immune responses after immunotherapy in glioblastoma using PET and MRI. Proc Natl Acad Sci USA. 2017;114:10220–5. doi: 10.1073/pnas.1706689114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ronald JA, Kim BS, Gowrishankar G, Namavari M, Alam IS, D'Souza A. et al. A PET imaging strategy to visualize activated T cells in acute graft-versus-host disease elicited by allogenic hematopoietic cell transplant. Cancer Res. 2017;77:2893–902. doi: 10.1158/0008-5472.CAN-16-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM. et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 178.Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [18F]FLT PET imaging. Leuk Res. 2011;35:310–6. doi: 10.1016/j.leukres.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R. et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med. 2017;9:eaag2196. doi: 10.1126/scitranslmed.aag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gambhir S, Herschman H, Cherry S, Barrio J, Satyamurthy N, Toyokuni T. et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–38. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Min JJ, Iyer M, Gambhir SS. Comparison of [18F]FHBG and [14C]FIAU for imaging of HSV1-tk reporter gene expression: adenoviral infection vs stable transfection. Eur J Nucl Med Mol Imaging. 2003;30:1547–60. doi: 10.1007/s00259-003-1238-6. [DOI] [PubMed] [Google Scholar]
- 182.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 183.Jacobson O, Weiss ID. CXCR4 chemokine receptor overview: biology, pathology and applications in imaging and therapy. Theranostics. 2013;3:1–2. doi: 10.7150/thno.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li J, Peng C, Guo Z, Shi C, Zhuang R, Hong X. et al. Radioiodinated pentixather for SPECT imaging of expression of the chemokine receptor CXCR4 in rat myocardial-infarction-reperfusion models. Anal Chem. 2018;90:9614–20. doi: 10.1021/acs.analchem.8b02553. [DOI] [PubMed] [Google Scholar]
- 185.Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget. 2014;6:5022–40. doi: 10.18632/oncotarget.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG. et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–30. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 187.Jacobson O, Weiss ID, Szajek L, Farber JM, Kiesewetter DO. 64Cu-AMD3100 - a novel imaging agent for targeting chemokine receptor CXCR4. Bioorg Med Chem. 2009;17:1486–93. doi: 10.1016/j.bmc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular imaging of CXCR4 receptor expression in human cancer xenografts with [64Cu]AMD3100 positron emission tomography. Cancer Research. 2010;70:3935–44. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zitvogel L, Pitt JM, Daillere R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16:759–73. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 190.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H. et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–9. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL. et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A. 2013;110:20176–81. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–9. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Altmann Y, McLaughlin S, Padgett MJ, Goyal VK, Hero AO, Faccio D. Quantum-inspired computational imaging. Science. 2018;361:eaat2298. doi: 10.1126/science.aat2298. [DOI] [PubMed] [Google Scholar]
- 194.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J. et al. Radiomics: the bridge between medical imaging and personalized medicine. Nature Rev Clin Oncol. 2017;14:749–62. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 195.Semerano L, Minichiello E, Bessis N, Boissier MC. Novel immunotherapeutic avenues for rheumatoid arthritis. Trends Mol Med. 2016;22:214–29. doi: 10.1016/j.molmed.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 196.James ML, Hoehne A, Mayer AT, Lechtenberg K, Moreno M, Gowrishankar G. et al. Imaging B cells in a mouse model of multiple sclerosis using 64Cu-Rituximab PET. J Nucl Med. 2017;58:1845–51. doi: 10.2967/jnumed.117.189597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu Y, Aguzzi A. Immunotherapy for neurodegeneration? Science. 2019;364:130–1. doi: 10.1126/science.aaw0685. [DOI] [PubMed] [Google Scholar]
- 198.Herline K, Prelli F, Mehta P, MacMurray C, Goni F, Wisniewski T. Immunotherapy to improve cognition and reduce pathological species in an Alzheimer's disease mouse model. Alzheimers Res Ther. 2018;10:54–72. doi: 10.1186/s13195-018-0384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]