Abstract
Background
Alcohol withdrawal syndrome (AWS) is a distressing and life‐threatening condition that usually affects people who are alcohol dependent when they discontinue or decrease their alcohol consumption. Baclofen shows potential for rapidly reducing symptoms of severe AWS in people with alcoholism. Treatment with baclofen is easy to manage and rarely produces euphoria or other pleasant effects, or craving for the drug. This is an updated version of the original Cochrane Review first published in 2011 and last updated in 2017.
Objectives
To assess the efficacy and safety of baclofen for people with AWS.
Search methods
We updated our searches of the following databases to June 2019: the Cochrane Drugs and Alcohol Group Specialised Register, CENTRAL, PubMed, Embase, and CINAHL. We also searched registers of ongoing trials. We handsearched the references quoted in the identified trials, and sought information from researchers, pharmaceutical companies, and relevant trial authors about unpublished or uncompleted trials. We placed no restrictions on language.
Selection criteria
We included all randomised controlled clinical trials (RCTs) evaluating baclofen versus placebo or any other treatment for people with AWS. We excluded uncontrolled, non‐randomised, or quasi‐randomised trials. We included both parallel group and cross‐over studies.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included four RCTs with 189 randomised participants (one RCT new for this update). None of the included studies reported the primary outcomes of alcohol withdrawal seizures, alcohol withdrawal delirium, or craving.
For the comparison of baclofen and placebo (1 study, 31 participants), there was no evidence of a difference in Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in eight‐hour periods from days one to five (very low‐quality evidence).
For the comparison of baclofen and diazepam (2 studies, 85 participants), there was no evidence of a difference in change from baseline to days 10 to 15 on CIWA‐Ar scores (very low‐quality evidence, meta‐analysis was not performed due to insufficient data). In one study (37 participants), there was no evidence of a difference in participants with at least one adverse event (risk difference (RD) 0.00, 95% confidence interval (CI) –0.10 to 0.10; very low‐quality evidence), dropouts (RD 0.00, 95% CI –0.10 to 0.10; very low‐quality evidence), and dropouts due to adverse events (RD 0.00, 95% CI –0.10 to 0.10; very low‐quality evidence).
For the comparison of baclofen and chlordiazepoxide (1 study, 60 participants), there was no evidence of a difference in difference from baseline to nine‐day decremental fixed‐dose intervention: CIWA‐Ar scores (mean difference (MD) 1.00, 95% CI 0.70 to 1.30; very low‐quality evidence), global improvement (MD 0.10, 95% CI –0.03 to 0.23; very low‐quality evidence), 14/60 participants with adverse events (RD 2.50, 95% CI 0.88 to 7.10; very low‐quality of evidence), dropouts (RD 0.00, 95% CI –0.06 to 0.06; very low‐quality evidence), and dropouts due to adverse events (RD 0.00, 95% CI –0.06 to 0.06; very low‐quality evidence). None of the RCTs provided information on random sequence generation or allocation concealment, therefore, we assessed them at unclear risk of bias. Two RCTs were not of double‐blind design and had a high risk of bias in blinding (Addolorato 2006; Girish 2016). One RCT had more than 5% dropouts with high risk of attrition bias (Lyon 2011). We could not assess reporting bias as none of the prepublished protocols were available.
Authors' conclusions
No conclusions can be drawn about the efficacy and safety of baclofen for the management of alcohol withdrawal because we found insufficient and very low‐quality evidence.
Plain language summary
Baclofen for alcohol withdrawal syndrome
Review question
This review attempted to evaluate the efficacy and safety of baclofen as a therapy for alcohol withdrawal syndrome (AWS) in people with alcoholism.
Background
AWS is a distressing and life‐threatening condition that usually affects people who are alcohol dependent when they discontinue or decrease their alcohol consumption. The medicine baclofen has demonstrated potential to reduce symptoms of severe AWS in people with alcoholism. Treatment with baclofen is easy to manage, without producing any obvious side effects. This is an updated version of the original Cochrane Review published in 2011 and last updated in 2017.
Search date
The evidence is current to June 2019.
Study characteristics
We searched scientific databases for clinical trials comparing baclofen with placebo (a pretend treatment) or another potentially useful medicine in people with AWS. We included four randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) with 189 participants. One study from the USA compared baclofen to placebo given over at least 72 hours. The 31 participants were mainly men with the average age 47 years. Two studies with 85 participants compared baclofen to diazepam (a calming medicine) for 10 consecutive days, or for 10‐day inpatient stay with flexibility to allow negotiation of the discharge date between day 10 and day 15. One study compared baclofen to chlordiazepoxide given for nine days, in which the 60 participants were all men with an average age of 38 years. None of studies reported any conflict of interest. Addolorato 2006 was supported by Associazione Ricerca in Medicina, Italy. Girish 2016 was supported by KIMS Hospital and Research Centre (Bangalore, India). Jhanwar 2014 reported no funding source. Lyon 2011 was supported by Duluth Clinic Foundation (MN, USA).
Key results
None of the included studies assess the main outcomes of the review, that is, alcohol withdrawal seizures (fits), alcohol withdrawal delirium (confused thinking and awareness), and craving. We are uncertain whether baclofen improves withdrawal symptoms and signs, and reduces side effects when compared with placebo or other medicines as the quality of the evidence was very low.
Quality of the evidence
The quality of the evidence from the studies was very low and results should be interpreted with caution. In the future, well‐designed, double‐blind (where neither the participant nor the researcher knows which treatment has been given until after the results have been collected) RCTs with large numbers of participants are required to test how effective and well tolerated baclofen is in people with AWS.
Summary of findings
Background
Description of the condition
Nearly 4% of the global disease burden can be attributed to alcohol consumption (Rabiee 2017). Alcohol withdrawal syndrome (AWS) is a distressing and life‐threatening condition that usually affects people who are alcohol dependent when they discontinue or decrease their alcohol consumption (Gortney 2016). It has been estimated that 8% of primary care and hospitalised patients have associated AWS (Ramos 2013). The most common manifestations include tremor, restlessness, insomnia, nightmares, paroxysmal sweats, tachycardia, fever, nausea, vomiting, hallucinations, increased agitation, tremulousness, and delirium. In severe cases, symptoms might progress to seizures and coma, or even cardiac arrest and death in 5% to 10% of people (Jesse 2017). Long‐term alcohol consumption causes such changes as reduced brain gamma aminobutyric acid (GABA) levels and GABA receptor sensitivity (Liang 2004), and activation of the glutamate system (Airagnes 2019), which lead to hyperactivity in the absence of alcohol. The advances in knowledge of neuroscience have prompted the use of drugs that act through GABA pathways for the treatment of AWS.
Description of the intervention
Baclofen is a GABA B (GABAB) receptor agonist with an approved indication to control spasticity (Creamer 2018). The drug suppresses AWS in rats made physically dependent on alcohol (Colombo 2000). Baclofen produces the effect through modulating the GABAB receptor, similar to gamma hydroxybutyrate. Moreover, the therapeutic properties of baclofen appear to be reduced abuse and dependence potential (Carter 2009; McDonald 2008), which are related to the modulation of the GABAB receptor. Based on the preclinical findings, open‐label trials showed that baclofen rapidly reduced symptoms of severe AWS in people with alcoholism (Addolorato 2002a). This observation was confirmed by a case of severe AWS complicated by delirium tremens that was successfully treated with baclofen (Cooney 2019). Treatment with baclofen is easy to manage and rarely produces euphoria or other pleasant effects, or craving for the drug. More importantly, baclofen may also be of benefit in the prophylaxis of AWS in humans (Stallings 2007).
How the intervention might work
The experimental evidence indicates that mesolimbic dopamine neurons might be associated with the mediation of alcohol intake and reinforcement (Weiss 2002). GABAB receptors are mainly located in the ventral tegmental area where mesolimbic dopamine neurons originate, both on the cell body of dopamine neurons and on the terminals of glutamatergic afferent neurons (Bowery 1987). Baclofen as a GABAB receptor agonist might exert an inhibitory action on the dopamine neurons (Westerink 1996), which may be the way that baclofen suppresses alcohol‐stimulated dopamine release and, in turn, dopamine‐mediated, alcohol‐reinforced and motivated behaviours. In addition, one hypothesis is that baclofen‐induced activation of GABAB receptors offsets AWS‐associated and enhanced function of N‐methyl‐D‐aspartate‐mediated glutamate excitatory neurotransmission, which results in an attenuation of AWS (Colombo 2000). Another possible mechanism is that baclofen can block the expression and sensitisation of anxiety‐like behaviour in animals because of GABAB‐ and GABAA‐related adaptive changes induced by repeated AWS (Knapp 2007).
Why it is important to do this review
Although benzodiazepines are commonly used as first‐line agents for the treatment of AWS (Amato 2010; Mayo‐Smith 1997), they are usually associated with unwanted adverse effects and addictive properties. Concerning anticonvulsants, it is suggested that carbamazepine may actually be more effective in treating AWS in comparison to benzodiazepines. However, adverse effects have not been rigorously evaluated (Minozzi 2010). The discovery of potentially useful and manageable drugs for the treatment of AWS is, therefore, of considerable practical importance (Leggio 2008). This review aimed to evaluate the efficacy and safety of treatment of AWS with baclofen.
Objectives
To assess the efficacy and safety of baclofen for people with AWS.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) evaluating baclofen versus placebo or any other treatment for people with AWS. We excluded uncontrolled, non‐randomised, or quasi‐randomised trials. We included both parallel group and cross‐over studies.
Types of participants
Inclusion criteria:
aged 18 to 75 years, no gender limitation;
met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Revised (DSMR‐IV) criteria for AWS;
agreed to abstain from alcohol for duration of study;
able to provide informed consent.
Exclusion criteria:
severe psychiatric diseases, for example, major unipolar depression or schizophrenia;
using baclofen at the time of study enrolment;
other active drug dependence in addition to alcohol, with the exception of nicotine;
other severe diseases, such as epilepsy, cardiac failure, diabetes, liver encephalopathy, kidney failure, and neoplastic diseases.
Types of interventions
Experimental intervention: baclofen.
Control intervention: placebo or any other pharmacological treatment, such as benzodiazepines.
Types of outcome measures
Primary outcomes
Efficacy outcomes
Alcohol withdrawal seizures, as number of participants experiencing seizures.
Alcohol withdrawal delirium, as number of participants experiencing delirium.
Alcohol withdrawal symptoms, as measured by the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) score.
Global improvement of overall AWS, as measured in prespecified scales (as number of participants with global improvement, global doctor's assessment of efficacy, participant's assessment of efficacy).
Craving, as measured by prespecified scales.
Safety outcomes
Adverse events, as number of participants experiencing at least one adverse event.
Severe, life‐threatening adverse events, as measured by number of participants experiencing severe, life‐threatening adverse events.
Acceptability outcomes
Dropouts.
Dropouts due to adverse events.
Secondary outcomes
Additional medication needed.
Length of stay in intensive care therapy.
Mortality.
Quality of life.
Search methods for identification of studies
Electronic searches
For the previous update of this review (Liu 2017), we searched the following electronic databases:
Cochrane Drugs and Alcohol Group Specialised Register (searched 7 March 2017);
CENTRAL (2017, Issue 4);
PubMed (January 2015 to 7 March 2017);
Embase (via embase.com) (January 2015 to 7 March 2017);
EBSCO CINAHL (January 2015 to 7 March 2017).
For this review update, we searched the following electronic databases:
Cochrane Drugs and Alcohol Group Specialised Register (searched 21 June 2019);
CENTRAL (2019, Issue 6);
PubMed (March 2017 to 21 June 2019);
Embase (via embase.com) (March 2017 to 21 June 2019);
EBSCO CINAHL (March 2017 to 21 June 2019).
The search used a combination of controlled vocabulary and free‐text terms relating to alcohol withdrawal in addiction with the Cochrane highly sensitive search strategy for identifying reports of RCTs (Higgins 2011). We developed the search strategy for PubMed and revised it for each database using the appropriate controlled vocabulary as applicable.
See Appendix 1, Appendix 2, Appendix 3, Appendix 4, and Appendix 5 for details of the search terms for each database.
We searched the following trials registries on 21 June 2019:
ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/);
the ISRCTN registry (www.isrctn.com);
Nederlands Trial Register (www.trialregister.nl);
European Clinical Trials Database (www.clinicaltrialsregister.eu);
UMIN Clinical Trials Registry (www.umin.ac.jp/ctr/);
Australian Clinical Trials Registry (www.anzctr.org.au).
Searching other resources
1. References
We inspected the reference lists in all studies that we identified for further relevant studies.
2. Personal contact
We sought information from researchers, pharmaceutical companies, and relevant trial authors about unpublished or uncompleted trials.
Where required, we contacted trial authors to request additional data. We did not systematically contact all authors for additional papers.
All searches included non‐English language literature and studies with English abstracts. When we believed they were likely to meet the inclusion criteria, studies were translated into English.
Data collection and analysis
Selection of studies
Two review authors (JL, LW) independently screened titles and abstracts of all the identified trials to determine if they met the inclusion criteria. We obtained the full text of all the possibly relevant studies for further consideration. Two review authors (JL, LW) independently evaluated the eligibility and methodological quality of these studies. We resolved any doubts by discussion or by consulting an independent party when necessary.
Data extraction and management
Two review authors (JL, LW) independently extracted eligible data from the published reports onto standardised forms and cross‐checked them for accuracy. We used checklists to independently record details, including participants characteristics (sociodemographic and related clinical information); details of the experimental and control interventions (medications and non‐pharmacological interventions); outcomes; adverse events and dropouts for all reasons; country where the studies were conducted; funding; and conflict of interest of study authors. The review authors resolved any disagreements by discussion and consensus.
Assessment of risk of bias in included studies
Two review authors (JL, LW) assessed the risk of bias using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane Review is a two‐part tool addressing the specific domains, namely random sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), and selective outcome reporting (reporting bias). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high, or unclear risk. To make these judgements, we used the criteria indicated in the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field (Higgins 2011). (See Table 4 for details.) The tool addresses the domains of sequence generation and allocation concealment (avoidance of selection bias) by a single entry for each study. We considered blinding of participants, personnel, and outcome assessors (avoidance of performance bias and detection bias) separately for objective outcomes (e.g. dropouts, use of substance of abuse measured by urine analysis, participants relapsed at the end of follow‐up, participants engaged in further treatments) and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, participant self‐reported use of substance, adverse effects, social functioning as integration at school or work, family relationships). We considered incomplete outcome data (avoidance of attrition bias) for all outcomes except for the dropouts from the treatment, which is very often the primary outcome measure in trials on addiction.
1. Criteria to assess risk of bias in randomised controlled trials and controlled clinical trials.
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) |
Low risk | Investigators described a random component in the sequence generation process, such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. |
| High risk | Investigators described a non‐random component in the sequence generation process, such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| Unclear risk | Insufficient information about sequence generation process to permit judgement of low or high risk. | |
| 2. Allocation concealment (selection bias) |
Low risk | Investigators enrolling participants could not have foreseen assignment because 1 of the following methods, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly have foreseen assignments because 1 of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk, such as if the method of concealment not described or not described in sufficient detail to allow a definite judgement. | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes |
Low risk | No blinding or incomplete blinding, but review authors judged that outcome was unlikely to be influenced by lack of blinding. Blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken. |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes |
Low risk | Participants and providers blinded and unlikely that blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and outcome was likely to have been influenced by lack of blinding. Blinding of key study participants and personnel attempted, but it was likely that blinding could have been broken, and outcome was likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk | No blinding of outcome assessment, but review authors judged that outcome measurement was unlikely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| 6. Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk | No blinding of outcome assessment, but review authors judged that outcome measurement was unlikely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that blinding could have been broken. |
| High risk | No blinding of outcome assessment, and outcome measurement was likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that blinding could have been broken, and outcome measurement was likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or dropout |
Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to introduce bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data were imputed using appropriate methods. All randomised participants were reported/analysed in group they were allocated to by randomisation irrespective of non‐compliance and cointerventions (intention to treat). |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. 'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated; reasons for missing data not provided; number of dropouts not reported for each group). | |
| 8. Selective reporting (reporting bias) | Low risk | Study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in review were reported in the prespecified way. Study protocol was not available, but it was clear that the published reports included all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk | Not all the study's prespecified primary outcomes were reported. ≥ 1 primary outcomes were reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified. ≥ 1 reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect). ≥ 1 outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis. Study report failed to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. |
Measures of treatment effect
We analysed dichotomous outcomes by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed with 95% confidence interval (CI). We analysed continuous outcomes by calculating the mean difference (MD) with 95% CI when the studies used the same instrument for assessing the outcome. We planned to calculate the standardised mean difference (SMD) with 95% CI when the studies used different instruments. If a trial (or group within a trial) reported no adverse events or dropouts, we calculated the risk difference (RD) instead of the RR with 95% CI. We analysed all data with Review Manager 5 software (Review Manager 2014).
Unit of analysis issues
We dealt with unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We attempted to contact the authors of the studies for missing data and further details. We used an intention‐to‐treat analysis, which consisted of all the randomised participants. We considered different scenarios (best and worst case) for taking into account missing data.
Assessment of heterogeneity
We analysed heterogeneity by means of the I² statistic (Higgins 2011), and the Chi² test. The cut‐off points to establish heterogeneity were I² values of more than 50% and a P value for the Chi² test of less than 0.1.
Assessment of reporting biases
We planned to examine the presence of publication bias using a funnel plot, if at least 10 studies were included in a meta‐analysis.
Data synthesis
Where we considered studies to be sufficiently similar, we conducted a meta‐analysis by pooling the appropriate data using Review Manager 5 (Review Manager 2014). We expressed dichotomised data as RRs with their 95% CI. If a trial (or group within a trial) reported no adverse events or dropouts, we calculated RDs instead of RRs with 95% CI. We calculated the overall effects using a random‐effects model regardless of the level of heterogeneity. We provided a descriptive summary of the results when substantial heterogeneity between the studies prevented us from combining outcome data.
Subgroup analysis and investigation of heterogeneity
We planned to analyse subgroups of studies categorised according to demographic characteristics (e.g. age and gender) and the dosage and duration of treatment with baclofen.
Sensitivity analysis
We planned to carry out sensitivity analyses to assess the robustness of a random‐effects model versus a fixed‐effect model and the inclusion or exclusion of studies at high risk of bias (e.g. inadequate allocation concealment and lack of blinded outcome assessors), as well as the use of different scenarios for missing data.
Summary of findings and quality of evidence (GRADE)
We assessed the overall quality of the evidence for the primary outcome using the GRADE system. GRADE developed a system for grading the quality of evidence (GRADE 2013), which takes into account issues not only related to internal validity but also to external validity, such as directness, consistency, imprecision of results, and publication bias. The 'Summary of findings' tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Grading is decreased for the following reasons.
Serious (–1) or very serious (–2) study limitation for risk of bias.
Serious (–1) or very serious (–2) inconsistency between study results.
Some (–1) or major (–2) uncertainty about directness (the correspondence between the population, the intervention, or the outcomes measured in the studies actually found and those under consideration in our systematic review).
Serious (–1) or very serious (–2) imprecision of the pooled estimate (–1).
Publication bias strongly suspected (–1).
Results
Description of studies
For substantive descriptions of the studies, see: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
In the previous review update (Liu 2017), we found and included three eligible studies (Addolorato 2006; Lyon 2011; Girish 2016), and one ongoing trial (NCT02052440).
On rerunning the searches in 2019 (Figure 1), we identified 52 records. Once duplicates had been removed, we had a total of 22 records. We excluded 21 records based on titles and abstracts. We included one new study (Jhanwar 2014). Agreement between the review authors on exclusion of the remaining studies was 100%. The previous ongoing trial was terminated due to insufficient funding to complete with low enrolment rate (NCT02052440).
1.
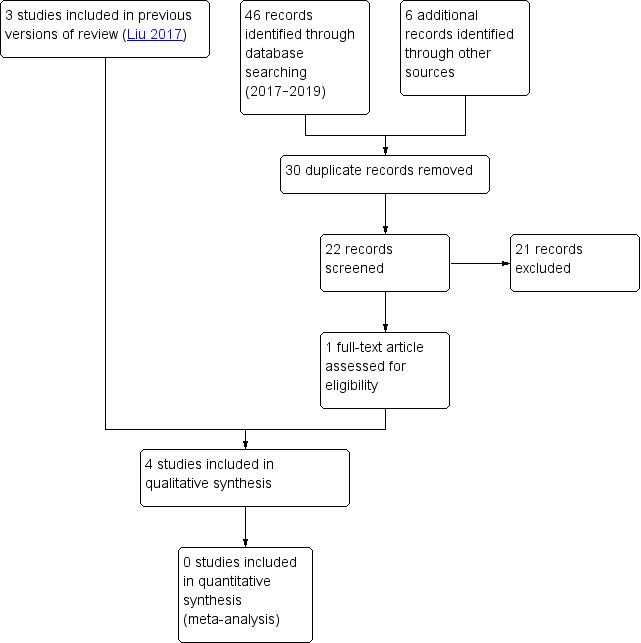
Study flow diagram.
Included studies
We included four RCTs with 189 randomised participants, which focused on the CIWA‐Ar score.
Addolorato 2006 was an RCT comparing baclofen versus diazepam for 10 consecutive days. It took place in Italy and was supported by a grant from the Associazione Ricerca in Medicina, Rome‐Bologna, Italy with no conflicts of interest.
Jhanwar 2014 was a randomised, parallel, double‐blind comparative study comparing on baclofen versus diazepam for 10 consecutive days and post medication at day 15. It took place in India with no conflicts of interest.
Lyon 2011 was a randomised, double‐blind, placebo‐controlled trial comparing baclofen versus placebo, orally every eight hours with observation for 72 hours or more. It took place in the USA and was supported by a grant from the Duluth Clinic Foundation with no conflicts of interest.
Girish 2016 was a randomised, open‐label, standard controlled, parallel trial. The intervention was nine‐day decremental fixed‐dose baclofen or chlordiazepoxide. It took place in India and was supported by pharmacology and psychiatry departments of KIMS Hospital and Research Centre with no conflicts of interest.
Excluded studies
We excluded studies for the following reasons.
The participants did not abstain from alcohol during the study (Addolorato 2002b; Garbutt 2010).
The participants had liver cirrhosis, which met the exclusion criterion 'liver encephalopathy' (Addolorato 2007; Leggio 2012; Morley 2013).
The trial was not an RCT (Garbutt 2007; Rigal 2015; Rolland 2017; Simioni 2016).
The participants did not meet the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria of AWS (Addolorato 2011; Beraha 2016; Farokhnia 2015; Franchitto 2014; Geisel 2016; Gupta 2017; Hauser 2017; Imbert 2015; Krupitsky 1993; Krupitsky 1995; Krupitsky 2015; Leggio 2013; Müller 2015; Pommier 2014; Ponizovsky 2015; Vourc'h 2016).
Risk of bias in included studies
See Figure 2 and Figure 3 and the Characteristics of included studies table for details.
2.
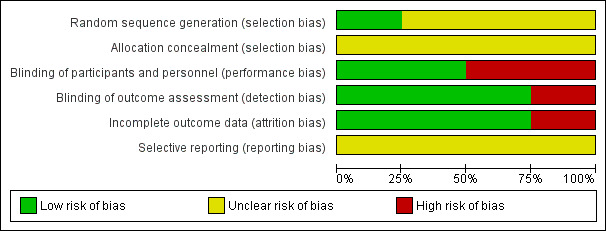
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
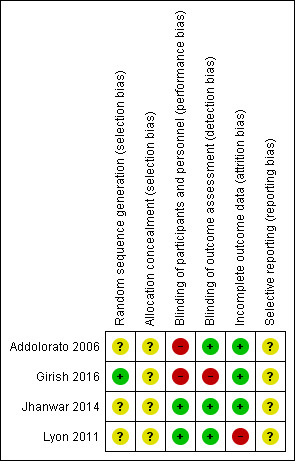
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Girish 2016 used a randomisation table (low risk of bias). Addolorato 2006 and Lyon 2011 did not report the methods of sequence generation (unclear risk of bias). Jhanwar 2014 allocated participants in chronological order into two groups by an even and odd method with an unclear risk of bias. None of four RCTs reported concealment. Therefore, we assessed them at unclear risk of bias.
Blinding
Addolorato 2006 had a single‐blind design where the investigators who assessed the CIWA‐Ar score were unaware of allocation. Therefore, we regarded detection bias as low risk and performance bias at high risk. Lyon 2011 and Jhanwar 2014 blinded participants and study personnel to treatment group with low risk of performance and detection bias. Girish 2016 was an open‐label study with high risk of performance and detection bias.
Incomplete outcome data
There were no dropouts in Addolorato 2006 and Girish 2016. In Jhanwar 2014, 96% participants completed trial in each group with low risk of bias. In Lyon 2011, only 31/44 randomised participants completed 72 hours of CIWA‐Ar assessment and the study was at high risk of bias.
Selective reporting
We could not assess reporting bias as none of the studies had prepublished protocols.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Baclofen compared with placebo for alcohol withdrawal.
| Baclofen compared with placebo for alcohol withdrawal | ||||||
|
Patient or population: people with alcohol withdrawal Settings: 2 tertiary‐care hospitals in Duluth, Minnesota Intervention: baclofen Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Baclofen | |||||
| AW seizures | Not reported | Not reported | — | — | — | — |
| AW delirium | Not reported | Not reported | — | — | — | — |
| AW symptoms (CIWA‐Ar score) | See comment | See comment | NA | 31 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No significant difference in CIWA‐Ar scores between baclofen and placebo groups in 8‐hour periods from days 1 to 5. |
| Global improvement | Not reported | Not reported | — | — | — | — |
| Craving | Not reported | Not reported | — | — | — | — |
| AEs | Not reported | Not reported | — | — | — | — |
| Severe AEs | Not reported | Not reported | — | — | — | — |
| Dropouts | Not reported | Not reported | — | — | — | — |
| Dropouts due to AEs | Not reported | Not reported | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse events; AW: alcohol withdrawal; CI: confidence interval; CIWA‐Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; NA: not available. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of attrition bias. bDowngraded two levels due to very small number of participant.
Summary of findings 2. Baclofen compared with diazepam for alcohol withdrawal.
| Baclofen compared with diazepam for alcohol withdrawal | ||||||
|
Patient or population: people with alcohol withdrawal Settings: alcohol treatment unit Intervention: baclofen Comparison: diazepam | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Baclofen | |||||
| AW seizures | Not reported | Not reported | — | — | — | — |
| AW delirium | Not reported | Not reported | — | — | — | — |
| AW symptoms (CIWA‐Ar score) | See comment | See comment | NA | 85 (2 study) | ⊕⊝⊝⊝ Very lowa,b |
Both baclofen and diazepam treatments significantly decreased CIWA‐Ar score with no significant differences between the 2 treatments (2‐way analysis of covariance: F[1,140] = 0.91, P > 0.05) in Addolorato 2006. The changes from baseline to day 10 in CIWA‐Ar Total score were 10.39 (SD 0.37) in baclofen group, and 10.33 (SD 0.36) in diazepam group (P > 0.10). The changes from baseline to day 15 in CIWA‐Ar Total score were 11 (SD 0.35) in baclofen group, and 10.8 (SD 0.38) in diazepam group (P > 0.10) (Jhanwar 2014). |
| Global improvement | Not reported | Not reported | — | — | — | — |
| Craving | Not reported | Not reported | — | — | — | — |
| AEs | 0 per 1000 | 0 per 1000 | RD 0.00 (–0.10 to 0.10) | 37 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| Severe AEs | Not reported | Not reported | — | — | — | — |
| Dropouts | 0 per 1000 | 0 per 1000 | RD 0.00 (–0.10 to 0.10) | 37 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| Dropouts due to AEs | 0 per 1000 | 0 per 1000 | RD 0.00 (–0.10 to 0.10) | 37 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse events; AW: alcohol withdrawal; CI: confidence interval; CIWA‐Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; NA: not available; RD: risk difference; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of performance bias. bDowngraded two levels due to very small number of participants.
Summary of findings 3. Baclofen compared with chlordiazepoxide for alcohol withdrawal.
| Baclofen compared with chlordiazepoxide for alcohol withdrawal | ||||||
|
Patient or population: people with alcohol withdrawal Settings: tertiary care hospital, Bengaluru Intervention: baclofen Comparison: chlordiazepoxide | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chlordiazepoxide | Baclofen | |||||
| AW seizures | Not reported | Not reported | — | — | — | — |
| AW delirium | Not reported | Not reported | — | — | — | — |
| AW symptoms (CIWA‐Ar score) | 0.133 (SD 0.434) | 1.133 (SD 0.730) | MD 1.00 (0.70 to 1.30) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
Relative effect was calculated at the endpoint (day 9), with no evidence of a difference. |
| Global improvement | 1.0 (SD 0.2) | 1.1 (SD 0.3) | MD 0.10 (–0.03 to 0.23) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| Craving | Not reported | Not reported | — | — | — | — |
| AEs | 133 per 1000 | 333 per 1000 | RR 2.50 (0.88 to 7.10) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| Severe AEs | Not reported | Not reported | — | — | — | — |
| Dropouts | 0 per 1000 | 0 per 1000 | RD 0.00 (–0.06 to 0.06) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| Dropouts due to AEs | 0 per 1000 | 0 per 1000 | RD 0.00 (–0.06 to 0.06) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
No evidence of a difference. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse events; AW: alcohol withdrawal; CI: confidence interval; CIWA‐Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; MD: mean difference; RD: risk difference; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of performance bias and detection bias. bDowngraded two levels due to very small number of participants.
Baclofen versus placebo
We found one study with 31 participants comparing baclofen with placebo (Lyon 2011).
Primary efficacy outcomes
Alcohol withdrawal seizures, alcohol withdrawal delirium
The study did not assess alcohol withdrawal seizures or alcohol withdrawal delirium.
Alcohol withdrawal symptoms (CIWA‐Ar score)
Authors reported that there was no evidence of a difference in CIWA‐Ar scores between the baclofen and placebo groups in eight‐hour periods from days one to five. There were no further data.
Global improvement or craving
The study did not assess global improvement or craving.
Primary safety outcomes
Adverse events, severe adverse events
The study did not assess adverse events or serious adverse events.
Primary acceptability outcomes
Dropouts and dropouts due to adverse events
The study did not assess dropouts or dropouts due to adverse events.
Secondary outcomes
Additional medication needed
The cumulative dose of lorazepam administered to the 31 participants ranged from 0 mg to 1035 mg in the 72 hours following randomisation, with a range of 1 mg to 1035 mg in the placebo group and 0 mg to 39 mg in the baclofen group. The eight participants who received the highest doses of lorazepam (20 mg or more) included 1/18 participants who received baclofen and 7/13 participants who received placebo (P = 0.004).
Length of stay in intensive care therapy, mortality, and quality of life
The study did not assess length of intensive therapy stay, mortality, or quality of life.
Baclofen versus diazepam
We found two studies with 85 participants comparing baclofen versus diazepam (Addolorato 2006; Jhanwar 2014).
Primary efficacy outcomes
Alcohol withdrawal seizures and alcohol withdrawal delirium
The studies did not assess alcohol withdrawal seizures or alcohol withdrawal delirium.
Alcohol withdrawal symptoms (CIWA‐Ar score)
Both baclofen and diazepam treatments significantly decreased the CIWA‐Ar score with no evidence of a difference between the two treatments (two‐way analysis of covariance: F[1,140] = 0.91, P > 0.05) (Addolorato 2006). The changes from baseline to day 10 in CIWA‐Ar Total score were 10.39 (standard deviation, (SD) 1.81) in the baclofen group and 10.33 (SD 1.76) in the diazepam group; P > 0.10). The changes from baseline to day 15 in CIWA‐Ar Total score were 11 (SD 1.71) in the baclofen group and 10.8 (SD 1.86) in the diazepam group (P > 0.10) (Jhanwar 2014). There was no evidence of a difference between baclofen and diazepam (MD 0.20, 95% CI –0.81 to 1.21; Analysis 1.1).
1.1. Analysis.
Comparison 1 Baclofen versus diazepam, Outcome 1 Alcohol withdrawal symptoms (CIWA‐Ar score).
Global improvement and craving
The studies did not assess global improvement or craving.
Primary safety outcomes
Adverse events
One study found no evidence of a difference in adverse events between baclofen and diazepam (RD 0.00, 95% CI –0.10 to 0.10; Analysis 1.2; Addolorato 2006). Jhanwar 2014 did not assess this outcome.
1.2. Analysis.
Comparison 1 Baclofen versus diazepam, Outcome 2 Adverse events.
Severe adverse events
The studies did not assess severe adverse events.
Primary acceptability outcomes
Dropouts and dropouts due to adverse events
One study found no evidence of a difference in dropouts and dropouts due to adverse events (Analysis 1.3; Analysis 1.4; Addolorato 2006). Jhanwar 2014 did not assess these outcomes.
1.3. Analysis.
Comparison 1 Baclofen versus diazepam, Outcome 3 Dropouts.
1.4. Analysis.
Comparison 1 Baclofen versus diazepam, Outcome 4 Dropouts due to adverse events.
Secondary outcomes
Additional medication needed
One study found no evidence of a difference in additional medication needed (Analysis 1.5; Addolorato 2006). Jhanwar 2014 did not assess this outcome.
1.5. Analysis.
Comparison 1 Baclofen versus diazepam, Outcome 5 Additional medication needed.
Length of stay in intensive care therapy, mortality, and quality of life
The studies did not assess length of intensive therapy stay, mortality, or quality of life.
Baclofen versus chlordiazepoxide
We found one study with 60 participants comparing baclofen versus chlordiazepoxide (Girish 2016).
Primary efficacy outcomes
Alcohol withdrawal seizures and alcohol withdrawal delirium
The study did not assess alcohol withdrawal seizures or alcohol withdrawal delirium.
Alcohol withdrawal symptoms (CIWA‐Ar score)
At day nine, there was a decrease in mean score of 1.133 (SD 0.730) for the baclofen group and a decrease in mean score of 0.133 (SD 0.434) for the chlordiazepoxide group (MD 1.00, 95% CI 0.70 to 1.30; Analysis 2.1).
2.1. Analysis.
Comparison 2 Baclofen versus chlordiazepoxide, Outcome 1 Alcohol withdrawal symptoms (CIWA‐Ar score).
Global improvement
The change in clinical global impression improvement was 1.1 (SD 0.3) for the baclofen group and 1.0 (SD 0.2) for the chlordiazepoxide group (MD 0.10, 95% CI –0.03 to 0.23; Analysis 2.2).
2.2. Analysis.
Comparison 2 Baclofen versus chlordiazepoxide, Outcome 2 Global improvement.
Craving
The study did not assess craving.
Primary safety outcomes
Adverse events and severe adverse events
There was no evidence of a difference in adverse events between the two groups (RR 2.50, 95% CI 0.88 to 7.10; Analysis 2.3).
2.3. Analysis.
Comparison 2 Baclofen versus chlordiazepoxide, Outcome 3 Adverse events.
The study did not assess severe adverse events.
Primary acceptability outcomes
Dropouts and dropouts due to adverse events
There was no evidence of a difference in dropouts (RD 0.00, 95% CI –0.06 to 0.06; Analysis 2.4) and dropouts due to adverse events (RD 0.00, 95% CI –0.06 to 0.06; Analysis 2.5).
2.4. Analysis.
Comparison 2 Baclofen versus chlordiazepoxide, Outcome 4 Dropouts.
2.5. Analysis.
Comparison 2 Baclofen versus chlordiazepoxide, Outcome 5 Dropouts due to adverse events.
Secondary outcomes
Additional medication needed
There was no evidence of a difference in additional medication needed (Analysis 2.6).
2.6. Analysis.
Comparison 2 Baclofen versus chlordiazepoxide, Outcome 6 Additional medication needed.
Length of stay in intensive care therapy, mortality, and quality of life
The study did not assess length of intensive therapy stay, mortality, or quality of life.
Discussion
Summary of main results
We included four RCTs with 189 participants. We found methodological flaws in all the RCTs. Concerning efficacy, we found no evidence of a difference in the severity of alcohol withdrawal symptoms as measured by changes of CIWA‐Ar score and additional medication needed in all the comparisons (Addolorato 2006; Girish 2016; Lyon 2011; Jhanwar 2014). Only Girish 2016 reported changes in clinical global impression improvement and severity and found no differences. For safety, we found no evidence of a difference in adverse events, severe adverse events, dropouts, and dropouts due to adverse events in any of the comparisons (Addolorato 2006; Girish 2016; Lyon 2011). None of the studies assessed the primary outcomes of seizures and delirium.
Overall completeness and applicability of evidence
One study compared baclofen with placebo in an inpatient setting for managing AWS, in relatively short episodes of treatment (at least 72 hours) (Lyon 2011). The participants were mainly men with a mean age of 47 years. Two studies compared baclofen with diazepam in an inpatient setting, with the treatment of AWS for 10 consecutive days (Addolorato 2006), or for a 10‐day inpatient stay with flexibility to allow negotiation of the discharge date between day 10 and day 15 (Jhanwar 2014). One study compared baclofen with chlordiazepoxide, in an inpatient setting and the treatment of AWS for a duration of nine days (Girish 2016). The participants were all men with a mean age of 38 years. Although the participants, interventions, and settings of the studies were comparable with the participants usually treated in clinical practice, more research is required.
Quality of the evidence
There were methodological limitations in all the included studies. Three RCTs did not provide information on random sequence generation or allocation concealment, therefore, we assessed them at unclear risk of bias (Addolorato 2006; Lyon 2011; Jhanwar 2014). Girish 2016 was at low risk of bias for random sequence generation. Two RCTs were not of double‐blind design and had a high risk of bias in blinding (Addolorato 2006; Girish 2016). One RCT had more than 5% dropouts with high risk of attrition bias (Lyon 2011). We could not assess reporting bias as none of the prepublished protocols were available. Furthermore, the sample size of randomised participants was too small to reach a robust conclusion. Therefore, we regarded the quality of evidence to be very low and the conclusions should be interpreted with caution.
Potential biases in the review process
The search for trials was rigorously performed based on the strategies in different electronic databases. We sought information from researchers, pharmaceutical companies, and relevant trial authors about unpublished or uncompleted trials. However, we could not exclude the possibility that we did not identify unpublished trials. The review included only four RCTs and we could not assess publication bias using funnel plots.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review in baclofen for the treatment of AWS. Our findings were similar to one Cochrane overview on pharmacological interventions for the treatment of AWS, which also found insufficient evidence on the effectiveness and safety of baclofen (Amato 2011).
Authors' conclusions
Implications for practice.
We could draw no conclusions about the efficacy and safety of baclofen for the management of alcohol withdrawal syndrome (AWS) because we found insufficient and very low‐quality evidence.
Implications for research.
We require more research, which would benefit from double‐blind randomised controlled trials where:
the participants meet Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Revised (DSMR‐IV) criteria for AWS;
the participants agree to abstain from alcohol during the study;
the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) score, a standard scale for measuring AWS, is applied.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2019 | New citation required but conclusions have not changed | New study included. The previous ongoing trial (NCT02052440) was terminated due to insufficient funding to complete with low enrolment rate. |
| 21 June 2019 | New search has been performed | New search |
History
Protocol first published: Issue 5, 2010 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 28 October 2012 | New citation required but conclusions have not changed | The previous ongoing study (NCT00597701) has been finished and included in this update (Lyon 2011). |
| 25 October 2012 | New search has been performed | New search has been performed. |
Acknowledgements
The review authors would like to acknowledge the help provided by the Cochrane Drugs and Alcohol group (cda.cochrane.org/).
Appendices
Appendix 1. Cochrane Drug and Alcohol Group Specialised Register search strategy
baclofen AND alcohol AND INREGISTER
Appendix 2. CENTRAL search strategy
Free text: (((alcohol) AND (withdraw* or detox* or abstinen* or abstain*)) AND (baclofen)))
Appendix 3. PubMed search strategy
alcohol‐related disorders [MeSH]
abuse[tiab] OR dependen*[tiab] OR disorder* OR consumption [tiab]
withdraw*[tiab] OR abstinen*[tiab] OR abstain*[tiab] OR detox*[tiab] OR neuropathy[tiab] OR delirium [tiab]
#1 OR #2 OR #3
alcohol [tiab]
#4 AND #5
Baclofen [MeSH]
Chlorophenyl GABA [tiab]
beta‐(p‐Chlorophenyl)‐gamma‐aminobutyric Acid [tiab]
"gamma‐amino butyric acid‐B receptor agonists"
Lioresal [tiab]
#7 OR #8 OR #9 OR #10 OR #11
randomized controlled trial [pt]
controlled clinical trial [pt]
random* [tiab]
placebo [tiab]
drug therapy [MeSH]
trial [tiab]
groups [tiab]
#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
animals [mh] NOT human [mh]
#20 NOT #21
#6 AND #12 AND #22
Appendix 4. Embase search strategy
'alcohol withdrawal'/exp
'withdrawal syndrome'/exp
(disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy):ti,ab
alcohol:ti,ab
#1 or #2 or #3
#4 and #5
'benzodiazepine derivative'/exp
(Baclofen or Chlorophenyl GABA or beta‐(p‐Chlorophenyl)‐gamma‐aminobutyric Acid or Lioresal).ti,ab
#7 or #8
random*:ti,ab
placebo:ti,ab
((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)):ti,ab
crossover*:ti,ab
'randomized controlled trial'/exp
'double blind procedure'/exp
'single blind procedure'/exp
'triple blind procedure'/exp
'latin square design'/exp
'crossover procedure'/exp
'Latin square design'/exp
'placebos'/exp
'multicenter study'/exp
#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
#6 and #9 and #23
limit 24 to human
Appendix 5. CINAHL search strategy
MH "alcohol related disorders"
MH "alcohol withdrawal delirium"
TX (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy)
TX alcohol
S1 or S2 or S3
S4 AND S5
MH "GABAB receptor agonist, Baclofen"
TX (Baclofen or Chlorophenyl GABA or beta‐(p‐Chlorophenyl)‐gamma‐aminobutyric Acid or gamma‐amino butyric acid‐B receptor agonists or Lioresal)
S7 or S8
MH "Random Assignment"
MH "Clinical Trials"
TX random*
TX placebo*
TX group*
TX (singl* or doubl* or tripl* or trebl*) and (mask* or blind*)
MH "crossover design"
TX crossover*
TX allocate*
TX assign*
S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19
S6 and S9 and S20
Data and analyses
Comparison 1. Baclofen versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol withdrawal symptoms (CIWA‐Ar score) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.81, 1.21] |
| 2 Adverse events | 1 | 37 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 3 Dropouts | 1 | 37 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 4 Dropouts due to adverse events | 1 | 37 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 5 Additional medication needed | 1 | 37 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
Comparison 2. Baclofen versus chlordiazepoxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol withdrawal symptoms (CIWA‐Ar score) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.70, 1.30] |
| 2 Global improvement | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.03, 0.23] |
| 3 Adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.88, 7.10] |
| 4 Dropouts | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 5 Dropouts due to adverse events | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 6 Additional medication needed | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.7 [0.94, 3.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Addolorato 2006.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: aged 18–75 years; alcohol consumption > 80 g alcohol/day during previous 24 hours; diagnosis of alcohol dependence according to DSM‐IV criteria (APA 1994). Only people with CIWA‐Ar score ≥ 10 (defined as moderate or severe AWS requiring pharmacological treatment) ultimately enrolled in study. Exclusion criteria: current presence of: delirium tremens or hallucinosis; severe psychiatric diseases; epilepsy; severe cardiac failure; diabetes mellitus; severe liver impairment; liver encephalopathy; kidney failure; neoplastic diseases; lack of co‐operating relatives; abuse of or dependence on other drugs, with the exception of nicotine. Baseline: baclofen group: 83.3% men; mean age 42.3 (SD 2.7) years; 18 alcoholics with alcohol consumption 130–440 g/day (mean 256.7 (SD 19.3) g/day); addiction range 3–39 years (mean 13.6 (SD 2.6) years); diazepam group: 89.5% men; mean age 42.0 (SD 2.4) years; 19 alcoholics with alcohol consumption 90–600 g/day (mean 191.3 (SD 28.9) g/day; P < 0.005, Mann‐Whitney test with respect to baclofen group); addiction range 3–39 years (mean 15.8 (SD 1.9) years; P > 0.05, Mann‐Whitney test with respect to baclofen group). |
|
| Interventions |
Baclofen group: baclofen 30 mg/day orally, divided in 3 daily administrations for 10 consecutive days (n = 18). Diazepam group: diazepam 0.5–0.75 mg/kg divided in 6 daily administrations for 10 consecutive days. Doses tapered by 25% daily from day 7 to day 10 (Lejoyeux 1998) (n = 19). |
|
| Outcomes | CIWA‐Ar administered once a day (immediately before the first daily administration of drug) on days 1, 2, 3, 4, 5, and 10. Baseline values were those collected on day 1 before first drug administration. A reduction in AST, ALT, GGT and MCV. | |
| Notes |
Funding source: supported by a grant from "Associazione Ricerca in Medicina," Rome‐Bologna, Italy. Conflict of interest: not mentioned Country: Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method used (i.e. random number table, computer random number generator, coin tossing, etc.). |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment unknown. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The whole study was performed on a single blind design; in particular, investigators who performed CIWA‐Ar at the different times of treatment were always the same and were unaware as to which drug was being administered to patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The whole study was performed on a single blind design; in particular, investigators who performed CIWA‐Ar at the different times of treatment were always the same and were unaware as to which drug was being administered to patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis of the efficacy of the 2 drugs on the severity of AWS was intended to be performed with the intention‐to‐treat principles." |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocols unavailable. |
Girish 2016.
| Methods | Randomised, open‐label, standard controlled, parallel trial | |
| Participants |
Inclusion criteria: either gender; aged 18–65 years; fulfilled DSM‐IV criteria for AWS; last alcohol intake within 24–48 hours preceding initiation of therapy; willingness to give written informed consent. Exclusion criteria: complicated AWS comprising any 1 or all of the following: delirium tremens, withdrawal seizures, and cognitive impairment (Wernicke‐Korsakoff syndrome); known psychiatric disorders; multi‐drug abuse (except nicotine); advanced hepatic, renal, and cardiovascular diseases; known allergy to any of study medications; recent use of drugs which lower the seizure threshold; conditions which can mask or affect the clinical parameters of AWS such as use of β‐blockers (propranolol), thyrotoxicosis, meningitis, and haemorrhage/head injury. Baseline: 60 men. Baclofen group: mean age 36.7 (SD 8.8) years; mean duration of hazardous consumption of alcohol 16.5 (SD 8.2) years; chlordiazepoxide group: mean age 40.0 (SD 10.1) years; mean duration of hazardous consumption of alcohol 16.9 (SD 7.7) years. |
|
| Interventions |
Baclofen group: 9‐day decremental fixed‐dose baclofen 10 mg (n = 30) Chlordiazepoxide group: 9‐day decremental fixed‐dose chlordiazepoxide 25 mg (n = 30) |
|
| Outcomes | Withdrawal symptoms assessed daily by CIWA‐Ar scores before the administration of morning dose. Symptom‐free days; Clinical Global Impression; participant satisfaction of AWS management | |
| Notes |
Funding source: supported by pharmacology and psychiatry departments of KIMS Hospital and Research Centre (Bangalore, India). Conflict of interest: not declared Country: India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were assigned either to the baclofen (n = 30) or to the chlordiazepoxide group (n = 30) based on the 1:1 randomization table." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment unknown. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was a randomized, open‐label, standard controlled, parallel group study of baclofen, and chlordiazepoxide in AWS." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This study was a randomized, open‐label, standard controlled, parallel group study of baclofen, and chlordiazepoxide in AWS." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed trial. |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocols unavailable. |
Jhanwar 2014.
| Methods | Randomised, parallel, double‐blind comparative study | |
| Participants |
Inclusion criteria: aged 18–60 years; no gender limitation; agree to abstain for alcohol for duration of study; willing to participate and provide informed consent; meet criteria for alcohol dependence (DSM‐IV‐TR) and mild‐to‐moderate AWS; have a clinically withdrawal assessment prior to study; medically acceptable for study treatment; no past or present physical disorder that was likely to deteriorate during participation; no other psychiatric condition or psychotropic medication 15 days prior to entering the study. Exclusion criteria: current diagnosis of any other substance‐dependence syndrome other than alcohol dependence (excluding nicotine and caffeine dependence); diagnosis of schizophrenia, bipolar disorder, or dementia; history of hepatic encephalopathy, ascites, diabetes, or renal disease; pregnant and lactating women; known sensitivity of previous adverse reaction to baclofen or diazepam. Baseline: 48 participants randomised; 24 in each group. People with severe AWS were excluded. |
|
| Interventions |
Baclofen group: baclofen 10 mg at 1 tablet twice a day for 5 days followed by 1 tablet once a day for the next 5 days. The inpatient unit offered a 10‐day inpatient stay with flexibility to allow negotiation of the discharge date between day 10 and day 15. Diazepam group: diazepam 10 mg at 1 tablet twice a day for 5 days followed by 1 tablet once a day for the next 5 days. The inpatient unit offered a 10‐day inpatient stay with flexibility to allow negotiation of the discharge date between day 10 and day 15. |
|
| Outcomes | AWS assessed by CIWA‐Ar scale on days 1 to day 10 and post medication at day 15. | |
| Notes |
Funding source: none Conflict of interest: not declared Country: India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated in chronological order and randomized into two groups by even and odd method." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment unknown. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Diazepam and Baclofen tablets were indistinguishable, having the same physical characteristics (e.g. size, colour, appearance). Study medications for both groups were dispensed in temper proof, packets that were similar in appearance. Label on the packets contained study code, patient’s serial number, manufacturing date and expiry date and space for date of dispensing. Hence, neither the patient nor the investigator was aware of the medication received by the patients. Only the pharmacist preparing the study medication was aware of the allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Diazepam and Baclofen tablets were indistinguishable, having the same physical characteristics (e.g. size, colour, appearance). Study medications for both groups were dispensed in temper proof, packets that were similar in appearance. Label on the packets contained study code, patient’s serial number, manufacturing date and expiry date and space for date of dispensing. Hence, neither the patient nor the investigator was aware of the medication received by the patients. Only the pharmacist preparing the study medication was aware of the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96% participants completed trial in each group. |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocols unavailable. |
Lyon 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Inclusion criteria: history of AWS or alcohol use suggestive of significant risk for AWS, and able to provide informed consent. Exclusion criteria: other active drug dependence in addition to alcohol; using baclofen at time of study enrolment; using benzodiazepines chronically at time of study enrolment; known baclofen or benzodiazepine sensitivity; unable to take oral medications; pregnant or breastfeeding; serum creatinine level ≥ 2.0 mg/dL; history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; unable to complete consenting procedures. Baseline: baclofen group: 76% men; 87.5% had history of AWS; mean age at admission 47.5 (SD 10.3) years; placebo group: 94.7% men; 87.5% had history of AWS; mean age at admission 46.1 (SD 11.9) years. |
|
| Interventions |
Baclofen group: baclofen 10 mg orally every 8 hours with observation for ≥ 72 hours (n = 19). Placebo group: placebo orally every 8 hours with observation for ≥ 72 hours (n = 25). |
|
| Outcomes | CIWA‐Ar score; need for high doses of benzodiazepine to control AWS | |
| Notes |
Funding source: supported by a grant from the Duluth Clinic Foundation (MN, USA). Conflict of interest: authors reported no conflict of interest. Country: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects and study personnel were blinded to treatment group (baclofen vs placebo)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects and study personnel were blinded to treatment group (baclofen vs placebo)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments." |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocols unavailable. |
ALT: alanine aminotransferase; AST: aspartate transaminase; AWS: alcohol withdrawal syndrome; CIWA‐Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition: Text Revision; GGT: gamma‐glutamyl transferase; MCV: mean corpuscular volume; n: number of participants; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Addolorato 2002b | Participants did not abstain from alcohol during study. |
| Addolorato 2007 | Participants had liver cirrhosis, which met exclusion criteria 'liver encephalopathy.' |
| Addolorato 2011 | Participants neither met DSM‐IV criteria of AWS nor abstained from alcohol during study. |
| Beraha 2016 | Participants did not meet DSM‐IV criteria of AWS. |
| Farokhnia 2015 | Participants did not meet DSM‐IV criteria of AWS. |
| Franchitto 2014 | Unknown if participants met DSM‐IV criteria of AWS or if they abstained from alcohol during study. |
| Garbutt 2007 | Comment on included study (Addolorato 2007). |
| Garbutt 2010 | Participants did not abstain from alcohol during study. |
| Geisel 2016 | Participants did not meet DSM‐IV criteria of AWS. |
| Gupta 2017 | Participants did not meet DSM‐IV criteria of AWS. |
| Hauser 2017 | Participants were veterans with chronic hepatitis C, which did not meet DSM‐IV criteria of AWS. |
| Imbert 2015 | Participants did not meet DSM‐IV criteria of AWS. |
| Krupitsky 1993 | Unknown if participants met DSM‐IV criteria of AWS or if they abstained from alcohol during study. |
| Krupitsky 1995 | Unknown if participants met DSM‐IV criteria of AWS or if they abstained from alcohol during study. |
| Krupitsky 2015 | Participants did not meet DSM‐IV criteria of AWS. |
| Leggio 2012 | Participants had liver cirrhosis, which met exclusion criteria 'liver encephalopathy.' |
| Leggio 2013 | Unknown if participants met DSM‐IV criteria of AWS or if they abstained from alcohol during study. |
| Morley 2013 | Participants had liver cirrhosis, which met exclusion criteria 'liver encephalopathy.' |
| Müller 2015 | Participants did not meet DSM‐IV criteria of AWS. |
| Pommier 2014 | Unknown if participants met DSM‐IV criteria of AWS or if they abstained from alcohol during study. |
| Ponizovsky 2015 | Participants did not meet DSM‐IV criteria of AWS. |
| Rigal 2015 | Not a randomised controlled trial. |
| Rolland 2017 | Not a randomised controlled trial. |
| Simioni 2016 | Not a randomised controlled trial. |
| Vourc'h 2016 | Participants did not meet DSM‐IV criteria of AWS. |
AWS: alcohol withdrawal syndrome; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.
Characteristics of ongoing studies [ordered by study ID]
CTRI.
| Trial name or title | A trial to compare safety and efficacy of Baclofen and Diazepam in Alcohol Withdrawal Syndrome |
| Methods | Randomized, Parallel Group, Active Controlled Trial |
| Participants | Meets criteria for alcohol dependence (DSM‐iv‐TR) (American Psychiatry Association‐1994) & mild to moderate alcohol withdrawal syndrome |
| Interventions | Baclofen vs Diazepam |
| Outcomes | The Revised Clinical Institute Withdrawal Assessment For Alcohol ( CIWA‐Ar ) Score |
| Starting date | 2011 May 29 |
| Contact information | Dr Ashutosh Chourishi, Department of Pharmacology ,R.D.Gardi Medical College Department of Pharmacology, R.D.Gardi Medical College, India. dr_chourishi@yahoo.co.in |
| Notes | Last refreshed on: 2019 June 17 |
Differences between protocol and review
We added "If a trial (or group within a trial) reported no adverse events or dropouts, we calculated risk differences (RD) instead of RRs with 95% CI." in "Measures of treatment effect."
Assessment of publication by funnel plot inspection was not performed because only four studies were included in the review.
Subgroup or sensitive analysis was not performed due to insufficient data.
Contributions of authors
JL and LW formulated the idea and developed the basis for the review.
JL took the lead in searching, identifying, and assessing studies; in data extraction and analyses; and in writing the full review.
LW gave general advice on the review and provided help in identifying trials, assessing studies, and extracting data.
JL supervised the quality assessment of the methodology.
JL and LW wrote and revised this review.
JL was responsible for updating the review.
Declarations of interest
JL: none.
LW: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Addolorato 2006 {published data only}
- Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. American Journal of Medicine 2006;119(3):276.e13‐8. [DOI] [PubMed] [Google Scholar]
Girish 2016 {published data only}
- Girish K, Vikram Reddy K, Pandit LV, Pundarikaksha HP, Vijendra R, Vasundara K, et al. A randomized, open‐label, standard controlled, parallel group study of efficacy and safety of baclofen, and chlordiazepoxide in uncomplicated alcohol withdrawal syndrome. Biomedical Journal 2016;39(1):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jhanwar 2014 {published data only}
- Jhanwar A, Patidar OP, Sharma RN, Chourishi A, Jain A. A double blind study for efficacy of diazepam and baclofen in the treatment of alcohol withdrawal syndrome. Journal of Pharmaceutical and Biomedical Sciences 2014;4(6):482‐8. [Google Scholar]
Lyon 2011 {published data only}
- Lyon JE, Khan RA, Gessert CE, Larson PM, Renier CM. Treating alcohol withdrawal with oral baclofen: a randomized, double‐blind, placebo‐controlled trial. Journal of Hospital Medicine 2011;6(8):469‐74. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Addolorato 2002b {published data only}
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomised controlled study. Alcohol and Alcoholism (Oxford, Oxfordshire) 2002;37(5):504‐8. [DOI] [PubMed] [Google Scholar]
Addolorato 2007 {published data only}
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind controlled study. Lancet 2007;370(9603):1915‐22. [DOI] [PubMed] [Google Scholar]
Addolorato 2011 {published data only}
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, et al. Dose–response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double‐blind, placebo‐controlled trial. Alcohol and Alcoholism (Oxford, Oxfordshire) 2011;46(3):312‐7. [DOI] [PubMed] [Google Scholar]
Beraha 2016 {published data only}
- Beraha EM, Salemink E, Goudriaan AE, Bakker A, Jong D, Smits N, et al. Efficacy and safety of high‐dose baclofen for the treatment of alcohol dependence: a multicentre, randomised, double‐blind controlled trial. European Neuropsychopharmacology 2016;26(12):1950‐9. [DOI] [PubMed] [Google Scholar]
Farokhnia 2015 {published data only}
- Farokhnia M, Edwards SM, Bollinger J, Amodio J, Zywiak WH, Tidey JW, et al. Baclofen as a pharmacotherapy for the treatment of concurrent alcohol and nicotine dependence: a double‐blind, placebo‐controlled, randomized trial. Neuropsychopharmacology 2015;40(6):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franchitto 2014 {published data only}
- Franchitto N, Pelissier F, Lauque D, Simon N, Lançon C. Self‐intoxication with baclofen in alcohol‐dependent patients with co‐existing psychiatric illness: an emergency department case series. Alcohol and Alcoholism (Oxford, Oxfordshire) 2014;49(1):79‐83. [DOI] [PubMed] [Google Scholar]
Garbutt 2007 {published data only}
- Garbutt JC, Flannery B. Baclofen for alcoholism. Lancet 2007;370:1884‐5. [DOI] [PubMed] [Google Scholar]
Garbutt 2010 {published data only}
- Garbutt JC, Kampov‐Polevoy AB, Gallop R, Kalka‐Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double‐blind, placebo‐controlled trial. Alcoholism, Clinical and Experimental Research 2010;34:1849‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geisel 2016 {published data only}
- Geisel O, Hellweg R, Müller CA. Serum levels of brain‐derived neurotrophic factor in alcohol‐dependent patients receiving high‐dose baclofen. Psychiatry Research 2016;240:177‐80. [DOI] [PubMed] [Google Scholar]
Gupta 2017 {published data only}
- Gupta M, Verma P, Rastogi R, Arora S, Elwadhi D. Randomized open‐label trial of baclofen for relapse prevention in alcohol dependence. American Journal of Drug and Alcohol Abuse 2017;43:324‐31. [DOI] [PubMed] [Google Scholar]
Hauser 2017 {published data only}
- Hauser P, Fuller B, Ho SB, Thuras P, Kern S, Dieperink E. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized clinical trial. Addiction 2017;112:1173‐83. [DOI] [PubMed] [Google Scholar]
Imbert 2015 {published data only}
- Imbert B, Alvarez J, Simon N. Anticraving effect of baclofen in alcohol‐dependent patients. Alcoholism, Clinical and Experimental Research 2015;39:1602‐8. [DOI] [PubMed] [Google Scholar]
Krupitsky 1993 {published data only}
- Krupitsky EM, Burakov AM, Ivanov VB, Krandashova GF, Lapin IP, Grinenko AJ, et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug and Alcohol Dependence 1993;33:157‐63. [DOI] [PubMed] [Google Scholar]
Krupitsky 1995 {published data only}
- Krupitsky EM, Burakov AM, Grinenko AIa, Borodkin IuS. Effect of pharmacotherapy of affective disorders on the psycho‐semantics of alcoholic patients. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova / Ministerstvo Zdravookhraneniia i Meditsinskoi Promyshlennosti Rossiiskoi Federatsii, Vserossiiskoe Obshchestvo Nevrologov [i] Vserossiiskoe Obshchestvo Psikhiatrov 1995;95:67‐71. [PubMed] [Google Scholar]
Krupitsky 2015 {published data only}
- Krupitsky EM, Rybakova KV, Kiselev AS, Alexeeva YV, Berntsev VA, Chekhlaty EI, et al. Double blind placebo controlled randomized pilot clinical trial of baclofen (Baclosan®) for alcohol dependence. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova / Ministerstvo Zdravookhraneniia i Meditsinskoi Promyshlennosti Rossiiskoi Federatsii, Vserossiiskoe Obshchestvo Nevrologov [i] Vserossiiskoe Obshchestvo Psikhiatrov 2015;115:53‐62. [DOI] [PubMed] [Google Scholar]
Leggio 2012 {published data only}
- Leggio L, Ferrulli A, Zambon A, Caputo F, Kenna GA, Swift RM, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addictive Behaviors 2012;37:561‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leggio 2013 {published data only}
- Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, et al. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacology, Biochemistry, and Behavior 2013;103:784‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morley 2013 {published data only}
- Morley KC, Leung S, Baillie A, Haber PS. The efficacy and biobehavioural basis of baclofen in the treatment of alcoholic liver disease (BacALD): study protocol for a randomised controlled trial. Contemporary Clinical Trials 2013;36:348‐55. [DOI] [PubMed] [Google Scholar]
Müller 2015 {published data only}
- Müller CA, Geisel O, Pelz P, Higl V, Krüger J, Stickel A, et al. High‐dose baclofen for the treatment of alcohol dependence (BACLAD study): a randomized, placebo‐controlled trial. European Neuropsychopharmacology 2015;25:1167‐77. [DOI] [PubMed] [Google Scholar]
Pommier 2014 {published data only}
- Pommier P, Debaty G, Bartoli M, Viglino D, Carpentier F, Danel V, et al. Severity of deliberate acute baclofen poisoning: a nonconcurrent cohort study. Basic & Clinical Pharmacology & Toxicology 2014;114:360‐4. [DOI] [PubMed] [Google Scholar]
Ponizovsky 2015 {published data only}
- Ponizovsky AM, Rosca P, Aronovich E, Weizman A, Grinshpoon A. Baclofen as add‐on to standard psychosocial treatment for alcohol dependence: a randomized, double‐blind, placebo‐controlled trial with 1 year follow‐up. Journal of Substance Abuse Treatment 2015;52:24‐30. [DOI] [PubMed] [Google Scholar]
Rigal 2015 {published data only}
- Rigal L, Legay Hoang L, Alexandre‐Dubroeucq C, Pinot J, Jeunne C, Jaury P. Tolerability of high‐dose baclofen in the treatment of patients with alcohol disorders: a retrospective study. Alcohol and Alcoholism (Oxford, Oxfordshire) 2015;50:551‐7. [DOI] [PubMed] [Google Scholar]
Rolland 2017 {published data only}
- Rolland B, Auffret M, Labreuche J, Lapeyre‐Mestre M, Dib M, Kemkem A, et al. Phone‐based safety monitoring of the first year of baclofen treatment for alcohol use disorder: the BACLOPHONE cohort study protocol. Expert Opinion on Drug Safety 2017;16:125‐32. [DOI] [PubMed] [Google Scholar]
Simioni 2016 {published data only}
- Simioni N, Preda C, Deken V, Bence C, Cottencin O, Rolland B. Characteristics of patients with alcohol dependence seeking baclofen treatment in France: a two‐centre comparative cohort study. Alcohol and Alcoholism (Oxford, Oxfordshire) 2016;51:664‐9. [DOI] [PubMed] [Google Scholar]
Vourc'h 2016 {published data only}
- Vourc'h M, Feuillet F, Mahe PJ, Sebille V, Asehnoune K, BACLOREA trial group. Baclofen to prevent agitation in alcohol‐addicted patients in the ICU: study protocol for a randomised controlled trial. Trials 2016;17:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
CTRI {unpublished data only}
- A trial to compare safety and efficacy of Baclofen and Diazepam in Alcohol Withdrawal Syndrome. Ongoing study 2011 May 29.
Additional references
Addolorato 2002a
- Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. American Journal of Medicine 2002;112:226‐9. [DOI] [PubMed] [Google Scholar]
Airagnes 2019
- Airagnes G, Ducoutumany G, Laffy‐Beaufils B, Faou AL, Limosin F. Alcohol withdrawal syndrome management: Is there anything new. La Revue de Médecine Interne 2019;40:373‐9. [DOI] [PubMed] [Google Scholar]
Amato 2010
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005063.pub3] [DOI] [PubMed] [Google Scholar]
Amato 2011
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008537.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
Bowery 1987
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 1987;20:365‐83. [DOI] [PubMed] [Google Scholar]
Carter 2009
- Carter LP, Koek W, France CP. Behavioral analyses of GHB: receptor mechanisms. Pharmacology & Therapeutics 2009;121:100‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2000
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, et al. Ability of baclofen in reducing alcohol intake and withdrawal severity: I – preclinical evidence. Alcoholism, Clinical and Experimental Research 2000;24:58‐66. [PubMed] [Google Scholar]
Cooney 2019
- Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome – a short review. Frontiers in Psychiatry 2019;9:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creamer 2018
- Creamer M, Cloud G, Kossmehl P, Yochelson M, Francisco GE, Ward AB, et al. Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. Stroke 2018;49:2129‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gortney 2016
- Gortney JS, Raub JN, Patel P, Kokoska L, Hannawa M, Argyris A. Risk for delirium tremens in patients with alcohol withdrawal syndrome. Cleveland Clinic Journal of Medicine 2016;83:67‐79. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 8 August 2017).
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jesse 2017
- Jesse S, Bråthen G, Ferrara M, Keindl M, Ben‐Menachem E, Tanasescu R, et al. Alcohol withdrawal syndrome: mechanisms, manifestations, and management. Acta Neurologica Scandinavica 2017;135:4‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knapp 2007
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety‐like behavior in an animal model of repeated stress and ethanol withdrawal. Alcoholism, Clinical and Experimental Research 2007;31:582‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leggio 2008
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non‐benzodiazepine GABAergic medications. Biological Psychiatry 2008;32:1106‐17. [DOI] [PubMed] [Google Scholar]
Lejoyeux 1998
- Lejoyeux M, Solomon J, Adès J. Benzodiazepine treatment for alcohol‐dependent patients. Alcohol and Alcoholism (Oxford, Oxfordshire) 1998;33:563‐75. [DOI] [PubMed] [Google Scholar]
Liang 2004
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. Journal of Pharmacology and Experimental Therapeutics 2004;310:1234‐45. [DOI] [PubMed] [Google Scholar]
Mayo‐Smith 1997
- Mayo‐Smith MF. Pharmacological management of alcohol withdrawal. A meta analysis and evidence‐based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA 1997;278:144‐51. [DOI] [PubMed] [Google Scholar]
McDonald 2008
- McDonald LM, Sheppard WF, Staveley SM, Sohal B, Tattersall FD, Hutson PH. Discriminative stimulus effects of tiagabine and related GABAergic drugs in rats. Psychopharmacology 2008;197:591‐600. [DOI] [PubMed] [Google Scholar]
Minozzi 2010
- Minozzi S, Amato L, Vecchi S, Davoli M. Anticonvulsants for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005064.pub3] [DOI] [PubMed] [Google Scholar]
NCT02052440
- NCT02052440. Preventing alcohol withdrawal syndrome with oral baclofen. clinicaltrials.gov/show/NCT02052440 (first received 3 February 2014).
Rabiee 2017
- Rabiee R, Agardh E, Coates MM, Allebeck P, Danielsson AK. Alcohol‐attributed disease burden and alcohol policies in the BRICS‐countries during the years1990‐2013. Journal of Global Health 2017;7:010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramos 2013
- Ramos R, Mallet T, Divittis A, Cohen R. Predictors of severity of alcohol withdrawal in hospitalized patients. Journal of Clinical Medicine Research 2013;5:376‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stallings 2007
- Stallings W, Schrader S. Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review. Journal of Oklahoma State Medical Association 2007;100:354‐60. [PubMed] [Google Scholar]
Weiss 2002
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. Journal of Neuroscience 2002;22:3332‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westerink 1996
- Westerink BH, Kwint H‐F, Vries JB. The pharmacology of mesolimbic dopamine neurons: a dual‐probe micro dialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. Journal of Neuroscience 1996;16:2605‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2010
- Liu J, Wang L. Baclofen for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011
- Liu J, Wang L. Baclofen for alcohol withdrawal. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008502.pub2] [DOI] [PubMed] [Google Scholar]
Liu 2013
- Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD008502.pub3] [DOI] [PubMed] [Google Scholar]
Liu 2015
- Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD008502.pub4] [DOI] [PubMed] [Google Scholar]
Liu 2017
- Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD008502.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


