Abstract
Circulating peroxiredoxin-4 (Prx4) is suggested as a prognosis marker as well as a regulator of many diseases. We aimed to examine 1) whether Prx4 is secreted from the liver in an animal model of sepsis and 2) effects of GYY4137, a hydrogen sulfide donor molecule, on septic liver injury as well as the hepatic secretion of Prx4. Rats (Wistar, male, 6 weeks old) were administered lipopolysaccharide (LPS, 15 mg/kg body weight, i.p.) with or without pre-administration of GYY4137 (50 mg/kg body weight, i.p.) and sacrificed 24 h after LPS administration. Hematoxylin-eosin and Elastica Masson-Goldner stains were used to evaluate hepatic injuries. Cytokine expression levels were determined by qPCR, and the levels of Prx4 in the serum and liver were determined by immunoblotting. Hepatocytes were isolated from rat liver, and the levels of Prx4 in the medium as well as the cells were determined 24 h after the administrations of LPS (1 µg/ml), tumor necrosis factor-α (TNFα, 50 ng/ml), or interleukin-1β (IL-1β, 10 ng/ml), with or without GYY4137 (300 µM). Hepatic inflammation and damage in LPS-administered rats were suppressed by GYY4137. An increase in plasma Prx4 level caused by LPS was observed, but the increase was attenuated by pre-administration of GYY4137. Prx4 was secreted from isolated hepatocytes after stimulation with LPS, TNFα, or IL-1β. GYY4137 attenuated the IL-1β-induced Prx4 secretion from hepatocytes. Secretion from hepatocytes is likely involved in the increase in circulating Prx4 during sepsis. GYY4137 attenuates not only hepatic injury but also Prx4 secretion.
Keywords: sepsis, hepatocytes, peroxiredoxin-4, GYY4137, inflammation
Peroxiredoxins (Prxs) are antioxidant enzymes involved in the elimination of hydrogen peroxide (H2O2)1, 2. There are at least 6 Prx members in mammals (Prx1–6). Prx4 is expressed in most tissues including liver and is the only secretory Prx3, 4. In cells, Prx4 is localized to the endoplasmic reticulum (ER), where it is involved in removing H2O2 generated during the folding of proteins5. In addition to its role in scavenging intracellular H2O2, increased plasma levels of Prx4 are reported in many diseases, including cardiovascular diseases6, diabetes7, and sepsis8.
Sepsis leads to dysfunctions in multiple organ including severe damage to the liver9. Although effective antidotes or treatments for sepsis have not been established to date, both carbon monoxide (CO) and hydrogen sulfide (H2S) have been shown to ameliorate many of the symptoms of sepsis when applied at effectively low doses10, 11, 12. We have also shown that cardiac as well as pulmonary damage in sepsis model rats can be mitigated by CORM-3, a carbon monoxide-releasing molecule13, 14.
In this study, we examined the effects of H2S on liver damage in an animal model of sepsis by use of GYY4137, a slow-releasing H2S donor15. We also examined the possibility that Prx4 secretion from hepatocytes might contribute to the reported increase in circulating Prx4 levels during sepsis.
All the animal experiments in this study were approved by the animal care and use committee of Tokyo Medical and Dental University. Rats (Wistar, male, 6 weeks old, ⁓250 g body weight) were divided into four groups (control, LPS, LPS+GYY4137, and GYY4137). LPS (E. coli, O55:B5, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline solution and administered intraperitoneally (i.p.) to rats at a final dose of 15 mg/kg body weight to create an animal model of sepsis16, 17. Control rats were received the same volume of saline solution. GYY4137 (Dojindo, Kumamoto, Japan) was administered i.p. to achieve a final dose of 50 mg/kg body weight 30 min prior to the administration of LPS. Blood was collected through cardiac puncture. For the analysis of plasma proteins, albumin was removed from the plasma using a Pierce Albumin Depletion Kit (Thermo Fisher Scientific, Waltham, MA, USA). Rat livers were excised, and the right lobe of the livers was cut at a thickness of 2 mm, fixed in 4% paraformaldehyde, and embedded in paraffin. Sections (3 µm thick) were affixed to slides, deparaffinized, and subjected to standard hematoxylin and eosin (H&E) and Elastica Masson-Goldner (EMG) stain protocols. The specimens were observed under a light microscope (AX-80, Olympus, Tokyo, Japan).
To evaluate the relative expression of genes in the liver, total RNA was extracted from rat liver tissues by use of TRIzol reagent (Thermo Fisher Scientific). cDNA was synthesized using oligo(dT)15 and SuperScript II reverse transcriptase (Thermo Fisher Scientific). Levels of mRNAs were determined by quantitative real-time reverse transcriptase-mediated PCR analysis (qPCR) by use of a StepOnePlus system (Thermo Fisher Scientific) based on SYBR Green. The relative levels of gene expression were calculated by the comparative Ct method. The primers used are listed in Table 1. To evaluate relative levels of proteins in the liver, tissues were lysed in a lysis buffer [consisting of 10 mM Tris-HCl (pH 7.4), 320 mM sucrose, 5 mM EDTA, 50 mM NaF, 2 mM Na3VO4, and protease inhibitor cocktail (Complete, Roche, Mannheim, Germany). Equal amounts of liver lysates were subjected to SDS-PAGE. After electrophoresis, the proteins were transferred to a PVDF membrane, blocked in TBST containing 5% skim milk, and incubated with following primary antibodies: anti-caspase-1 (ab179515, Abcam, Cambridge, MA, USA), anti-E-cadherin (610181, BD Bioscience, San Jose, CA, USA), anti-GAPDH (MAB374, Merck Millipore, Burlington, MA, USA), anti-actin (A2066, Sigma-Aldrich), and anti-Prx4 (SC-376668, Santa Cruz Biotechnology, Dallas, TX, USA). After further incubation with a peroxidase-conjugated anti-IgG secondary antibody (Promega, Madison, MI, USA) and visualization of the antigens by ECL reagents (Thermo Fisher Scientific), the relative levels of antigens in the samples were determined by an image analyzer (CS analyzer 4, Atto, Tokyo, Japan). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was performed as described previously18. In brief, protein bands were excised from CBB stained gels, digested by trypsin, and subjected to TOF-MS (ultrafleXtreme, Bruker, Billerica, MA, USA). The Mascot search engine (Matrix Science) was used to identify proteins. Rat hepatocytes were isolated and maintained as described previously19. Isolated hepatocytes were maintained in Williams medium E (Sigma-Aldrich) supplemented with 10% FBS, streptomycin, and penicillin. LPS (1 µg/ml), TNFα (50 ng/ml, human recombinant, Sigma-Aldrich), or IL-1β (10 ng/ml, mouse recombinant, Sigma-Aldrich) was added to the medium, and the cells were incubated for 24 h. GYY4137 (300 µM) was added 30 min prior to the addition of IL-1β. Hepatocytes and the conditioned medium were collected and analyzed by immunoblotting. Student’s t-test and the Tukey-Kramer post hoc test were used throughout this study. P<0.05 was considered statistically significant.
Table 1. Primers Used in This Study.
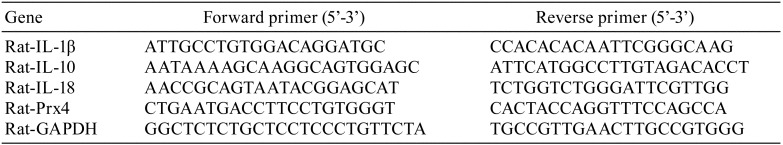
We previously reported that many symptoms of sepsis were observed in the rats administered LPS (15 mg/kg, i.p. 24 h)13, 14, 20. In agreement with our previous reports, H&E staining of livers from rats administered LPS showed inflammation and sinusoid dilation, both of which were suppressed by pre-administration of GYY4137 (50 mg/kg, Fig. 1A). There was infiltration of more neutrophils and fewer macrophages in sinusoids of the LPS-administered rat liver (Fig. 1A). Hepatocytes were anisokaryotic, and arrangement of the cells seemed to be nonuniform in LPS groups compared with the control group (Fig. 1A). This hepatocyte damage matched well with that in our previous reports, in which increased serum ALT levels were observed in LPS-administered rats20, 21. There were no differences of EMG staining in any liver sections from the control, LPS, LPS+GYY4137, and GYY4137 groups, confirming that there were no signs of increasing connective tissue on this time scale (24 h) after LPS administration (Fig. 1A). In agreement with the accumulation of neutrophils in the sinusoid (Fig. 1A), qPCR analysis showed that the expression levels of pro-inflammatory cytokines (IL-1β and IL-18) were significantly increased in the LPS groups (Fig. 1B). LPS also increased the expression of IL-10 (Fig. 1B), which has anti-inflammatory roles. The simultaneous occurrence of both pro- and anti-inflammatory responses is a feature of sepsis22. Interestingly, GYY4137 suppressed the LPS-induced upregulation of pro-inflammatory cytokines (IL-1β and IL-18) but not that of anti-inflammatory cytokines (IL-10) (Fig. 1B). These results show that GYY4137 attenuates pro-inflammatory but not anti-inflammatory responses in the septic liver, confirming the anti-inflammatory properties of GYY4137.
Fig. 1.
Histochemical and molecular biological analysis of livers from rats administered lipopolysaccharide (LPS) with or without GYY4137. Rats were administered LPS (15 mg/kg, i.p.) with or without pre-administration of GYY4137 (50 mg/kg, i.p.); the livers were excised 24 h after LPS administration. (A) Hematoxylin and eosin (H&E) and Elastica Masson-Goldner (EMG) staining of liver sections. Arrows indicate neutrophil accumulation in sinusoids. Black bar, 50 µm. White bar, 200 µm. (B) Quantitative real-time reverse transcriptase-mediated PCR (qPCR) analysis of mRNA levels in the liver. GAPDH served as an endogenous control. Each bar shows the mean and standard error (SE) (n=4). *P<0.05 by Tukey-Kramer post hoc test. **P<0.01 by Tukey-Kramer post hoc test. ***P<0.001 by Tukey-Kramer post hoc test.
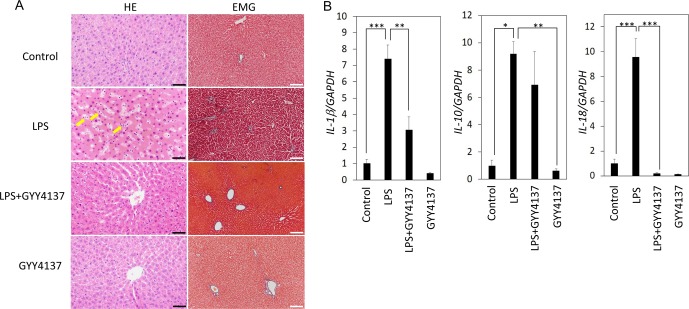
Given the indications of inflammation as well as sinusoid dilation in the LPS-administered rat liver, we examined the activation of inflammatory caspase (caspase-1) as well as the status of the cell-cell adhesion protein E-cadherin by immunoblotting. As shown in Fig. 2 (A and B), generation of the activated p10/12 form of caspase-1 was observed in the liver of LPS group rats but not in liver of LPS+GYY4137 group rats. Similar to caspase-1 activation, degradation of E-cadherin was observed in the LPS group (Fig. 2A and B). However, the degradation was mitigated in the LPS+GYY4137 and GYY4137 groups compared with the LPS group (Fig. 2A and B). Caspase-1 activation in the LPS group and its suppression in the LPS+GYY4137 group coincide well with the expression levels of IL-1β and IL-18 (Fig. 1B). E-cadherin degradation in the liver of the LPS group indicates that damage of epithelial integrity might be related to dilation of the sinusoid.
Fig. 2.
Immunoblot analysis of caspase-1 and E-cadherin in the livers from rats administered lipopolysaccharide (LPS) with or without GYY4137. Livers were collected from rats 24 h after the administration of LPS (15 mg/kg, i.p.) with or without GYY4137 (50 mg/kg, i.p.). (A and B) Immunoblot analyses of caspase-1 and E-cadherin are shown. GAPDH served as an endogenous control. Each bar shows the mean and SE (n=4). **P<0.01 by Tukey-Kramer post hoc test. ***P<0.001 by Tukey-Kramer post hoc test.
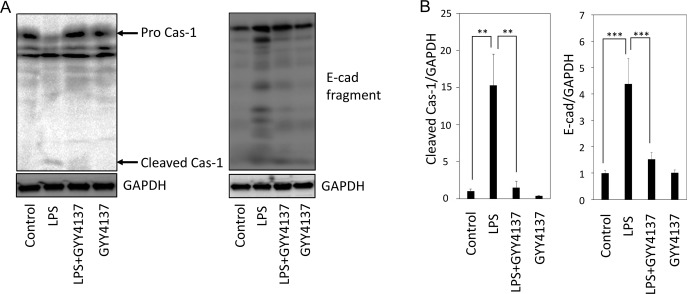
We next examined Prx4 in the liver of rats administered LPS with or without GYY4137. Examination of the protein and mRNA levels for Prx4 revealed that, although Prx4 mRNA levels increased, there was no increase in the protein levels by LPS (Fig. 3A and B). To examine extracellular secretion of Prx4, serum from the blood of rats was depleted of albumin and subjected SDS-PAGE. CBB staining of the gel showed several bands that were not only obviously upregulated in the LPS group but also remained at basal levels in the LPS+GYY4137 group (Fig. 3C). These protein bands were identified as C-reactive peptide (CRP), haptoglobin, and serotransferrin by MALDI-TOF-MS analysis, providing serological evidence for the occurrence of inflammation in LPS-administered rats and its amelioration by GYY4137 (Fig. 3C). Immunoblot analysis of the serum revealed that Prx4 levels were increased in the LPS group but not in the LPS+GYY4137 group (Fig. 3D).
Fig. 3.
Analysis of proteins in the liver and plasma of rats administered lipopolysaccharide (LPS) with or without GYY4137. Livers and plasma were collected from rats 24 h after the administration of LPS (15 mg/kg, i.p.) with or without GYY4137 (50 mg/kg, i.p.). (A and B) Immunoblot (A) and quantitative real-time reverse transcriptase-mediated PCR (qPCR) (B) analyses of peroxiredoxin-4 in rat livers. Each bar shows the mean and SE (n=4). ***P<0.001 by Tukey-Kramer post hoc test. (C) CBB staining of proteins in rat plasma. Serum albumin was removed from the samples using an albumin depletion kit. Bands marked with arrows were subjected to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis. (D) Immunoblot analysis of Prx4 in the plasma. A representative immunoblot from 4 biological replicates is shown.
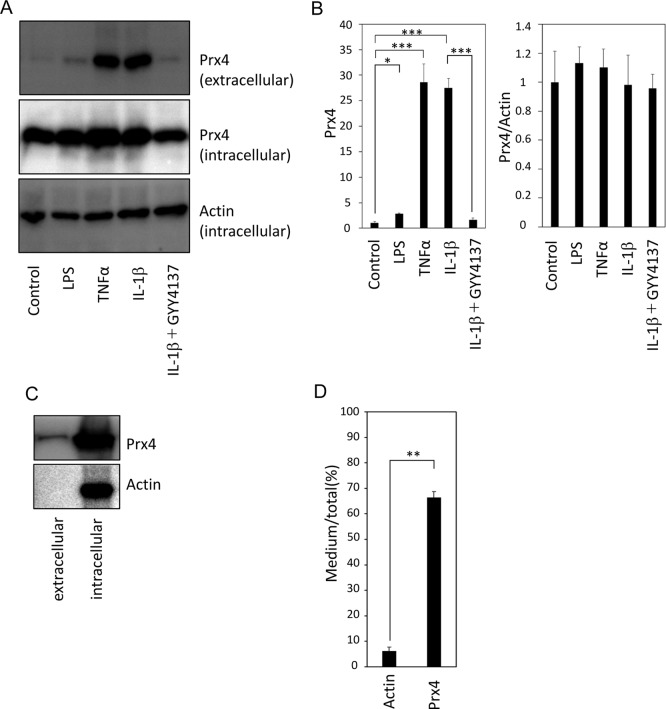
We isolated hepatocytes and stimulated them with LPS as well as TNFα or IL-1β. Extracellular levels of Prx4 were significantly increased after stimulation with 1 µg/ml LPS for 24 h (Fig. 4A and B). Interestingly, both TNFα and IL-1β were more potent inducers of Prx4 secretion than LPS (Fig. 4A and B). Furthermore, pretreatment with GYY4137 attenuated the IL-1β-induced secretion of Prx4 (Fig. 4A and B). Similar to the findings in the rat liver, the intracellular levels of Prx4 were unchanged under all conditions examined (Fig. 4A and B). After stimulation with IL-1β for 24 h, nearly 70% of the Prx4 was detected in the medium (Fig. 4C). In contrast, less than 4% of the actin was detected in the medium, confirming that the presence of Prx4 in the medium was not due to cell death.
Fig. 4.
Secretion of Prx4 from cultured hepatocytes in response to lipopolysaccharide (LPS), tumor necrosis factor-α (TNFα), or interleukin-1β (IL-1β). Primary cultures of rat hepatocytes were stimulated with LPS (1 µg/ml), TNFα (50 ng/ml), or IL-1β (10 ng/ml) with and without pretreatment with GYY4137 (300 µM). (A and B) Immunoblot analyses of intracellular and extracellular Prx4 levels are shown. Actin was served as an endogenous control. Each bar shows the mean and SE (n=4). *P<0.05 by Tukey-Kramer post hoc test. ***P<0.001 by Tukey-Kramer post hoc test. (C and D) Percentages of extracellular Prx4 per total Prx4 in IL-1β-stimulated hepatocyte culture. Each bar shows the mean and SE (n=4). **P<0.01 by Student’s t-test.
We have shown previously that the secretion of mitochondrial contents from hepatocytes is involved in local and/or systemic inflammation during sepsis19, 20. Prxs including Prx4 are suggested to represent damage-associated molecular patterns (DAMPs)23, 24. Indeed, it has been shown that extracellular Prx4 induces inflammatory responses in macrophages through ligation of the TLR4 receptor24. We also showed that GYY4137 can suppress hepatic secretion of Prx4 (Fig. 4). This observation implies that the anti-inflammatory effects of hydrogen sulfide might contribute to inhibition of the release/secretion of DAMPs, such as Prx4, from hepatocytes. Liver inflammation is a process triggered by hepatocyte injuries and subsequent release of DAMPs, which in turn leads to the activation of immune cells such as neutrophils and macrophages25. Although its mechanism still remains to be elucidated, anti-inflammatory effects of hydrogen sulfide are largely attributed to its effects on immune cells. Our current report thus shed light on the role of hydrogen sulfide against the release of DAMPs from hepatocytes, representing another point of anti-inflammatory action of hydrogen sulfide.
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by MEXT KAKENHI (Grant Number 16K09203 to Ka.U. and Grant Number 17H04147 to T.A.).
References
- 1.Schulte J. Peroxiredoxin 4: a multifunctional biomarker worthy of further exploration. BMC Med. 9: 137 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada S, and Guo X. Peroxiredoxin 4 (PRDX4): Its critical in vivo roles in animal models of metabolic syndrome ranging from atherosclerosis to nonalcoholic fatty liver disease. Pathol Int. 68: 91–101. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Okado-Matsumoto A, Matsumoto A, Fujii J, and Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J Biochem. 127: 493–501. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto A, Okado A, Fujii T, Fujii J, Egashira M, Niikawa N, and Taniguchi N. Cloning of the peroxiredoxin gene family in rats and characterization of the fourth member. FEBS Lett. 443: 246–250. 1999. [DOI] [PubMed] [Google Scholar]
- 5.Tavender TJ, and Bulleid NJ. Peroxiredoxin IV protects cells from oxidative stress by removing H2O2 produced during disulphide formation. J Cell Sci. 123: 2672–2679. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbasi A, Corpeleijn E, Postmus D, Gansevoort RT, de Jong PE, Gans RO, Struck J, Schulte J, Hillege HL, van der Harst P, Peelen LM, Beulens JW, Stolk RP, Navis G, and Bakker SJ. Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J Am Heart Assoc. 1: e002956 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerrits EG, Alkhalaf A, Landman GW, van Hateren KJ, Groenier KH, Struck J, Schulte J, Gans RO, Bakker SJ, Kleefstra N, and Bilo HJ. Serum peroxiredoxin 4: a marker of oxidative stress associated with mortality in type 2 diabetes (ZODIAC-28). PLoS One. 9: e89719 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulte J, Struck J, Köhrle J, and Müller B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 35: 460–465. 2011. [DOI] [PubMed] [Google Scholar]
- 9.Strnad P, Tacke F, Koch A, and Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 14: 55–66. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, and Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 18: 854–856. 2004. [DOI] [PubMed] [Google Scholar]
- 11.Ryter SW, and Choi AM. Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found Symp. 280: 165–175, discussion 175–181. 2007. [PubMed] [Google Scholar]
- 12.Norris EJ, Larion S, Culberson CR, and Clemens MG. Hydrogen sulfide differentially affects the hepatic vasculature in response to phenylephrine and endothelin 1 during endotoxemia. Shock. 39: 168–175. 2013. [DOI] [PubMed] [Google Scholar]
- 13.Unuma K, Aki T, Nagano S, Watanabe R, and Uemura K. The down-regulation of cardiac contractile proteins underlies myocardial depression during sepsis and is mitigated by carbon monoxide. Biochem Biophys Res Commun. 495: 1668–1674. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Unuma K, Aki T, Noritake K, Funakoshi T, and Uemura K. A CO-releasing molecule prevents annexin A2 down-regulation and associated disorders in LPS-administered rat lung. Biochem Biophys Res Commun. 487: 748–754. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, and Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 117: 2351–2360. 2008. [DOI] [PubMed] [Google Scholar]
- 16.Nemzek JA, Hugunin KM, and Opp MR. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med. 58: 120–128. 2008. [PMC free article] [PubMed] [Google Scholar]
- 17.Fink MP. Animal models of sepsis. Virulence. 5: 143–153. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aki T, Unuma K, Noritake K, Kurahashi H, Funakoshi T, and Uemura K. Interaction of carbon monoxide-releasing ruthenium carbonyl CORM-3 with plasma fibronectin. Toxicol In Vitro. 50: 201–209. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Unuma K, Aki T, Funakoshi T, Hashimoto K, and Uemura K. Extrusion of mitochondrial contents from lipopolysaccharide-stimulated cells: Involvement of autophagy. Autophagy. 11: 1520–1536. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unuma K, Aki T, Matsuda S, Funakoshi T, Yoshida K, and Uemura K. Elimination and active extrusion of liver mitochondrial proteins during lipopolysaccharide administration in rat. Hepatol Res. 43: 526–534. 2013. [DOI] [PubMed] [Google Scholar]
- 21.Unuma K, Aki T, Matsuda S, Funakoshi T, Yoshida K, and Uemura K. Inducer of heme oxygenase-1 cobalt protoporphyrin accelerates autophagy and suppresses oxidative damages during lipopolysaccharide treatment in rat liver. Hepatol Res. 43: 91–96. 2013. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 112(Suppl): 321S–329S. 1997. [DOI] [PubMed] [Google Scholar]
- 23.Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K, and Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 18: 911–917. 2012. [DOI] [PubMed] [Google Scholar]
- 24.Zhao LX, Du JR, Zhou HJ, Liu DL, Gu MX, and Long FY. Differences in proinflammatory property of six subtypes of peroxiredoxins and anti-inflammatory effect of ligustilide in macrophages. PLoS One. 11: e0164586 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner C, Galluzzi L, Kepp O, and Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 59: 583–594. 2013. [DOI] [PubMed] [Google Scholar]