Key Teaching Points.
-
•
Myocarditis is a rare but important complication of immune checkpoint inhibitor therapy and may manifest as fulminant heart failure or arrhythmia.
-
•
A favorable outcome is possible in life-threatening immune-mediated myocarditis with prompt recognition and aggressive management.
-
•
Multiple strategies may be required for rhythm management in cases of severe fulminant myocarditis.
Introduction
The management of malignancy with immunotherapy is a rapidly evolving field and immune checkpoint inhibitors (ICI) are being used and trialed in an increasing number of malignancies. Monoclonal antibodies that target programmed death-1 (PD-1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) enable the patient to generate an immune response to tumor cells, but have also generated T cell–mediated autoimmune responses described in several organs. To date, the most common autoimmune side effects have been colitis, hepatitis, and pneumonitis. Myocarditis is a rare complication of immune checkpoint inhibitor therapy and is more commonly described in the context of combination therapy with ipilimumab (anti-CTLA-4 antibody) and nivolumab (anti-PD-1 antibody).1, 2, 3 In most cases the myocarditis is fatal3 and although some isolated cases report some positive treatment outcomes,4, 5 the best management of cardiac complications is not well established. A recent case series comprising 35 patients with ICI-induced myocarditis reported 7 cases of nivolumab-induced myocarditis, of which 100% experienced a major cardiovascular event.6 We report the first successful management of ventricular storm precipitated by checkpoint inhibitor–mediated fulminant myocarditis, using a combination of antiarrhythmics, antitachycardia and overdrive pacing, direct current cardioversion, and sedation with concurrent immunosuppression, including rabbit-anti-thymocyte globulin (r-ATG).
Case report
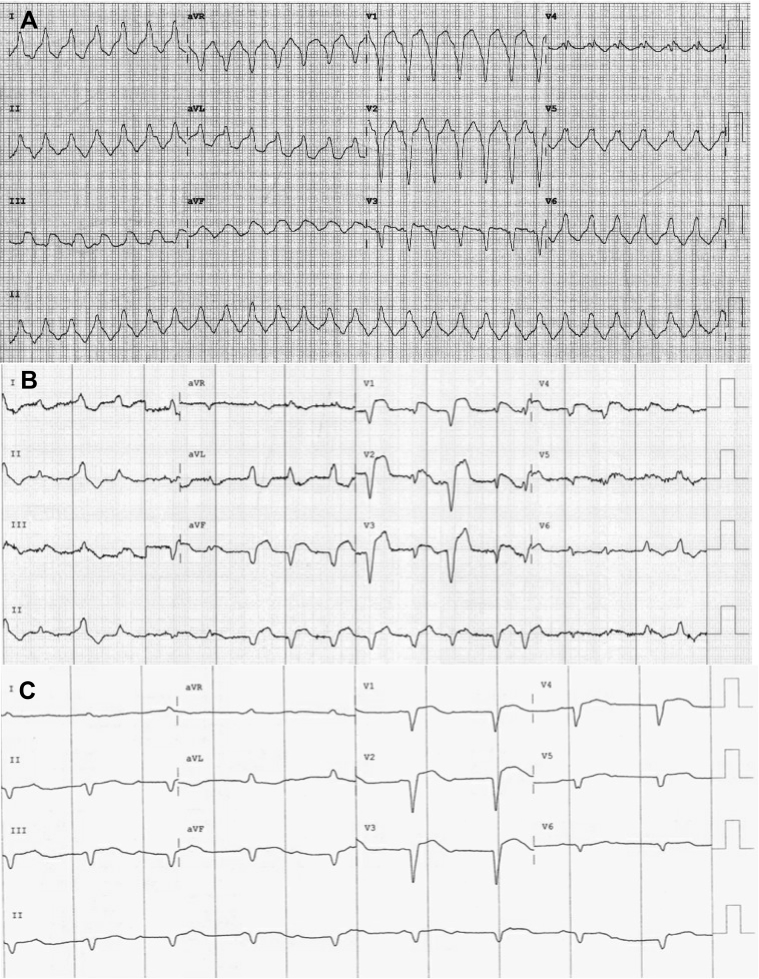
A 65-year-old woman presented to an emergency department having been unwell for 2 days. She reported intermittent lightheadedness, dyspnea, and lethargy on a background of stage III esophageal carcinoma, hypertension, hypercholesterolemia, diabetes mellitus, and prior history of smoking. Her therapy for her esophageal adenocarcinoma included neoadjuvant chemotherapy/radiotherapy (carboplatin, paclitaxel) and surgical resection completed 4 months prior to current presentation. She had an estimated median survival of greater than 1 year and was enrolled in a randomized controlled trial on nivolumab. Following presentation she was unblinded to confirm she was on the active treatment arm and had received her second dose of nivolumab 2 weeks prior to her presentation. Shortly after presentation she became hypotensive and tachycardic with a 12-lead electrocardiogram, revealing a regular broad complex tachycardia with left bundle branch block pattern consistent with ventricular tachycardia (VT) at a rate of 156 beats per minute (Figure 1). The arrhythmia reverted spontaneously to atrial fibrillation with a left bundle branch block; however, she remained hypotensive despite fluid resuscitation. Cardiac enzymes were elevated, including a high sensitivity troponin I (5828 ng/L) and creatinine kinase (842 U/L). The patient was transferred urgently to a tertiary referral center for further investigation and management.
Figure 1.
Electrocardiograms demonstrating A: ventricular tachycardia on initial presentation, B: atrial fibrillation, C: complete heart block.
Further investigations were performed to exclude alternative causes of the troponin rise. Coronary angiography demonstrated minor coronary disease, a ventilation-perfusion scan excluded pulmonary embolus, and a transthoracic echocardiogram demonstrated global left ventricular systolic impairment with a left ventricular ejection fraction (LVEF) of 35%, consistent with a myocarditis. In contrast, a baseline echocardiogram prior to the management of her malignancy demonstrated normal left ventricular function. The patient’s full blood count, electrolytes, and renal, liver, and thyroid function tests were normal. No abnormalities were detected on autoimmune serology. A cardiac magnetic resonance imaging was aborted owing to recurrence of VT mid-scan.7 Cardiac biopsy was not performed as it was felt that the risks of the procedure outweighed the benefits, given the patient’s clinical condition.
Over the subsequent 4 days the patient was commenced on a beta-blocker, amiodarone, and pulse methylprednisolone (1 g/day intravenously). Her clinical situation deteriorated further from day 4 after presentation, with refractory ventricular storm and intermittent third-degree A-V block. Bolus doses and infusions of intravenous amiodarone and lignocaine failed to quiesce her recurrent VT. A transvenous temporary pacing wire was inserted on day 5 of the admission for pace termination of sustained VT, which was performed on multiple occasions. On 1 occasion the VT exceeded the maximum pacing rate of the temporary pacing generator and the patient became profoundly hypotensive. She was sedated, ventilated, and cardioverted twice on day 5 and sedation was maintained for 4 days to help facilitate control of the ventricular storm.
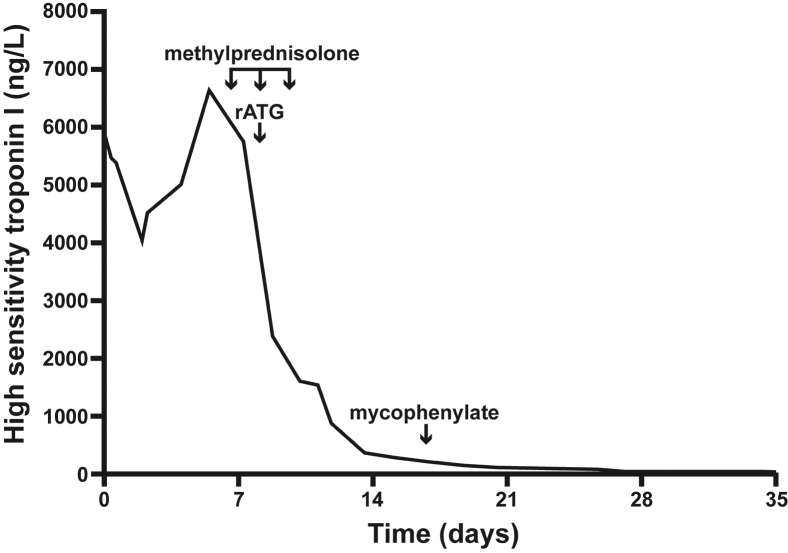
The troponin peaked at 6632 ng/L on day 4, but remained elevated, and a decision was made to escalate immunosuppressive therapy on day 5. On day 2 of steroid therapy a single dose of Genzyme r-ATG (Sanofi Genzyme, Cambridge, MA) was administered (1.5 mg/kg) with premedication (loratadine and hydrocortisone). Administration of r-ATG was complicated by severe hypotension, requiring inotropic support with vasopressin and noradrenaline, and no further doses were administered. Abrogation of the patient’s lymphocyte count (0 cells/L) occurred 1 day after the administration of r-ATG. Pulse methylprednisolone 1 g daily was continued for 3 days, followed by tapered course of prednisone initiated at 1 mg/kg/day and weaned completely after 7 months. Mycophenolate mofetil was commenced 5 days after r-ATG as adjunctive therapy and a steroid-sparing agent, initially 1 g twice daily tapered completely after 7 months.
The patient’s clinical state improved, with no further episodes of VT after day 5, and her left ventricular ejection fraction improved to 45%–50% on day 20 of the admission, with a fall in the troponin to 28 ng/L (Figure 2). She had an internal cardiac defibrillator implanted prior to discharge and has had good functional recovery after a brief period of inpatient rehabilitation. Prednisone and mycophenolate was slowly weaned over the next 6 months, but unfortunately, despite control of her ventricular storm and myocarditis, the patient had a recurrence of her malignancy and was managed with a palliative approach, dying 19 months after her initial presentation.
Figure 2.
Reduction of troponin level following the administration of rabbit-anti-thymocyte globulin (rATG): troponin level during course of treatment.
Discussion
ICIs are effectively used in the treatment of melanoma and cancers of the lung cancer, kidney, and head and neck.8 Ipilimumab induces an antitumor response by inhibiting CTLA-4, resulting in the activation and proliferation of a higher number of effector T cells, regardless of TCR specificity, and reducing regulatory T cell–mediated suppression of T-cell responses, control of peripheral T-cell tolerance, and autoimmunity via the CTLA-4 and PD-1 pathways.9 Nivolumab-induced PD-1 blockade works to restore the immune function of T cells in the periphery that have been turned off following extended or high levels of PD ligand-1 antigen exposure, as in advanced cancer.10 The potential for subsequent activation of autoreactive T cells causes immune-related adverse events (irAEs). Skin, gut, endocrine, lung, and musculoskeletal irAEs are relatively common, whereas cardiovascular, hematologic, renal, neurologic, and ophthalmologic irAEs are much less frequently observed. The cardiovascular complications include life-threatening myocarditis, conduction abnormalities, and ventricular storm. Recognition of the cardiac complications of immune checkpoint inhibitors is increasing, with a recent observational, retrospective pharmacovigilance study identifying 122 patients with immune checkpoint inhibitor–induced myocarditis, 58 of which had nivolumab monotherapy.3 Despite this, the majority of published reports still suggest that the prognosis is poor, and experience in the best management remains limited. Consistent with this, Salem and colleagues3 reported a 50% mortality rate for ICI-associated myocarditis.
Several case reports or small case series have now also been reported describing cardiac complications while on nivolumab monotherapy, most commonly myocarditis and conduction abnormalities. Only 3 of the case reports identified were complicated by ventricular arrhythmias, of which 2 cases had a fatal outcome. One report describes a fatal case of VT and bradycardic arrest in a patient with normal LVEF (65%) treated with nivolumab for small cell lung carcinoma.11 Another report describes a case of new-onset acute decompensated heart failure and recurrent VT following nivolumab therapy.12 In this case the patient died 2 days after discharge from a presumed ventricular arrhythmia. A third case was the only case of nivolumab-induced myocarditis complicated by ventricular arrhythmia identified that had a favorable outcome.13 The patient in this report had episodes of nonsustained VT and an episode of sustained VT requiring cardioversion, but not ventricular storm as in our case. Similarly, this patient was treated with antiarrhythmics, beta blockers, steroids, immunosuppression, and anti-thymocyte globulin. Two other recent cases of nivolumab-induced myocarditis, not complicated by ventricular arrhythmia, also demonstrate how aggressive management can lead to a favorable outcome.4, 5 Management in these cases including aggressive hemodynamic support with extracorporeal membrane oxygenation, intra-aortic balloon pump, heart failure management, pacing, and aggressive management to halt the immunological process, including methylprednisolone, infliximab, plasmapheresis, intravenous immunoglobulin, and tacrolimus.
Cardiac complications and arrhythmias are also rarely eported with other immune checkpoint inhibitor therapies. Salem and colleagues3 report 22 cases of ventricular arrhythmias with ICIs at a rate of 0.07%.3 One case series of cardiac complications on ICI comprised 8 patients.1 Six patients received ipilimumab, 1 pembrolizumab (another anti-PD-1 antibody), and 1 combination therapy (nivolumab and ipilimumab). Further reports of cardiac complications have been reported with combination immuno-checkpoint inhibitors (nivolumab and ipilimumab). Johnson2 reported 2 fatal cases on combination therapy. The first patient had a normal LVEF (73%) but developed a troponin rise and complete heart block. The second patient had a troponin elevation, mildly reduced LVEF (50%), and a conduction delay.
The alternate diagnoses for myocarditis include viral infection and autoimmune disease, which are excluded on routine laboratory investigation, but recognition and therapy of an irAE should not be delayed for the results of these investigations for a life-threatening presentation. Management of the cardiac irAE includes 3 concurrent arms of therapy: (1) immediate management for tachy- and bradyarrhythmias; (2) management of cardiac failure, as many of these are rapidly progressing and potentially life threatening; and (3), prompt recognition that the process is attributable to ICI therapy with early implementation of immunosuppressive therapy. This is achieved by discontinuing the causative agent and rapid initiation of high-dose steroids with or without steroid-sparing agents. r-ATG appeared in this case to be highly effective at abrogating the T cell population and helping to control the immune response. While it cannot be conclusively determined that the clinical response was a direct result of administration of r-ATG, the time course of clinical response and fall in troponin, along with immediate reduction in T cell population, suggests that the administration of r-ATG did indeed play a role. Given the potential adverse effects of r-ATG, including the possibility of cancer recurrence, as demonstrated in this case, this may be best reserved for cases refractory to first-line immunosuppression.
One limitation of this report is that it is a single case study from 1 center. Autoimmune and viral causes of the myocarditis, troponin rise, and arrhythmias were excluded on laboratory investigation. A cardiac magnetic resonance imaging was not completed owing to the occurrence of VT. The echocardiogram, however, did demonstrate global left ventricular dysfunction in a pattern consistent with myocarditis. An endomyocardial biopsy may have revealed lymphocytic infiltrates with predominance of cytotoxic CD8 T cells and decreased FoxP3+ regulatory T cells as previously described in ICI myocarditis14 and excluded causes such as cardiac sarcoidosis, giant cell myocarditis, or idiopathic granulomatosis. Polymerase chain reaction studies might have been useful to exclude coxsackievirus, human herpesvirus 6, and other adenoviruses. However, in cases attributable to ICI myocarditis, peripheral viral serology is negative.1 Despite these limitations, the temporal relationship between nivolumab therapy and myocarditis in our patient, the absence of clinical and laboratory evidence of an autoimmune or viral cause, and the response to r-ATG and immunosuppressive therapy is highly suggestive that the diagnosis was ICI cardiac toxicity.
Conclusion
This case report illustrates a case of immune-mediated myocarditis, a rare but important complication of ICI. It highlights the tachy- and bradyarrhythmias that can complicate such a presentation. A favorable outcome is possible in life-threatening presentations with prompt recognition of the diagnosis and effective and aggressive rhythm management, including pace termination of arrhythmias and potent immunosuppression.
Acknowledgments
The authors wish to acknowledge the medical and nursing staff of the intensive care and coronary care units of Royal North Shore Hospital for the clinical services provided to the patient. They are grateful for the Department of Pharmacy at Royal North Shore Hospital for the supply of r-ATG administered to the patient.
References
- 1.Heinzerling L., Ott P.A., Hodi F.A. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunotherapy Cancer. 2016;4:50–61. doi: 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem J., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arangalage D., Deylon J., Lermuzeaux M. Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann Intern Med. 2017;167:683–684. doi: 10.7326/L17-0396. [DOI] [PubMed] [Google Scholar]
- 5.Frigeri M., Meyer P., Banfi C. Immune checkpoint inhibitor-associated myocarditis: a new challenge for cardiologists. Can J Cardiol. 2018;34:92.e1–92.e3. doi: 10.1016/j.cjca.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaheen O., Ghibour A., Alsaid B. Esophageal cancer metastases to unexpected sites: a systematic review. Gastroenterol Res Pract. 2017;2017:1657310. doi: 10.1155/2017/1657310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchbinder E., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 10.Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 11.Gibson R., Delaune J., Szady A., Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-216228. bcr2016216228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassam T., Ramasubbu K. Fatal myocarditis: a rare but life threatening adverse effect of nivolumab chemotherapy. Chest. 2017;152:692A. [Google Scholar]
- 13.Tay R., Blackley E., McLean C. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer. 2017;117:921–924. doi: 10.1038/bjc.2017.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brustle K., Heindecker B. Checkpoint inhibitor induced cardiotoxicity: managing the drawbacks of our newest agents against cancer. Oncotarget. 2017;8:106165–106166. doi: 10.18632/oncotarget.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]