Introduction
Atrioventricular nodal reentrant tachycardia (AVNRT) is a frequently encountered tachycardia. It is generally associated with a favorable prognosis. Polymorphic ventricular tachycardia (VT), on the other hand, may be associated with an adverse prognosis and can result in ventricular fibrillation (VF). We present 3 cases of otherwise healthy patients with typical AVNRT triggering polymorphic VT.
Case report
Case 1
The first patient is a 35-year-old woman who presented with palpitations and near syncope. There was no relevant past medical history, or family history. Electrocardiograms (ECGs) showed a narrow QRS-complex tachycardia at a rate of 250 beats per minute (bpm). The supraventricular tachycardia (SVT) degenerated spontaneously into polymorphic VT (Figure 1A). The onset of the ventricular arrhythmia may have coincided with a subtle increase in cycle length of the tachycardia or impending spontaneous termination. The polymorphic VT converted into sinus tachycardia. No drugs were administered. ECG in sinus rhythm was normal (Figure 1B). Electrolytes, echocardiogram, cardiac magnetic resonance imaging, computed tomography coronary angiography, and exercise testing were normal. She subsequently underwent an electrophysiology (EP) study in the conscious state, during which typical AVNRT (240 bpm) was induced using atrial extrastimuli with isoprenaline infusion. His-synchronous ventricular premature beats (VPBs) did not perturb SVT, thereby excluding concealed accessory pathway. Ventricular overdrive pacing resulted in a VAHV response with a long postpacing interval consistent with typical AVNRT. The SVT was allowed to sustain for 10 minutes to see if the polymorphic VT would occur spontaneously. However, no ventricular arrhythmia was observed and the SVT was pace-terminated. The slow pathway was ablated. Following ablation, no tachycardia was inducible. Specifically, ventricular arrhythmia was not inducible with burst pacing and up to 2 extrastimuli (S1 400 ms, S2 220 ms, S3 210 ms), from 2 sites in the right ventricle with and without isoprenaline. She was not started on any medication. She has not experienced further arrhythmic symptoms during a follow-up of 5 months. A cardiogenetic expert was consulted and genetic testing was deferred, given the expected low additional value in the absence of an overt phenotype for an inherited arrhythmia syndrome.
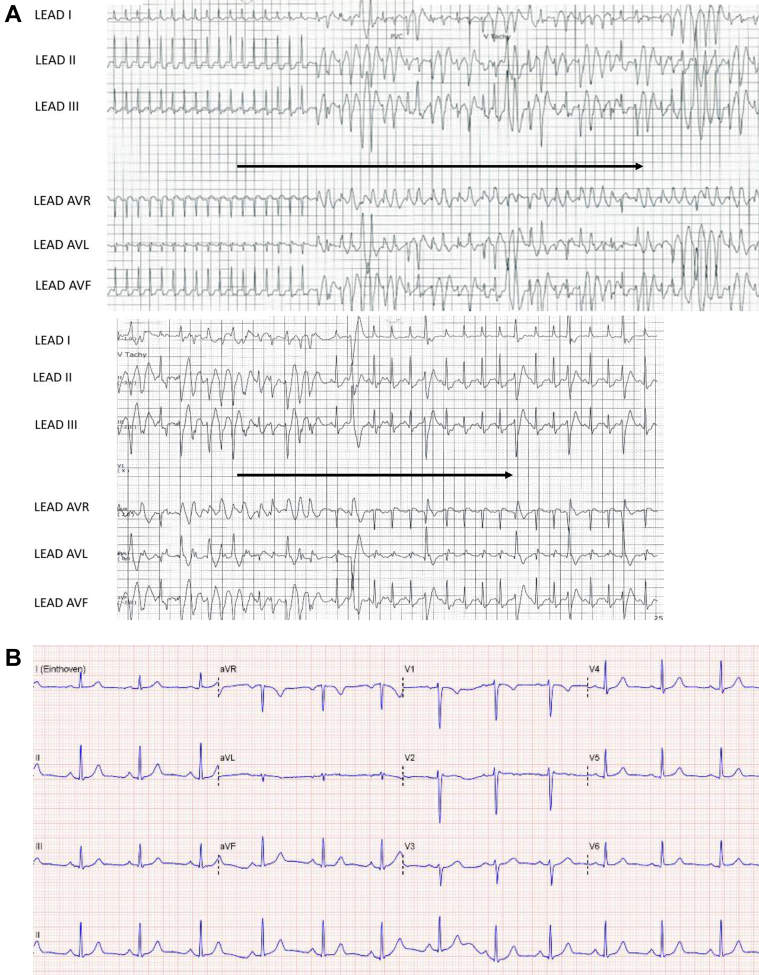
Figure 1.
Patient 1. A: Rhythm strip on admission showing supraventricular tachycardia of 250 beats per minute, triggering polymorphic ventricular tachycardia (VT). This run of polymorphic VT lasted 13.2 seconds and converted to sinus rhythm spontaneously. The coupling interval of the initiating beat was 310 ms. Following conversion to sinus rhythm multiple premature ventricular contractions (PVCs) were observed with 2 morphologies. The first PVC had a right inferior axis whereas the subsequent PVCs had left superior axis (similar to the PVC initiating polymorphic VT). B: Normal 12-lead electrocardiogram during sinus rhythm.
Case 2
The second patient is a 40-year-old woman who also presented with palpitations and lightheadedness. There was no relevant past medical history or family history. She presented with SVT at a rate of 250 bpm, which triggered a run of polymorphic VT (Figure 2). There was no evidence of bradycardia or pause preceding the VT. No medication was administered and electrolytes were normal. Coronary angiogram, echocardiography, cardiac magnetic resonance imaging, exercise testing, and adrenaline challenge were normal. An EP study was performed under sedation. Accessory pathways were excluded. Dual atrioventricular (AV) nodal pathways were present and typical AVNRT (250 bpm) was induced with atrial extrastimuli without isoprenaline infusion. His-synchronous VPBs did not perturb the tachycardia. The SVT terminated spontaneously after 5 minutes. No spontaneous ventricular arrhythmia was observed. The slow pathway was ablated and SVT was not inducible afterward. VT was not inducible with burst pacing, extrastimuli (S1 400 ms, S2 220 ms, S3 210 ms, S4 200 ms) with and without isoprenaline. The patient has remained arrhythmia free for the past 40 months without medications. Clinical genetic testing has been deferred.
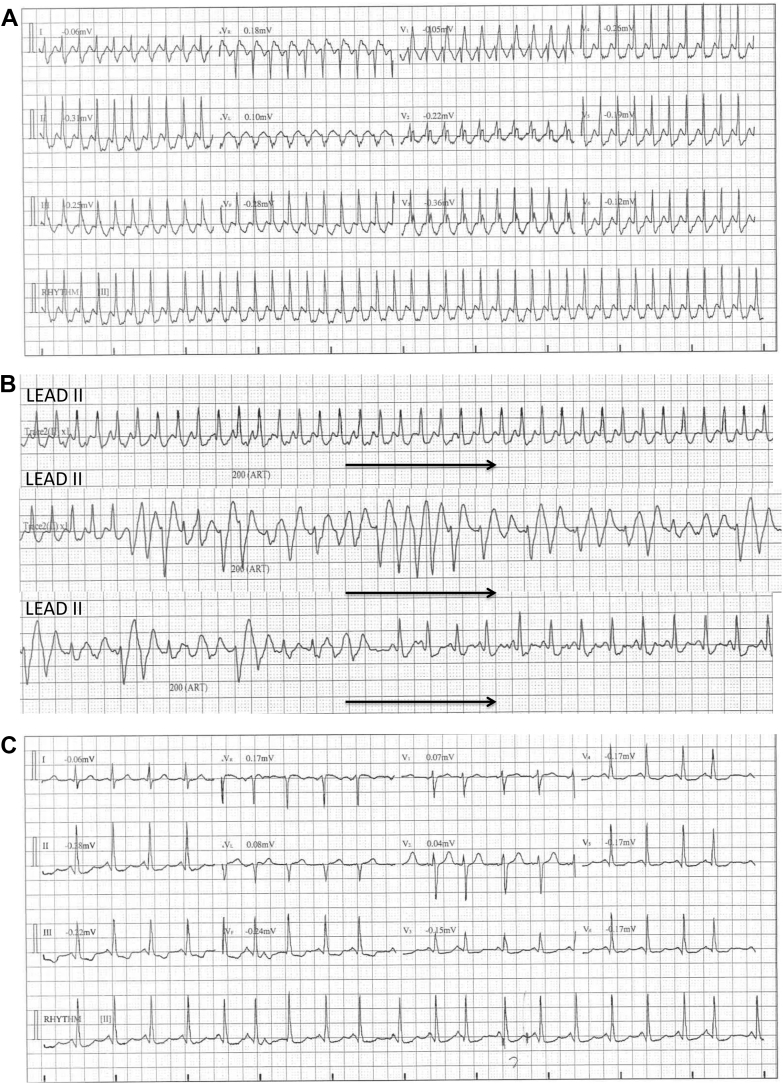
Figure 2.
Patient 2. A: A 12-lead electrocardiogram showing very rapid regular tachycardia of 250 beats per minute (bpm) with typical right bundle branch block. B: Single-lead (lead II) rhythm strip showing regular supraventricular tachycardia at a rate of 250 bpm degenerating into polymorphic ventricular tachycardia (VT) of 12.5 seconds’ duration. The coupling interval of the first VT beat is 230 ms. This episode terminates spontaneously into sinus tachycardia. C: A 12-lead electrocardiogram post spontaneous reversion to sinus rhythm.
Case 3
The third patient is a 48-year-old woman who underwent a slow pathway ablation for typical AVNRT 2 years ago. There was no other relevant medical history or family history. She was taking levothyroxine for hypothyroidism. Following the ablation, she experienced recurrent palpitations and presented with rapid SVT of 260 bpm. In the emergency department, the SVT triggered a nonsustained run of polymorphic VT that transitioned to a more organized pleomorphic VT before spontaneously converting to sinus rhythm (Figure 3). No medication was administered and electrolytes were normal. Her ECG in sinus rhythm was normal. Echocardiogram, coronary computed tomography scan, and exercise testing were normal. During the repeat EP study, there was dual AV nodal physiology without evidence of accessory pathway. SVT at a rate of 250 bpm was induced on isoprenaline with atrial burst pacing. His-synchronous VPBs did not perturb tachycardia. The response to ventricular overdrive pacing was compatible with typical AVNRT. The SVT was pace-terminated after 6 minutes. No spontaneous ventricular arrhythmia was observed following the SVT. The slow pathway was successfully modified and tachycardia was not inducible following ablation. VT could not be induced with ventricular pacing (S1 400 ms, S2 260 ms, S3 240 ms) with and without isoprenaline. She was not started on medication and she has not experienced further arrhythmic issues during a follow-up of 5 months. Genetic testing was deferred following cardiogenetic consultation.
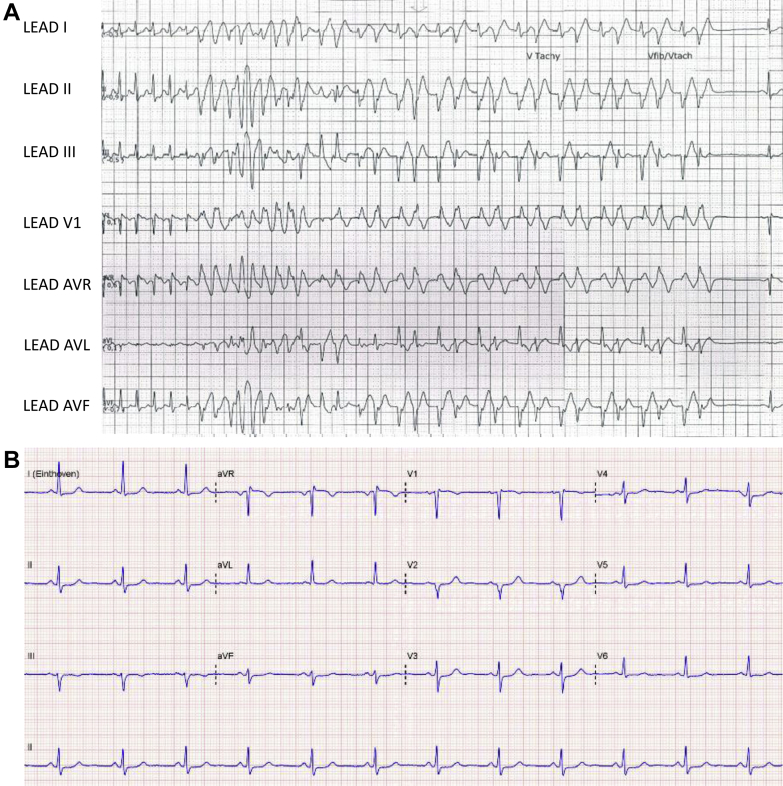
Figure 3.
Patient 3. A: Rhythm strip at presentation, demonstrating regular supraventricular tachycardia of 260 beats per minute triggering a run of polymorphic ventricular tachycardia lasting for 8.5 seconds before spontaneous termination. The coupling interval of the initiating beat is 220 ms. B: Baseline 12-lead electrocardiogram during sinus rhythm.
Discussion
Polymorphic VT can deteriorate into VF and can result in sudden death. Underlying causes of polymorphic VT include coronary artery disease, anomalous coronary anatomy, cardiomyopathy, and inherited arrhythmia syndromes. Idiopathic polymorphic VT is rare.1
Our patients underwent extensive cardiac evaluation, effectively excluding overt cardiomyopathy, coronary artery disease, and coronary anomaly. An inherited channelopathy such as long QT syndrome or catecholaminergic polymorphic VT (CPVT) would also be unlikely in the setting of normal baseline and stress ECGs and negative family history. However, it has been reported that latent CPVT can be brought out by adrenaline even when the stress test is negative.2 In addition, a possible association has been recently reported between Brugada syndrome and AVNRT, and Brugada ECG changes may be absent in the baseline ECG. Hasdemir and colleagues3 performed an ajmaline challenge in patients undergoing ablation for AVNRT without any sign of Brugada syndrome on baseline electrocardiogram; Brugada syndrome was unmasked in 26 of 96 patients with AVNRT (27.1%) compared to in 3 of 66 control patients.
AVNRT is commonly seen in daily practice. AVNRT is often well tolerated and it is rarely life threatening.4 In particular, AVNRT degenerating into polymorphic VT is rarely seen. Likewise, during invasive EP studies, polymorphic VT is a very rare observation even when the atrium is stimulated at rates beyond 250 bpm or during induced tachycardia with or without beta-agonists.
There are publications suggesting an association between AVNRT and idiopathic VT. Wylie and colleagues5 found in a retrospective study that among 33 patients with idiopathic VT, 23 of them had dual AV nodal physiology. The prevalence of dual AV nodal pathways with echo beats and sustained AVNRT in this group of 33 patients was 24% and 21%, respectively. So AVNRT or at least dual AV nodal physiology may be associated with idiopathic VT. The etiology of this association is unclear, but one of the hypotheses is that a persisting embryonic conduction branch, the so-called “dead-end tract,” may form a connection between the AV nodal region and the focus for idiopathic VT.6
A few articles have reported polymorphic VT in structurally normal hearts induced by Valsalva maneuvers7 and by carotid sinus massage8 without preceding bradycardia. These observations support a hypothesis that ventricular arrhythmia can be triggered by a complex interplay of the sympathetic and parasympathetic activity of the autonomic nervous system induced by these maneuvers. Moreover, Tan and colleagues9 have reported the occurrence of ventricular ectopy varying from premature ventricular beats to nonsustained VT in two-thirds of 187 cases of SVT in which adenosine was administered. This might be due to delayed afterdepolarizations caused by adenosine or it might be an effect of the preceding SVT.
In the present cases of otherwise healthy patients, structural heart disease was ruled out and no vagal maneuver or medication was administered. The clinical and electrophysiological findings are summarized in Table 1. Although there have been prior reports of polymorphic VT and VF induced by right ventricular outflow tract arrhythmias,10, 11 reports of AVNRT spontaneously degenerating into polymorphic VT are scarce, with only 2 cases reported.12, 13 To our knowledge this is the first reported case series. A difference between our patients and the previous reports is the heart rate of the SVT. In all 3 patients reported in the current series, the heart rate was 250 bpm or higher, while in the 2 cases reported previously the heart rate was 220 bpm and 200 bpm, respectively.
Table 1.
Summary of clinical and electrophysiological findings
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age | 35 | 40 | 48 |
| Sex | Female | Female | Female |
| Rate of SVT (bpm) | 250 | 250 | 260 |
| Initiating beat | |||
| Coupling interval (ms) | 310 | 230 | 220 |
| Axis | Superior | Superior | RBBB, superior |
| QRS duration (ms) | 110 | 100 | 100 |
| PMVT | |||
| Duration (s) | 13.2 | 12.5 | 8.5 |
| Postreversion PVCs | Yes | No | No |
| Axis | Left superior | N/A | N/A |
| Electrophysiology study | |||
| Sedation | Nil | Yes | Nil |
| Method of induction | Atrial extrastimuli (on isoprenaline) | Atrial extrastimuli (no isoprenaline) | Atrial burst pacing (on isoprenaline) |
| Rate of induced SVT (bpm) | 240 | 250 | 250 |
| Septal VA interval (ms) | 12 | 15 | 0 |
| Effect of His-synchronous PVC | Nil | Nil | Nil |
| Duration of induced SVT | 10 minutes | 5 minutes | 6 minutes |
| Termination | Pace-terminated | Spontaneous AV block | Pace-terminated |
| VT induction | |||
| Minimum pacing CL (ms) | 280 | 250 | 300 |
| Extrastimuli, S1,2,3,4 (ms) | 400,220,210 | 400,220,210,200 | 400,260,240 |
| VT induction result | No VT | No VT | No VT |
| Exercise testing | |||
| Peak rate (% age-pred max) | 183 (97%) | 179 (99%) | 143 (80%) |
| PVC/VT | No PVC/VT | No PVC/VT | 1 PVC in recovery (RBBB morphology, superior axis) |
AV = atrioventricular; bpm = beats per minute; CL = cycle length; N/A = not applicable; PMVT = polymorphic ventricular tachycardia; Pred max = predicted maximum; PVC = premature ventricular contraction; RBBB = right bundle branch block; SVT = supraventricular tachycardia; VA = ventriculoatrial; VT = ventricular tachycardia.
The mechanism is not known. Although the patients did not exhibit a clear phenotype for a classic Mendelian inherited channelopathy, one wonders whether these patients may have genetic polymorphism that predisposes them to polymorphic VT when stimulated at very high rates. In up to 20% of patients with idiopathic ventricular fibrillation (IVF), a family history of sudden cardiac death or IVF is present, and this suggests that at least some cases of IVF are hereditary.14 Ischemia caused by a prolonged duration of very rapid SVT is another consideration even though coronary artery disease was excluded and functional ischemia was not observed during induced SVT (of several minutes’ duration) or rapid burst pacing during isoprenaline testing. It is also possible that abnormal calcium homeostasis triggering VT may be an inherent issue or induced by prolonged periods of tachycardia. However, the absence of ventricular ectopy or arrhythmia during exercise testing and burst pacing on isoprenaline infusion would argue against abnormal calcium homeostasis akin to CPVT. Another possible mechanism may be fascicular or bundle branch reentry, even though such reentrant beats were not observed during the EP studies. Interestingly, underlying genetic culprit mutations were recently identified for 6 cases of apparent idiopathic bundle branch reentrant VT.15
The present cases highlight the possibility of polymorphic VT being induced by SVT in patients without overt underlying heart disease, with the common feature being very rapid heart rate (≥250 bpm). Therefore, this may provide the impetus for a more aggressive approach (including ablation) in such cases.
In all 3 cases the potential triggering stimulus was removed by ablating the AVNRT and no tachyarrhythmia was inducible afterward. It is unknown if these patients should be further protected by offering prophylactic beta-blockers. There is also no evidence to inform the discussion about the value of genetic testing. Finally, there is clinical equipoise in terms of defibrillator implantation in this setting. Patients should be engaged in shared decision making after a thoughtful discussion of the potential benefits (prophylaxis against recurrent life-threatening arrhythmia) and risks (inappropriate shocks, infection, lead failure). It is interesting that in all 3 cases, the patients declined defibrillator implantation. Factors guiding the decisions included the self-limiting nature of the clinical ventricular arrhythmias, the ablation of the precipitating SVT, the absence of overt cardiomyopathy or channelopathy, and the potential long-term harm of defibrillators in young patients.
Key Teaching Points.
-
•
Polymorphic ventricular tachycardia (PMVT) may occur following supraventricular tachycardia in patients without overt structural heart disease.
-
•
Very rapid supraventricular tachycardia (≥250 beats per minute) may portend a higher risk of PMVT.
-
•
A more aggressive approach (including ablation) may be considered in such cases.
References
- 1.Mechleb B.K., Haddadin T.Z., Iskandar S.B., Abboud L.N., Fahrig S.A. Idiopathic polymorphic ventricular tachycardia with normal QT interval in a structurally normal heart. Pacing Clin Electrophysiol. 2006;29:791–796. doi: 10.1111/j.1540-8159.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 2.Sy R.W., Gollob M.H., Klein G.J. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2011;8:864–871. doi: 10.1016/j.hrthm.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 3.Hasdemir C., Payzin S., Kocabas U. High prevalence of concealed Brugada syndrome in patients with atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2015;12:1584–1594. doi: 10.1016/j.hrthm.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Page R.L., Joglar J.A., Caldwell M.A. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506–e574. doi: 10.1161/CIR.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 5.Wylie J.V., Jr., Milliez P., Germano J.J. Atrioventricular nodal reentrant tachycardia associated with idiopathic ventricular tachycardia: clinical and electrophysiologic characteristics. J Electrocardiol. 2007;40:94–99. doi: 10.1016/j.jelectrocard.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 6.De Vries L., Hendriks A., Szili-Torok T. The "dead-end tract" and its role in arrhythmogenesis. J Cardiovasc Dev Dis. 2016;3:11. doi: 10.3390/jcdd3020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Mattia L., Brieda M., Del Bianco F., Dametto E., Nicolosi G.L. Polymorphic ventricular tachycardia induced by Valsalva manoeuvre in a patient with paroxysmal supraventricular tachycardia. Europace. 2012;14:767–768. doi: 10.1093/europace/eur371. [DOI] [PubMed] [Google Scholar]
- 8.Deepak S.M., Jenkins N.P., Davidson N.C., Bennett D.H., Mushahwar S.S. Ventricular fibrillation induced by carotid sinus massage without preceding bradycardia. Europace. 2005;7:638–640. doi: 10.1016/j.eupc.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Tan H.L., Spekhorst H.H., Peters R.J., Wilde A.A. Adenosine induced ventricular arrhythmias in the emergency room. Pacing Clin Electrophysiol. 2001;24:450–455. doi: 10.1046/j.1460-9592.2001.00450.x. [DOI] [PubMed] [Google Scholar]
- 10.Noda T., Shimizu W., Taguchi A. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J Am Coll Cardiol. 2005;46:1288–1294. doi: 10.1016/j.jacc.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 11.Viskin S., Antzelevitch C. The cardiologists' worst nightmare, sudden death from "benign" ventricular arrhythmias. J Am Coll Cardiol. 2005;46:1295–1297. doi: 10.1016/j.jacc.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moondra V., Sangha R., Greenberg M.L. Spontaneous deterioration of atrioventricular nodal reentrant tachycardia to polymorphic ventricular tachycardia in the absence of heart disease. Pacing Clin Electrophysiol. 2011;34:e14–e17. doi: 10.1111/j.1540-8159.2010.02737.x. [DOI] [PubMed] [Google Scholar]
- 13.Açıkgöz N., Kılıç A., Jata B., Köse S. Degeneration of atrioventricular nodal reentrant tachycardia into polymorphic ventricular tachycardia: a case report. Turk Kardiyol Dern Ars. 2011;39:143–146. doi: 10.5543/tkda.2011.01032. [DOI] [PubMed] [Google Scholar]
- 14.Alders M., Koopmann T.T., Christiaans I. Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am J Hum Genet. 2009;84:468–476. doi: 10.1016/j.ajhg.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts J.D., Gollob M.H., Young C. Bundle branch re-entrant ventricular tachycardia. Novel genetic mechanisms in a life-threatening arrhythmia. JACC Clin Electrophysiol. 2017;3:276–288. doi: 10.1016/j.jacep.2016.09.019. [DOI] [PubMed] [Google Scholar]