Introduction
Bradycardia is common among patients with severe anorexia nervosa (AN). Though generally reversible,1 marked bradycardia may be worrisome to clinicians with limited experience treating eating disorders. While most patients are asymptomatic, a significant minority present with presyncope, lightheadedness, fatigue, and exercise intolerance that could be construed as concordant with current guidelines for permanent pacemaker (PPM) implantation.2 However, symptoms may not reflect bradycardia per se, but rather extreme malnutrition, deconditioning, and hypovolemia associated with restricted caloric intake and purging. Guidelines on the evaluation and management of patients with bradycardia enumerate many potentially reversible causes of sinus node dysfunction, but do not mention severe AN, nor do they proscribe PPM among eating disorder populations.2
The degree of bradycardia can be extreme, with junctional escape rhythms as low as 20 beats per minute (bpm) and may be an indication for hospitalization.3 In a prior inpatient case series the mean heart rate was 44 bpm, with 69% of patients less than 50 bpm.4 Several mechanisms have been postulated to explain bradycardia: increased parasympathetic tone, decreased intracardiac glycogen stores, and myocardial atrophy with resultant structural changes, including fibrosis, on gadolinium-enhanced magnetic resonance imaging.5, 6, 7, 8, 9
Despite functional and structural myocardial changes, bradycardia may principally reflect reduced metabolic demand and cardiac output as a compensatory response to starvation. Weight restoration, therefore, is the mainstay of treatment, with normalization of heart rate upon achieving 85%–90% of ideal body weight (IBW).10 Despite this natural history, profound bradycardia is often distressing to caregivers and may lead to unnecessary PPM placement and attendant iatrogenic morbidity, including exacerbation of existing distorted body self-image. Given this background, we present 4 AN patients transferred to our medical stabilization unit with marked bradycardia, all of whom had manifestations typical of “symptomatic bradycardia.” Two were initially referred by outside facilities for PPM, and 2 had recent pacemaker implants, which we ultimately extracted. Our analysis received exemption from the Colorado Multiple Institutional Review Board (Protocol 19-0547).
Case report
Case 1
A 24-year-old woman with AN presented for refeeding to an outside hospital. She complained of cold and exercise intolerance, fatigue, and generalized weakness without associated lightheadedness or dizziness. She did not take any medications.
Upon admission, her heart rate was 30 bpm and PPM placement was recommended for symptomatic bradycardia. During triage for transfer to our medical stabilization unit we requested that PPM be deferred. Upon presentation to our center, she weighed 36.5 kg (59.3% IBW), with a body mass index (BMI) of 12.6 kg/m2. Supine blood pressure was 58/42 mm Hg with a heart rate of 28 bpm. She was hypothermic (34.6°C), was cachectic, and exhibited slowed speech. She had distant heart sounds, normal jugular venous pressure, and superficial erosion of her skin from prolonged placement of external adherent pacemaker pads. Thyroid studies were normal. An electrocardiogram showed sinus bradycardia at a rate of 29 bpm.
The patient was placed on continuous telemetry monitoring and refeeding was initiated in the intensive care unit. Later that evening, the patient’s heart rate dropped to 22 bpm while sleeping. Transthoracic echocardiogram showed normal left ventricular size and function, global thinning of her left ventricle with reduced left ventricular mass index, and no valvular pathology. Exercise treadmill testing demonstrated mildly reduced exercise capacity but a normal chronotropic response.
Heart rate gradually increased with weight restoration and all symptoms potentially attributable to bradycardia resolved. Upon discharge, her heart rate had increased to 50–60 bpm during sleep and 70–80 bpm when awake and she weighed 39.8 kg (64.8% IBW), a BMI of 13.7 kg/m2.
Case 2
A 19-year-old man with AN was transferred from an outside medical facility for emergent medical stabilization. He began suffering from an eating disorder about 1 year prior to admission when he started restricting calories and exercising regularly as way to “get in shape” for high school track. He consumed only 500–1000 kcal/day and induced vomiting after larger meals. He ran 4–6 miles/day and biked 20 miles/day. He reported generalized weakness and exertional fatigue corresponding with heart rates between 20 and 30 bpm. He denied dizziness, syncope, or presyncope. He was recommended to undergo PPM placement. However, arrangements were made to transfer via air ambulance to our institution prior to implantation.
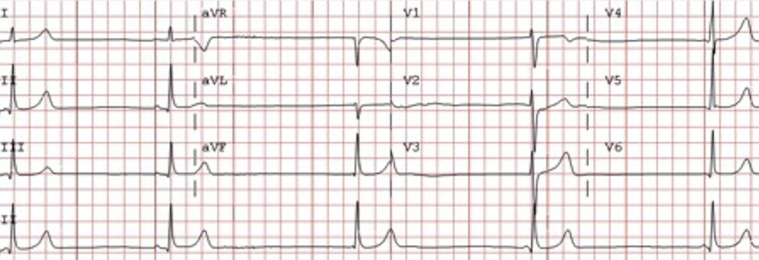
Upon presentation to our center, the patient weighed 52.8 kg (64.9% IBW) with a BMI of 15.7 kg/m.2 Blood pressure was 84/58 mm Hg with a heart rate of 27 bpm at rest and 39 bpm with standing. Cardiac examination was normal. Admission electrocardiogram showed extreme bradycardia at 27 bpm with sinus rhythm and junctional escape complexes (Figure 1). Transthoracic echocardiography was unremarkable.
Figure 1.
Patient 2 admission electrocardiogram.
He was placed on continuous telemetry monitoring and refeeding was initiated. Resting heart rates ranged between 20 and 30 bpm. Symptoms of weakness and fatigue subsequently improved and corresponded to an increase in his resting heart rate to 40–50 bpm with weight restoration. At the time of discharge, he weighed 58.1 kg (71.4% IBW), a BMI of 17.3 kg/m2.
Case 3
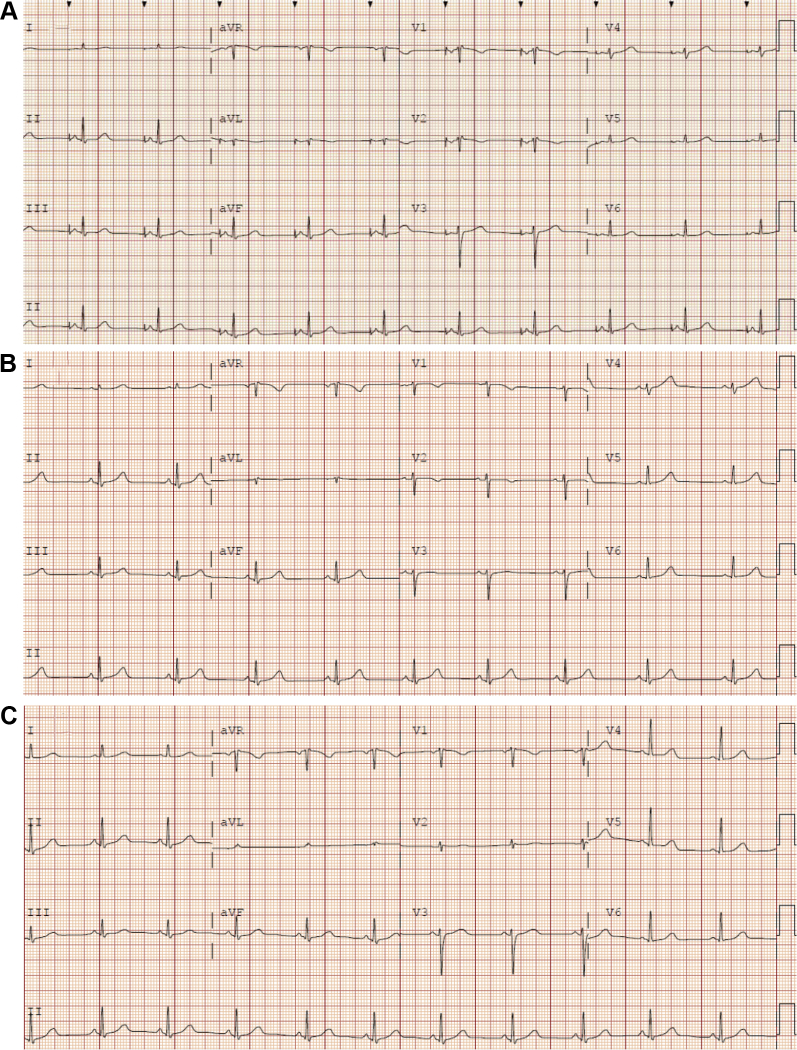
A 32-year-old woman with AN reported intermittent chest pain and was referred to an emergency department. While in the emergency department, she was noted to have sinus bradycardia with a heart rate of 25 bpm. She weighed 31.3 kg (67% IBW), with a BMI of 13.3 kg/m2. She was admitted to the intensive care unit with a primary diagnosis of “symptomatic bradycardia.” An echocardiogram revealed normal biventricular systolic function. She underwent exercise stress testing and her heart rate increased to 90 bpm. She subsequently underwent an invasive electrophysiology study. This demonstrated resting bradycardia with a sinus node cycle length of 1354 ms, with normal sinus node recovery time and normal corrected sinus node recovery time at various cycle lengths (≤1411 ms and ≤171 ms, respectively). Nonetheless, a single–atrial lead PPM system was implanted. She was discharged and referred to our center. During the flight, she developed progressive, pleuritic chest pain. On admission, a chest radiograph demonstrated a moderate-sized pneumothorax on the side of the pacemaker generator, which resolved after placement of a thoracostomy tube. While she was hospitalized, the lower rate limit of her PPM was adjusted from 60 bpm to 45 bpm. With refeeding and improvement in BMI (15 kg/m2), she no longer manifested atrial pacing on telemetry. Device interrogation noted pacing less than 1% of the time, which occurred exclusively while sleeping. After extensive discussion with the patient, we removed the pacemaker generator and extracted the atrial lead less than 1 month post implantation. She was discharged on hospital day 19 with a heart rate of 70 bpm, a weight of 36.2 kg (78% IBW), and a BMI of 15.3 kg/m2 (Figure 2).
Figure 2.
Patient 3 electrocardiograms. A: Presenting atrial-paced rhythm. B: Sinus bradycardia after reducing the device lower rate limit. C: Normal sinus rhythm after weight restoration and device extraction.
Case 4
A 63-year-old woman with AN presented for medical stabilization. She weighed 27 kg (52% IBW), representing a BMI of 10.46 kg/m2. She exhibited an atrial paced rhythm at 60 bpm with a blood pressure of 77/51 mm Hg. One year prior to presentation, she had complained to her primary care doctor of exertional fatigue and was ultimately referred to an electrophysiologist, who implanted a single-lead atrial PPM for “irreversible sinus bradycardia.” Prior to implantation she underwent ambulatory Holter monitoring, which demonstrated sinus rhythm with a minimum rate of 37 bpm, average rate of 51 bpm, and maximum rate of 93 bpm. The maximum R-R interval was 1.96 seconds. There were no reported symptoms. At that time, she weighed 29.9 kg (57% IBW, BMI of 11.4 kg/m2). Her postprocedure course was complicated by significant discomfort from pectoral muscle stimulation, which resolved with device reprogramming.
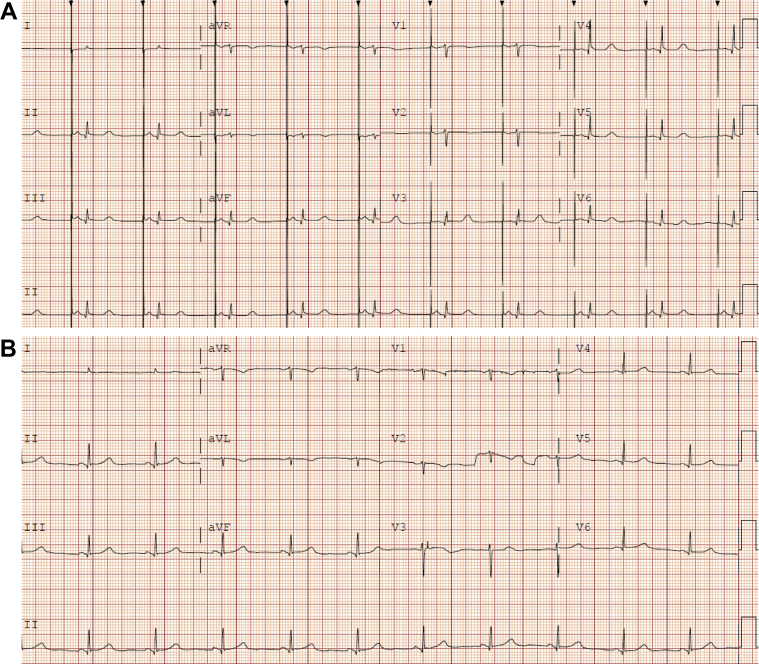
At our center, she weighed 30.6 kg (BMI of 12 kg/m2, 58% IBW) and was noted to intermittently be in normal sinus rhythm without atrial pacing. Her lower rate limit was reduced to 45 bpm and inpatient refeeding was continued. With improvement of her body mass to 33.8 kg (BMI 13.2 kg/m2, 64% IBW), she no longer manifested atrial pacing on telemetry. After discussion with the patient, we elected to remove the PPM lead and generator, in the operating room owing to the length of time the pacemaker lead had been in place (Figure 3).
Figure 3.
Patient 4 electrocardiograms. A: Presenting atrial-paced rhythm. B: Normal sinus rhythm after weight restoration and device extraction.
Discussion
AN has the highest mortality among all psychiatric illnesses.10 It is characterized by profound restriction of caloric intake relative to energy expenditures. Patients with AN have an intense fear of gaining weight with an attendant disturbance in body image perception, resulting in a total lack of recognition of the seriousness of their low body weight. Because AN is a relatively uncommon disorder, with a lifetime prevalence rate of 0.9% for female and 0.3% for male individuals,11 it is infrequently encountered by general cardiologists, electrophysiologists, and general practitioners. AN poses a substantial risk for premature death.12, 13 However, the etiology of sudden cardiac death in AN has not been definitively linked to severe bradycardia, given the absence of long-term arrhythmia monitoring. Profound bradycardia is a nearly universal finding in severe AN and the associated symptoms of AN overlap with “symptomatic bradycardia” seen in traditional sinus node dysfunction patients where a class I indication for PPM is noted in current guidelines.2
It is important to note that symptoms routinely attributable to bradycardia are common in patients with AN, including syncope, near-syncope, lightheadedness, fatigue, and exercise intolerance. These symptoms are often nonspecific and may result from severe malnutrition, dehydration, hypoglycemia, and cardiopulmonary deconditioning. As such, “symptomatic bradycardia” alone should not be considered a class I indication for PPM implantation among patients with severe AN. As noted in this case series, patients often demonstrate preserved chronotropic competence in formal exercise testing, which should be considered particularly reassuring among symptomatic patients with persistent junctional rhythm.6 Definitive data for best practices for monitoring and treatment of bradycardia in AN are lacking; however, consensus opinion points toward the safety of a restrained approach with medical management of the underlying severe malnutrition. In 30 years of treating patients with extreme AN on our medical stabilization unit, we have never seen the need for PPM, even among patients with BMI <10 kg/m2. This is not to say that PPM is never indicated. Shared decision making based on goals of care, preferences, and values remains essential. However, the best available evidence suggests that weight restoration remain the initial approach, with the recognition that symptoms of bradycardia and AN are inextricably overlapped. Thus, this decision-making dialogue should generally be pursued after restoration of IBW unless life-threatening hemodynamic consequences of bradycardia are present. We contend that although AN is not included as a reversible cause of bradycardia in current consensus society guidelines,2 future iterations might consider inclusion along with the current proviso for athletic training.
One additional consideration in this patient population is body self-image. Particularly among women with low BMI, PPM implantation may further distort body image, worsen self-esteem, and possibly exacerbate caloric restriction behaviors. Finally, with the markedly reduced BMI and thinning of the myocardium, patients with severe AN may be especially at risk for complications including pneumothorax (as observed in patient 3), perforation, erosion, or long-term vascular complications, since severe AN patients are often frail and much younger than the typical PPM population. Given this context, we recommend a high threshold for device implantation, restricted to patients with evidence of high-grade atrioventricular block (eg, Mobitz II or complete heart block) rather than sinus bradycardia alone. Although there is formal psychiatric training in eating disorders, there is no international training or curriculum for physicians addressing the medical complications of eating disorders. Cardiologists will continue to receive referrals for profound sinus bradycardia in patients with severe AN and should approach this as a potentially reversible condition.
Conclusion
With regard to the question to pace or not to pace in AN, we contend that permanent pacing is very rarely indicated, and temporary pacing should only be considered in the setting of life-threatening hemodynamic compromise. Although current guidelines mention a number of reversible causes of bradycardia, this series highlights the utility of being proscriptive regarding AN, given an overlapping clinical presentation that mimics classic “symptomatic bradycardia” yet is noncardiogenic in origin.
Key Teaching Points.
-
•
Profound bradycardia is a common, widely reported finding in anorexia nervosa.
-
•
It can be difficult to differentiate symptoms of bradycardia from those of anorexia nervosa itself: fatigue, exercise intolerance, lightheadedness, and presyncope.
-
•
Sinus bradycardia resolves with nutritional rehabilitation and improved body weight by treating the underlying anorexia nervosa. Pacemaker implantation is rarely indicated.
-
•
Pacemaker implantation may further distort body image in anorexia nervosa patients and may be associated with an increased risk of complications.
-
•
Future guidelines should consider anorexia nervosa as a potentially reversible cause of sinus node dysfunction.
Footnotes
Dr Mehler receives funding support from the Denver Health Foundation.
References
- 1.Mont L., Castro J., Herreros B. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry. 2003;42:808–813. doi: 10.1097/01.CHI.0000046867.56865.EB. [DOI] [PubMed] [Google Scholar]
- 2.Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:e51–e156. doi: 10.1016/j.jacc.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Sachs K.V., Harnke B., Mehler P.S., Krantz M.J. Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord. 2016;49:238–248. doi: 10.1002/eat.22481. [DOI] [PubMed] [Google Scholar]
- 4.Yahalom M., Spitz M., Sandler L., Heno N., Roguin N., Turgeman Y. The significance of bradycardia in anorexia nervosa. Int J Angiol. 2013;22:83–94. doi: 10.1055/s-0033-1334138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanbur N.O., Goldberg E., Pinhas L., Hamilton R.M., Clegg R., Katzman D.K. Second-degree atrioventricular block (Mobitz Type I) in an adolescent with anorexia nervosa: intrinsic or acquired conduction abnormality. Int J Eat Disord. 2009;42:575–578. doi: 10.1002/eat.20647. [DOI] [PubMed] [Google Scholar]
- 6.Krantz M.J., Gaudiani J.L., Johnson V.W., Mehler P.S. Exercise electrocardiography extinguishes persistent junctional rhythm in a patient with severe anorexia nervosa. Cardiology. 2011;120:217–220. doi: 10.1159/000335481. [DOI] [PubMed] [Google Scholar]
- 7.Romano C., Chinali M., Pasanisi F. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr. 2003;77:308–312. doi: 10.1093/ajcn/77.2.308. [DOI] [PubMed] [Google Scholar]
- 8.Gottdiener J.S., Gross H.A., Henry W.L., Borer J.S., Ebert M.H. Effects of self-induced starvation on cardiac size and function in anorexia nervosa. Circulation. 1978;58:425–433. doi: 10.1161/01.cir.58.3.425. [DOI] [PubMed] [Google Scholar]
- 9.Oflaz S., Yucel B., Oz F. Assessment of myocardial damage by cardiac MRI in patients with anorexia nervosa. Int J Eat Disord. 2013;46:862–866. doi: 10.1002/eat.22170. [DOI] [PubMed] [Google Scholar]
- 10.Shamim T., Golden N.H., Arden M., Filiberto L., Shenker I.R. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health. 2003;32:73–77. doi: 10.1016/s1054-139x(02)00533-5. [DOI] [PubMed] [Google Scholar]
- 11.Hudson J.I., Hiripi E., Pope H.G.J., Kessler R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcelus J., Mitchell A.J., Wales J., Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 13.Westmoreland P., Krantz M.J., Mehler P.S. Medical complications of anorexia nervosa and bulimia. Am J Med. 2016;129:30–37. doi: 10.1016/j.amjmed.2015.06.031. [DOI] [PubMed] [Google Scholar]