Abstract
It is common for multiple autoimmune diseases to occur in the same patient. However, autoimmune blistering diseases (AIBD) do not commonly associate with dermatomyositis (DM). We performed a literature review and found 12 previous reports that may be attributed to misdiagnosis, underreporting, or true rarity of association. Herein, we present a case of pemphigus vulgaris and a case of mucous membrane pemphigoid associated with DM and review the related literature. AIBD-associated interstitial lung disease, genetic predisposition, potential environmental triggers of both AIBD and DM, drug-related triggers, and paraneoplastic processes are discussed. Dermatologists must be vigilant for a second autoimmune disease in patients with AIBD that may have therapeutic implications.
Keywords: Dermatomyositis, interstitial lung disease, pemphigus, pemphigoid, autoimmune, blistering
Introduction
Autoimmune bullous diseases (AIBD) are organ-specific autoimmune disorders of the skin and mucous membranes. Target antigens in AIBD are located in the adhesion proteins between epidermal keratinocytes (pemphigus group of diseases) or at the dermoepidermal junction (pemphigoid group of diseases; Schmidt and Zillikens, 2013, Di Zenzo et al., 2016). Clinical entities in both disease groups have been described on the basis of identification of target autoantigens.
In contrast, dermatomyositis (DM) is a heterogeneous autoimmune disease that affects primarily the skin and muscles, with potential lung, joint, or cardiac involvement. DM can be associated with systemic lupus erythematosus, rheumatoid arthritis, secondary Sjogren’s syndrome, and solid organ tumors, including the lung, breast, ovarian, and gastric neoplasms (Thompson et al., 2017).
It is common for multiple autoimmune diseases to occur in the same patient; however, AIBDs do not commonly associate with DM (Table 1; Kridin, 2018a, Kridin, 2018b, Schmidt and Zillikens, 2013). There are limited reports on this association, which may be attributed to misdiagnosis, underreporting, or true rarity of association. Herein, we report on two additional cases of AIBD associated with DM—one patient with pemphigus vulgaris (PV) and another with mucous membrane pemphigoid (MMP)—along with a review of the related literature.
Table 1.
Diseases associated with autoimmune bullous diseases and dermatomyositis
| Disease | Associated diseases |
|---|---|
| Pemphigus vulgaris/foliaceus (Bai et al., 2016, Black and Marshman, 2011, Fujimoto et al., 2014, Kartan et al., 2017, Narbutt, 2003, Thongprasom et al., 2013) | Autoimmune thyroid disease, rheumatoid arthritis, type 1 diabetes, psoriasis, Parkinson’s disease, hematologic malignancies |
| Bullous pemphigoid (Kartan et al., 2017, Yanagi et al., 2007) | Psoriasis, neurologic disorders, psychiatric conditions, solid or hematological malignancies |
| Mucous membrane pemphigoid (Kartan et al., 2017) | Solid malignancies (anti–laminin-332 MMP), rheumatoid arthritis, systemic lupus erythematosus |
| Anti-p200 pemphigoid (Kridin, 2018b) | Psoriasis |
| Epidermolysis bullosa acquisita (Kridin, 2018b) | Inflammatory bowel disease, hematological malignancies, systemic lupus erythematosus |
| Linear immunoglobulin A dermatosis (Kridin, 2018b) | Lymphoproliferative diseases, ulcerative colitis |
| Dermatitis herpetiformis (Kalovidouris et al., 1989, Kridin, 2018b) | Autoimmune thyroiditis, type 1 diabetes, lupus erythematosus, Sjögren syndrome, vitiligo, primary biliary cirrhosis, pernicious anemia, alopecia areata, neuropathies |
| Dermatomyositis (Yang et al., 2015) | Systemic lupus erythematosus, rheumatoid arthritis, Sjogren syndrome, solid organ tumors (lung, breast, ovarian, and gastric neoplasms) |
Case 1
A 51-year-old woman presented with a history of PV, which was first diagnosed 8 months earlier. She initially had erosions on the tongue, buccal mucosa, and pharynx, as well as blisters on her trunk. Her medical history was relevant for Hashimoto’s thyroiditis, idiopathic trace proteinuria, facial erythema, diffuse nonscarring alopecia, and multifocal arthralgias. Notable laboratory test results included antinuclear antibody 1:160, + Sjögren's syndrome antibodies, and + anti-thyroperoxidase and + anti-thyroglobulin antibodies. Testing on skin biopsies from the breast and back showed typical suprabasal acantholysis with tombstone appearance of basal cells.
The patient was treated with low-dose prednisone, but control of the disease was not achieved, and the patient was referred with recalcitrant oral and pharynx erosions. At that time, the patient reported dysphagia, proximal muscle weakness, myalgias, and arthralgias. Upon examination, subtle clinical findings were suggestive of DM, including dilated capillary loops and hemorrhage on the proximal nail folds (Fig. 1), poikilodermatous changes on the neck, and faint erythema and hyperpigmentation over the extensor elbows and dorsal knuckles (Gottron’s sign). Additional workup showed normal creatinine kinase and aldolase levels. A myositis panel demonstrated + Sjögren's syndrome antibodies-60.
Fig. 1.
Dilated capillary loops and hemorrhage on the proximal nail folds (Case 1).
Case 2
An 82-year-old man presented with MMP limited to the oral mucosa, which was first diagnosed 14 years earlier (in 2004) through gingival biopsy testing. He was initially treated with dapsone (50-100 mg/day), prednisone (10-30 mg/day), and topical clobetasol. Mycophenolate mofetil was added in 2006, allowing taper of systemic corticosteroids. Five years ago, due to disease quiescence, mycophenolate mofetil was gradually tapered and discontinued in November 2013. The patient was maintained on a combination of dapsone and topical clobetasol. In August 2014, he was noted to have crackling by chest auscultation and low oxygen saturation. He was referred to pulmonology, diagnosed with chronic obstructive pulmonary disease, and initiated on overnight oxygen supplementation.
Over the next 2 years, the patient had progressive shortness of breath, nonproductive cough, exertional muscle fatigue, and increased oxygen requirements. Pulmonary function testing was notable for forced expiratory volume in 1 second/forced vital capacity of 71% predicted and a diffusion capacity of carbon monoxide of 42% predicted. A chest computed tomography scan demonstrated bibasilar reticulation, septal thickening, and centrilobular emphysema, consistent with mixed pulmonary fibrosis and emphysema. In October 2017, due to worsening pulmonary symptoms, the patient was initiated on prednisone 30 mg.
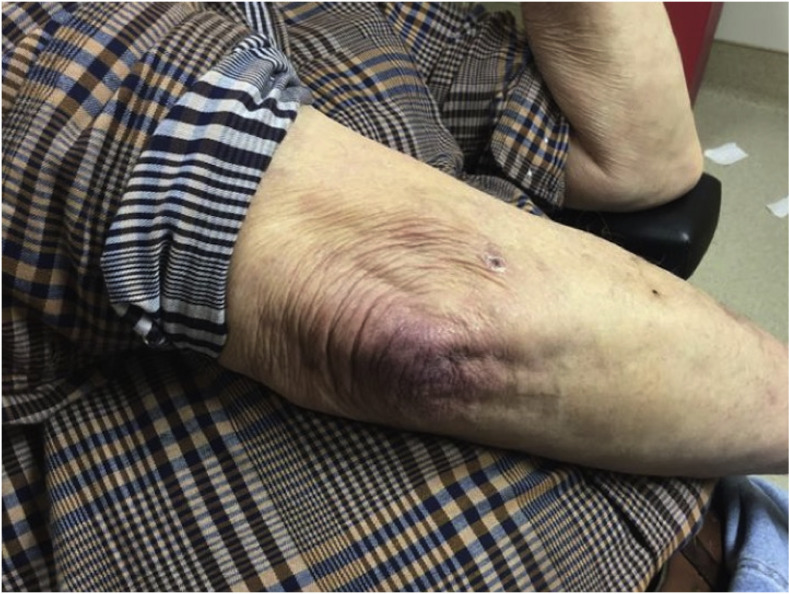
Upon examination, dilated capillaries with dropout in the proximal nail fold and prominent erythema on the extensor elbows and overlying the knuckles (Gottron’s sign) were noted (Fig. 2). A skin biopsy of the extensor elbow demonstrated vascular ectasia with mild perivascular lymphocytic infiltrate and scattered dyskeratotic cells at the dermo-epidermal junction.
Fig. 2.
Prominent erythema on the extensor elbows and overlying the knuckles (Gottron’s sign) (Case 2).
Laboratory test results of both cases are presented in Table 2.
Table 2.
Diagnostic examination results of present cases
| Histopath of bullous lesion | DIF | ELISA | Histopath of inflammatory skin lesion that could be DM | PFTs | HLA | |
|---|---|---|---|---|---|---|
|
Case 1 (PV and classic DM) |
Acantholytic dermatosis (Right breast, 4/26/2018) | IgG and C3 deposition intercellularly (left upper back, 4/26/2018) |
Dsg 1: 126.4075 (> 36) Dsg 3: 59.28889 (> 37) (10/05/2018) |
N/A | Order active | N/A |
|
Case 2 (MMP and DM) |
Consistent with pemphigoid (right buccal and gingival mucosa -2004) |
N/A | Vascular ectasia and perivascular inflammation, with rare dyskeratotic cells at the dermoepidermal junction, compatible with connective tissue disorder (Right elbow, 6/12/2018) |
Mild combined restriction and obstruction, with severely reduced diffusing capacity of the lungs for carbon monoxide 8/24/2018 Pulm: combined pulmonary fibrosis and emphysems (has smoking history also). Only fulfilled probable UIP (ILD). Lung biopsy is definitive. |
N/A | |
DIF, direct immunofluorescence; DM, dermatomyositis; Dsg, desmoglein; ELISA, enzyme-linked immunosorbent assay; DIF, HLA, human leukocyte antigen; Ig, immunoglobulin; ILD, interstitial lung disease, MMP, mucous membrane pemphigoid; N/A, not available; PFT, pulmonary function testing; PV, pemphigus vulgaris; UIP, usual interstitial pneumonia
Discussion
The first case report of any association of AIBD with DM was a case of dermatitis herpetiformis (DH) in a 67-year-old woman in whom DM preceded the manifestations of DH by 4 years (White, 1982). Eleven subsequent cases of AIBD associated with DM have since been reported. In total, four cases of bullous pemphigoid (BP), three cases of DH, two cases of PV, two cases of pemphigus foliaceus, and one case of linear immunoglobulin (Ig) A dermatosis have been reported (Table 3; Barrows-Wade and Jordon, 1992, Black and Marshman, 2011, Fujimoto et al., 2014, Garcia et al., 2017, Glover and Leigh, 1992, Kalovidouris et al., 1989, Narbutt, 2003, Thongprasom et al., 2013, Tsukada, 2003, White, 1982, Yanagi et al., 2007).
Table 3.
Dermatomyositis in association with autoimmune blistering diseases in the present literature
| Case | Citation | AIBD | Age, years | Sex | Timing of DM diagnosis | DM type | Treatment | Associated malignancy |
|---|---|---|---|---|---|---|---|---|
| 1 | Narbutt, 2003 | PF | 11 | Male | 2 years earlier | Juvenile | Prednisolone; hydroxychloroquine; cyclosporine; methotrexate; cyclophosphamide | None |
| 2 | Black and Marshman, 2011 | PV | 76 | Female | 40 months earlier | Classic | Prednisolone; methotrexate | None |
| 3 | Thongprasom et al., 2013 | PV | 36 | Female | 4 years later | Amyopathic | Prednisolone; dapsone; chloroquine; methotrexate; colchicine; celecoxib | None |
| 4 | Fujimoto et al., 2014 | PF | 39 | Female | 29 months earlier | Amyopathic | Topical steroidal agents | None |
| 5 | Present case | PV | 51 | Female | 8 months later | Classic | Prednisone | None |
| 6 | Glover and Leigh, 1992 | BP | 65 | M | Simultaneously | Classic | Prednisolone; azathioprine | |
| 7 | Tsukada, 2003 | BP | 70 | Male | 8 months later | Classic | Prednisolone; methotrexate; minocycline | None |
| 8 | Yanagi et al., 2007 | BP | 81 | Female | Simultaneously | Classic | Topical steroidal agents | Colon carcinoma |
| 9 | Garcia et al., 2017 | BP | 69 | Female | Simultaneously | Amyopathic + ILD | Prednisolone; rituximab | Gastric MALT lymphoma |
| 10 | Present case | MMP | 82 | Male | 5 years later | Amyopathic + ILD | Prednisone; dapsone; mycophenolate mofetil | None |
| 11 | Barrows-Wade and Jordon, 1992 | Linear IgA dermatosis | 56 | Female | Simultaneously | Classic | Dapsone; prednisolone | None |
| 12 | White, 1982 | DH | 67 | Female | 3 years earlier | Classic | None | |
| 13 | Kalovidouris et al., 1989 | DH | 34 | Male | 7 years later | Classic | Dapsone; prednisolone | None |
| 14 | Kalovidouris et al., 1989 | DH | 33 | Male | 4 years later | Classic | None |
AIBD, autoimmune bullous disease; BP, bullous pemphigoid; DH, dermatitis herpetiformis; DM, dermatomyositis; Ig, immunoglobulin; ILD, interstitial lung disease; MALT, mucosa-associated lymphoid tissue; MMP, mucous membrane pemphigoid; PF, pemphigus foliaceus; PV, pemphigus vulgaris
Among all 14 cases, there was no sex predominance (six men, eight women). DM was diagnosed before (four of 14 patients), simultaneous with (four of 14 patients), or after (six of 14 patients) the diagnosis of AIBD. Most cases had features of classic DM (nine of 14 patients). No muscle involvement was reported in four of 14 cases (amyopathic DM). Associated malignancy was reported only in two cases of BP (colon carcinoma and gastric mucosa-associated lymphoid tissue lymphoma; Garcia et al., 2017, Yanagi et al., 2007).
In the present literature review, none of the 14 patients with AIBD and DM had mucosal lesions attributed to DM. In DM, mucosal involvement, clinically represented as dysphagia, is reported in cohorts of patients with anti-nuclear matrix protein 2 DM and anti-small ubiquitin-like modifier-activating enzyme 1/2 autoantibody-positive DM (Wolstencroft and Fiorentino, 2018).
AIBD-associated interstitial lung disease (ILD) is rare, and linked with relapse and treatment-resistant AIBD (Usuba et al., 1989). In these cases, ILD has an improved response to systemic corticosteroids and a relatively better prognosis when compared with RA- or DM-associated ILD (Bai et al., 2016, Yoshioka et al., 2012).
In these patients, the clinical differential diagnosis should always include the pulmonary disease that accompanies paraneoplastic pemphigus/paraneoplastic multiorgan syndrome and represents a serious cause of morbidity/mortality. The mechanism of AIBD-associated ILD is unknown. There is speculation that cell adhesion proteins in the respiratory epithelial cells are similar to cutaneous desmosomes, or hemidesmosomes may cross-react with skin-directed autoantibodies. Yoshioka et al. (2012) described a case of BP-associated interstitial pneumonia in which linear deposition of IgG and C3 along the basement membrane of the alveoli was shown by direct immunofluorescence. Whether ILD is a pulmonary manifestation of BP requires further investigation.
Both AIBD and DM may affect genetically predisposed individuals. The most common human leukocyte antigen haplotypes associated with AIBD are summarized in Table 4 (Kárpáti, 2012, Kridin, 2018a, Kridin, 2018b, Schmidt and Zillikens, 2013, Thompson et al., 2017). Similar human leukocyte antigen associations have been observed in DM and BP, MMP, and DH.
Table 4.
Human leukocyte antigen associations in autoimmune bullous diseases and dermatomyositis
| Disease | Human leukocyte antigen association |
|---|---|
| Pemphigus vulgaris/foliaceus | DRB1*0402, DRB1*1401, DQB1*0302 (Caucasians) DRB1*14, DQB1*0503 (Japanese) A10, B38 |
| Bullous pemphigoid | DQB1*0301, DRB1*04, DRB1*1101 DQB1*0302 (Japanese) |
| Mucous membrane pemphigoid | DQB1*0301, DRB1*04, DRB1*11, DRB1*1503 |
| Epidermolysis bullosa acquisita | DR2 (African Americans) DRB1*1503, DRB1*13 (Koreans) |
| Linear immunoglobulin A dermatosis | B8, Cw7, DR3.3 |
| Dermatitis herpetiformis | DQ2 with DQA1* 0501/DQB1*0201, DQA1*0501, DQB1*0202, DRB1*03, DRB1*05/07 DQ8 with DQA1*0301/DQB1*0302, DRB1*4 |
| Dermatomyositis | DRB1*0301 or DQA1*0501 (Caucasians) B7, DRB1*0301 (Asians) DQA1*0501 (anti-Jo-1) DRB1*07 or DQA*0201 (anti-Mi-2) |
Potential environmental triggers of both AIBD and DM have not been conclusively identified (Black and Marshman, 2011). Some authors hypothesize that continuous epidermal damage from DM or sun-induced photodamage might unmask previously immunoprivileged antigens (i.e., epitope spreading phenomenon), leading to further autoimmune responses (Fujimoto et al., 2014, Glover and Leigh, 1992, Tsukada, 2003). However, the temporal relationship is not always consistent with this mechanism, particularly when blisters are present at the onset of DM.
Drug-related triggers may contribute in some cases because both DM and AIBD have been associated with medication exposure (Table 5; Delbaldo et al., 2002, Gaffney et al., 2018, Golberg and Harman, 2015, Seidler and Gottlieb, 2008, Stavropoulos et al., 2014, Vassileva, 1998). Medications may act as haptens, modify the antigenicity of structural proteins, or affect the immune tolerance against components of the adhesion structures among keratinocytes or at the dermoepidermal junction (Delbaldo et al., 2002). None of the presented cases had a history of environmental and/or drug triggers.
Table 5.
Drugs associated with autoimmune bullous diseases and dermatomyositis
| Disease | Associated medications |
|---|---|
| Pemphigus vulgaris/foliaceus | Captopril, penicillamine, gold, aspirin, rifampin, levodopa, calcium channel blockers, sulfasalazine |
| Bullous pemphigoid | Thiol-containing anti-hypertensive drugs, diuretics (especially furosemide), gliptins, penicillamine, sulfasalazine, phenacetin, nonsteroidal antiinflammatory drugs, salicylates, anti-tumor necrosis factor agents, vaccines |
| Mucous membrane pemphigoid | Gliptins, diphtheria-tetanus vaccination, atenolol, nonsteroidal antiinflammatory drugs |
| Linear immunoglobulin A dermatosis | Vancomycin, captopril, amiodarone, penicillins, cephalosporins, diclofenac, captopril, glibenclamide |
| Epidermolysis bullosa acquisita | Vancomycin |
| Dermatitis herpetiformis | Anti-tumor necrosis factor agents, gonadotropin-releasing hormone analog, leuprolide |
| Dermatomyositis | Hydroxyurea, penicillamine, statins, cyclophosphamide, Bacillus Calmette–Guérin vaccine, ipilimumab, proton pump inhibitors, lacosamide, capecitabine, anti-tumor necrosis factor agents, interferon-alfpha, nonsteroidal antiinflammatory drugs, zoledronic acid |
Paraneoplastic processes may be linked to autoimmunity in both AIBD and DM (Atzmony et al., 2017, Kartan et al., 2017). In BP, MMP, and linear IgA dermatosis, autoantibodies directed against epidermal keratinocyte adhesion proteins may cross-react with antibodies against tumor antigens. Another hypothesis is that the tumor may produce antigens that directly damage the dermoepidermal junction, leading to the formation of anti–basement membrane zone autoantibody production (Atzmony et al., 2017, Kartan et al., 2017). In DM, there may be expression of common autoantigens between tumor and muscle tissue, with immune response directed against tumor cells that also targets similar autoantigens in muscle tissue (i.e., molecular mimicry), resulting in muscle damage (Qiang et al., 2017, Yang et al., 2015). In the reported cases of AIBD associated with DM, a paraneoplastic process was observed in just two of 14 patients.
Conclusion
Dermatologists must be vigilant for a second autoimmune disease in patients with AIBD that may have therapeutic implications. These presentations may be subtle and insidious at the time of onset.
Footnotes
Sources of support: This work was supported by the U.S. Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development).
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- Atzmony L., Mimouni I., Reiter O., Leshem Y.A., Taha O., Gdalevich M. Association of bullous pemphigoid with malignancy: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(4):691–699. doi: 10.1016/j.jaad.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Bai Y.X., Chu J.G., Xiao T., Chen H.D. Pemphigus vulgaris-associated interstitial lung disease. Dermatol Ther. 2016;29(4):228–232. doi: 10.1111/dth.12342. [DOI] [PubMed] [Google Scholar]
- Barrows-Wade L., Jordon R.E. Linear IgA bullous dermatosis associated with dermatomyositis. Arch Dermatol. 1992;128:413–414. [PubMed] [Google Scholar]
- Black M., Marshman G. Dermatomyositis and pemphigus vulgaris: Association or coincidence? Australas J Dermatol. 2011;52(2):11–14. doi: 10.1111/j.1440-0960.2010.00646.x. [DOI] [PubMed] [Google Scholar]
- Delbaldo C., Chen M., Friedli A., Prins C., Desmeules J., Saurat J.H. Drug-induced epidermolysis bullosa acquisita with antibodies to type VII collagen. J Am Acad Dermatol. 2002;46(5 Suppl):161–164. doi: 10.1067/mjd.2002.107774. [DOI] [PubMed] [Google Scholar]
- Di Zenzo G., Amber K.T., Sayar B.S., Müller E.J., Borradori L. Immune response in pemphigus and beyond: Progresses and emerging concepts. Semin Immunopathol. 2016:57–74. doi: 10.1007/s00281-015-0541-1. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Takayama S., Hamaguchi Y., Fujimoto M., Tanaka T. Pemphigus foliaceus associated with anti-NXP2 autoantibody-positive dermatomyositis. Acta Derm Venereol. 2014;94(4):478–479. doi: 10.2340/00015555-1756. [DOI] [PubMed] [Google Scholar]
- Gaffney R.G., Tarazi M., Werth V.P. Drug-induced dermatomyositis after lacosamide: A case report. JAAD Case Rep. 2018;4(6):584–585. doi: 10.1016/j.jdcr.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia B., Dabouz F., Pascal L., Gillard M., Modiano P. Dermatomyosite amyopathique avec anticorps anti-MDA-5, associée à une pemphigoïde bulleuse, un syndrome de Sjögren et un lymphome de type MALT. Ann Dermatol Venereol. 2017;144(10):629–633. doi: 10.1016/j.annder.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Glover M., Leigh I. Dermatomyositis pemphigoides: A case with coexistent dermatomyositis and bullous pemphigoid. J Am Acad Dermatol. 1992;27(5):849–852. doi: 10.1016/0190-9622(92)70264-g. [DOI] [PubMed] [Google Scholar]
- Golberg O., Harman K.E. Drug-induced pemphigus. Eur Handb Dermatological Treat Third Ed. 2015;29(4):725–730. [Google Scholar]
- Kalovidouris A.E., Miller F.W., Lawley T.J. Polymyositis/dermatomyositis associated with dermatitis herpetiformis. Arthritis Rheum. 1989;32(9):1179–1181. doi: 10.1002/anr.1780320920. [DOI] [PubMed] [Google Scholar]
- Kárpáti S. Dermatitis herpetiformis. Clin Dermatol. 2012;30(1):56–59. doi: 10.1016/j.clindermatol.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Kartan S., Shi V.Y., Clark A.K., Chan L.S. Paraneoplastic pemphigus and autoimmune blistering diseases associated with neoplasm: Characteristics, diagnosis, associated neoplasms, proposed pathogenesis, treatment. Am J Clin Dermatol. 2017;18(1):105–126. doi: 10.1007/s40257-016-0235-z. [DOI] [PubMed] [Google Scholar]
- Kridin K. Pemphigus group: Overview, epidemiology, mortality, and comorbidities. Immunol Res. 2018;66(2):255–270. doi: 10.1007/s12026-018-8986-7. [DOI] [PubMed] [Google Scholar]
- Kridin K. Subepidermal autoimmune bullous diseases: Overview, epidemiology, and associations. Immunol Res. 2018;66(1):6–17. doi: 10.1007/s12026-017-8975-2. [DOI] [PubMed] [Google Scholar]
- Narbutt J. Pemphigus foliaceus in an 11-year-old boy with dermatomyositis: Simple coincidence or familial immunological background? Br J Dermatol. 2003:838–839. doi: 10.1046/j.1365-2133.2003.05256.x. [DOI] [PubMed] [Google Scholar]
- Qiang J.K., Kim W.B., Baibergenova A., Alhusayen R. Risk of malignancy in dermatomyositis and polymyositis: A systematic review and meta-analysis. J Cutan Med Surg. 2017;21(2):131–136. doi: 10.1177/1203475416665601. [DOI] [PubMed] [Google Scholar]
- Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- Seidler A.M., Gottlieb A.B. Dermatomyositis induced by drug therapy: A review of case reports. J Am Acad Dermatol. 2008;59(5):872–880. doi: 10.1016/j.jaad.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Stavropoulos P.G., Soura E., Antoniou C. Drug-induced pemphigoid: A review of the literature. J Eur Acad Dermatol Venereol. 2014;28(9):1133–1140. doi: 10.1111/jdv.12366. [DOI] [PubMed] [Google Scholar]
- Thompson C., Piguet V., Choy E. The pathogenesis of dermatomyositis. Br J Dermatol. 2017:1–7. doi: 10.1111/bjd.15607. [DOI] [PubMed] [Google Scholar]
- Thongprasom K., Prasongtanskul S., Fongkhum A., Iamaroon A. Pemphigus, discoid lupus erythematosus, and dermatomyositis during an 8-year follow-up period: A case report. J Oral Sci. 2013;55(3):255–258. doi: 10.2334/josnusd.55.255. [DOI] [PubMed] [Google Scholar]
- Tsukada Y. Bullous pemphigoid associated with dermatomyositis successfully controlled with minocycline. Clin Exp Dermatol. 2003;28:554–564. doi: 10.1046/j.1365-2230.2003.01355.x. [DOI] [PubMed] [Google Scholar]
- Usuba Y., Aiba S., Hashimoto K., Tanita Y., SK A fatal case of pemphigus vulgaris with interstitial pneumonia occurring during gold therapy. Nihon Hifuka Gakkai Zasshi. 1989:725–730. [in Japanese] [PubMed] [Google Scholar]
- Vassileva S. Drug-induced pemphigoid: Bullous and cicatricial. Clin Dermatol. 1998;16:379–387. doi: 10.1016/s0738-081x(98)00008-x. [DOI] [PubMed] [Google Scholar]
- White S.W. Dermatomyositis and dermatitis herpetiformis. Arch Dermatol. 1982;118:599–601. [PubMed] [Google Scholar]
- Wolstencroft P.W., Fiorentino D.F. Dermatomyositis clinical and pathological phenotypes associated with myositis-specific autoantibodies. Curr Rheumatol Rep. 2018;20:28. doi: 10.1007/s11926-018-0733-5. [DOI] [PubMed] [Google Scholar]
- Yanagi T., Kato N., Yamane N., Osawa R. Bullous pemphigoid associated with dermatomyositis and colon carcinoma. Clin Exp Dermatol. 2007;32(3):291–294. doi: 10.1111/j.1365-2230.2007.02368.x. [DOI] [PubMed] [Google Scholar]
- Yang Z., Lin F., Qin B., Yan L., Renqian Z. Polymyositis/dermatomyositis and malignancy risk: A metaanalysis study. J Rheumatol. 2015;42(2):282–291. doi: 10.3899/jrheum.140566. [DOI] [PubMed] [Google Scholar]
- Yoshioka D., Ishii H., Uchida T., Fujiwara S., Umeki K., Sakamoto N. Interstitial pneumonia associated with bullous pemphigoid. Chest. 2012;141(3):795–797. doi: 10.1378/chest.11-1241. [DOI] [PubMed] [Google Scholar]