Summary
Although in recent years there has been an increased awareness of the widespread nature of biofluorescence in the marine environment, the diversity of the molecules responsible for this luminescent phenotype has been mostly limited to green fluorescent proteins (GFPs), GFP-like proteins, and fluorescent fatty acid-binding proteins (FABPs). In the present study, we describe a previously undescribed group of brominated tryptophan-kynurenine small molecule metabolites responsible for the green biofluorescence in two species of sharks and provide their structural, antimicrobial, and spectral characterization. Multi-scale fluorescence microscopy studies guided the discovery of metabolites that were differentially produced in fluorescent versus non-fluorescent skin, as well as the species-specific structural details of their unusual light-guiding denticles. Overall, this study provides the detailed description of a family of small molecules responsible for marine biofluorescence and opens new questions related to their roles in central nervous system signaling, resilience to microbial infections, and photoprotection.
Subject Areas: Chemistry, Natural Product Chemistry, Natural Product Discovery
Graphical Abstract
Highlights
-
•
We describe a new form of biofluorescence from the skin of catsharks
-
•
Bromo-tryptophan-kynurenines are biofluorescent and show antimicrobial activities
-
•
Specific dermal denticles in the chain catshark act as optical light-guides
-
•
This study opens questions related to biological function of shark fluorescence
Chemistry; Natural Product Chemistry; Natural Product Discovery
Introduction
Biofluorescence is a widespread phenomenon in the marine environment, which results from the absorbance of the dominant ambient blue ocean light and its re-emittance at longer, lower-energy wavelengths, visually resulting in green, orange, and red fluorescence. Following the seminal 1962 discovery of green fluorescent protein (GFP) from Aequorea victoria, a hydrozoan medusa (Shimomura et al., 1962), fluorescent proteins (FPs) have since been described from cnidarians (Gruber et al., 2008, Matz et al., 1999), crustaceans (Meyers et al., 2004), and cephalochordates (Deheyn et al., 2007). The discovery of GFP has led to numerous breakthroughs in biomedical science (Gruber and Pieribone, 2006) following the exhibition of its heterologous expression (Chalfie et al., 1994) and mutations that led to brighter and spectrally modulatable variants (Tsien, 1998). GFP-like fluorescent proteins have since been identified in a wide range of other cnidarians, including corals, anemones, hydroids, pennatulids, and corallimorpharians and are now recognized as a ubiquitous metazoan protein superfamily (Shagin et al., 2004).
Within fishes, biofluorescence was only recently reported to be a phylogenetically widespread and phenotypically variable phenomenon that encompasses at least 16 orders, 50 families, 105 genera, and over 180 species (Sparks et al., 2014). Yet among this multitude of biofluorescent fish species, the chemistry had only been elucidated in the eels, Anguilla japonica (Hayashi and Toda, 2009, Kumagai et al., 2013), Kaupichthys hyoproroides, and Kaupichthys n. sp (Gruber et al., 2015), all of which are bilirubin-inducible fluorescent proteins. Here, we focus on the chemical mechanism of biofluorescence in two species of elasmobranchs, both in the family Scyliorhinidae, with Cephaloscyllium ventriosum (the swell shark) being endemic to the eastern Pacific (Compagno, 1984) and Scyliorhinus retifer (the chain catshark) from the western Atlantic (Castro, 2011).
Regional biofluorescence of the skin of these sharks has previously been shown to exhibit a higher intensity of the green-dominated fluorescence in the lighter, beige-colored areas, compared with the darker reticulated lines for the chain catshark or the dark spots for the swell shark (Figure 1) (Gruber et al., 2016). Through comparative metabolic profiling studies between these two distinctly pigmented tissue types, we report the discovery of a suite of small molecule metabolites stimulated in the light skin tissue, relative to the dark skin tissue from these two shark species. Specifically, high-resolution mass spectrometry (MS), UV-visible spectroscopy, multidimensional nuclear magnetic resonance (NMR), and fluorescence spectroscopy were used to unambiguously identify and characterize a family of brominated tryptophan-kynurenine metabolic products that are responsible for the biofluorescence phenotype in these two species of shark. Although GFP, GFP-like proteins, and bilirubin-binding fluorescent proteins have been reported from a variety of invertebrate (Gruber et al., 2008, Matz et al., 1999) and vertebrate (Gruber et al., 2015, Hayashi and Toda, 2009, Kumagai et al., 2013) taxa, these brominated tryptophan-kynurenine metabolites from elasmobranchs represent a previously undescribed family of biofluorescent small molecule metabolites.
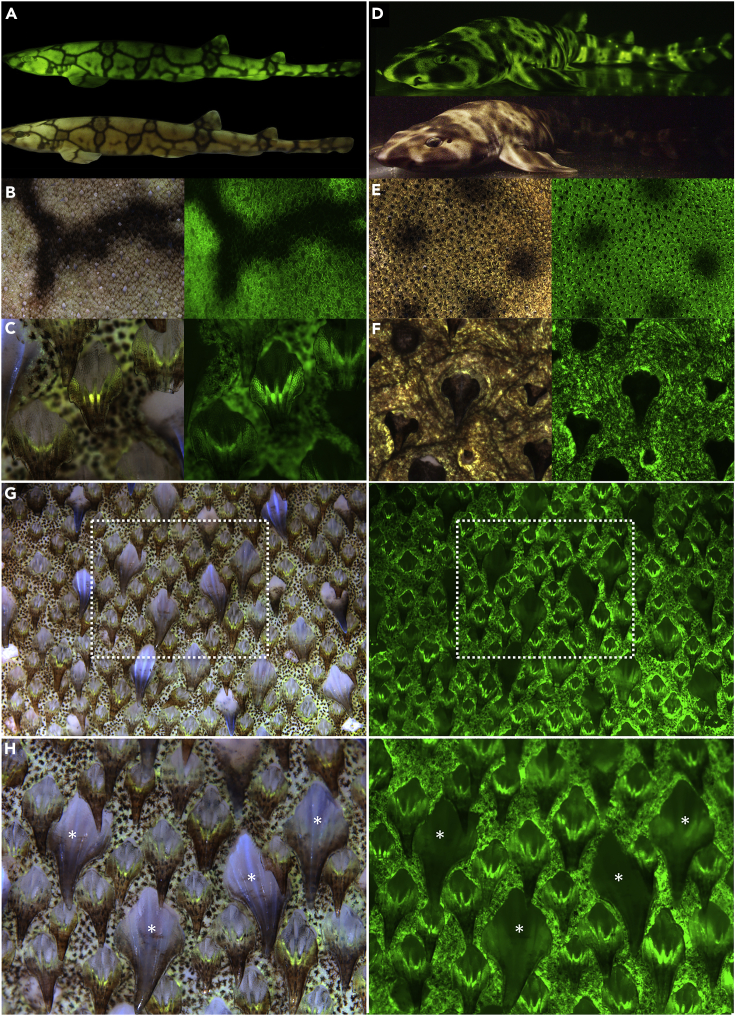
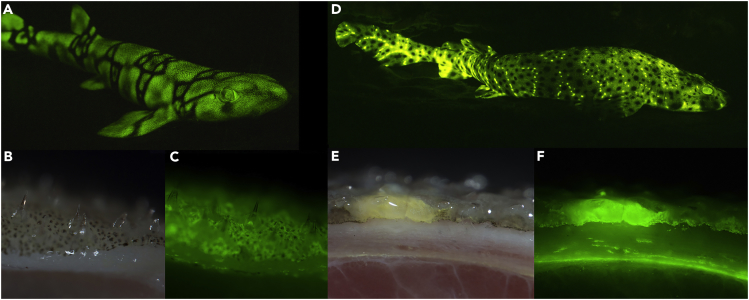
Figure 1.
Fluorescent Shark Imaging
(A–H) Bright-field and fluorescence imaging of Scyliorhinus retifer (A–C) and Cephaloscyllium ventriosum (D–F). In contrast to the dark denticles from C. ventriosum, those from S. retifer act as efficient optical waveguides, effectively channeling the fluorescence signal along their length. S. retifer has a second type of larger, non-light-guiding denticles (G and H), and bright-field (left) and fluorescence (right) imaging reveals that the larger non-light-guiding denticles can easily be distinguished through bright-field imaging (due to their ability to weakly scatter blue light from their longitudinal surface ridges). A patch of both denticle types is shown in (G), whereas the larger non-light-guiding denticles are highlighted with asterisks in (H). Image widths: B, 20 mm; C, 1.2 mm; E, 20 mm; F, 1.5 mm; G, 7 mm; H, 3.3 mm.
Results
Skin Tissue Structure in Microscopy
C. ventriosum has small intensely green fluorescent spots over much of the body (Video S1), which appear light beige under white light. S. retifer, in contrast, exhibits an alternating light and dark reticulated pigmentation pattern, but it lacks the brightly fluorescent spots characteristic of C. ventriosum (Figure 1). Excised skin pieces from C. ventriosum and S. retifer were examined in their native state using a dual-camera Zeiss Axio zoom v16 fluorescence microscope. For sequential data collection from identical areas, the bright-field images were acquired with a Zeiss 506 color digital camera and the green fluorescence images were acquired with a Hamamatsu Flash 4.0 black and white digital camera. We note a surprising finding that the dermal denticles of C. ventriosum are not fluorescent, whereas those of S. retifer act as efficient optical waveguides, channeling the fluorescent signal along their length (Figure 1). Further analysis of S. retifer denticle structural diversity revealed that in addition to the smaller light-guiding denticles, a second type of larger, non-light-guiding denticles are also present (Figures 1G and 1H). This shows that the green fluorescence is not only targeted to varying regions of the shark but also that the targeting is fine-tuned to specific denticle types within a shark species. It has been previously demonstrated that these shark species possess the visual apparatus to detect biofluorescence (484 and 488-nm monochromat visual pigments in C. ventriosum and S. retifer, respectively) and that there are pronounced sexually dimorphic fluorescent patterns (Gruber et al., 2016). The green biofluorescence thus creates greater visual contrast for these sharks at depth, due to the primarily blue mesophotic marine environment (Gruber et al., 2016). It has also been shown that there are species-specific emission patterns in other fluorescent fish species, leading to the suggestion that biofluorescence functions in intraspecific communication and assists camouflage (Sparks et al., 2014). These observations of biofluorescent targeting to specific denticle types further advances our understanding of the complexity of this unique biofluorescence in the shark skin and the possible optical functions it may play. It is also noteworthy, that the deep-sea lanternshark (Etmopterus spinax) has also been shown to utilize its mineralized spines for the transmission of bioluminescent signals (Claes et al., 2013).
Brominated Metabolites in Swell Shark Skin Tissue
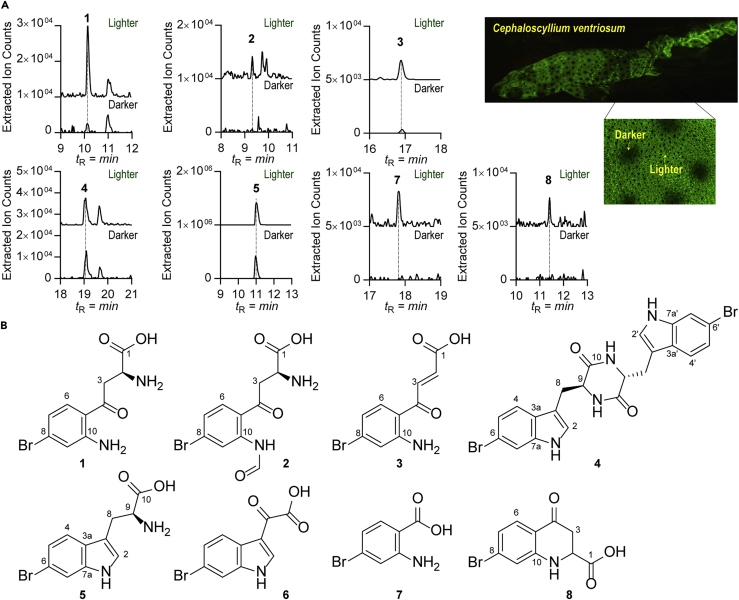
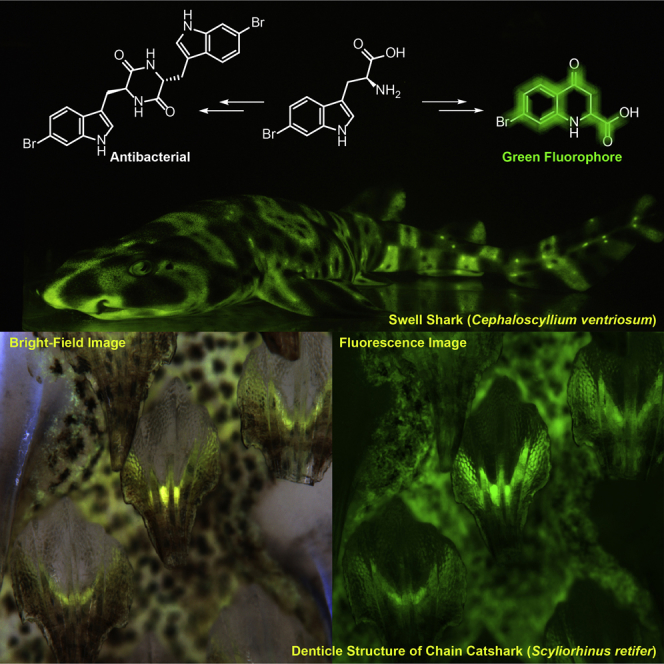
To establish a molecular basis for shark skin biofluorescence, we first analyzed small molecule extracts of dark and light skin tissue samples from the swell shark (C. ventriosum). Skin tissues were dissected using a fluorescence microscope (Zeiss-Axio Zoom V16 stereo fluorescent microscope) affixed with a Nikon D4 camera. The organic crude extracts of skin tissue samples were analyzed using an ultrahigh-performance liquid chromatography (UPLC) system equipped with a photodiode array detector and a high-resolution electrospray ionization-quadrupole time-of-flight-mass spectrometer (HR-ESI-QTOF-MS) (Figures 2A and S1). We first analyzed two major metabolites 4 and 5 eluting at retention times 19.2 and 11.2 min, respectively, which were similarly present in both the dark and light tissue samples (Figures 2A and S2). Their high-resolution mass spectra in positive mode showed an isotope distribution pattern attributable to the presence of bromine atoms at m/z 528.9875, 530.9859, and 532.9843 (relative intensity 1:2:1) for 4 and at m/z 283.0109 and 285.0090 (relative intensity 1:1) for 5, indicating the molecular formulas of 4 and 5 to be C22H18Br2N4O2 and C11H11BrN2O2, respectively (Table S1 and Figure S1). In addition, UV-visible spectra of these peaks shared a characteristic indole chromophore, suggesting the presence of a brominated tryptophan moiety known to be fluorescent (Figures S7A and S8A) (Schnepel et al., 2016). The chemical structures of these metabolites isolated from swell shark skin were fully elucidated using 1H and gradient-enhanced 2D NMR (correlation spectroscopy, heteronuclear single-quantum coherence with adiabatic pulses, and heteronuclear multiple-bond correlation with adiabatic pulses) and Marfey's analysis (Figures S7, S8, S15, and S16, and Table S3). The NMR interpretation of these metabolites revealed resonances and correlations attributable to a 6-bromo tryptophan-derived diketopiperazine (4) and its monomer substrate (5), respectively (Figure 2B). Marfey's analysis on 4 and 5 allowed us to establish their structures as (9S, 9′R)-6-bromo-tryptophan-derived diketopiperazine and 9S-bromo-tryptophan, respectively (Figures S7C and S8C).
Figure 2.
Comparative Metabolic Profiles of Skin Extracts from Cephaloscyllium ventriosum
(A) Differential extracted ion counts chromatograms corresponding to eight representative metabolites from light and dark areas of skin.
(B) Chemical structures of metabolites 1–8. 1–4 and 8 represent previously unknown metabolites.
We then focused on small molecules differentially produced in light and dark skin tissue samples. We detected five major metabolites (1–3, 7, and 8) that were significantly stimulated in the light samples relative to the dark samples (Figure 2A). The UV-visible spectrum of metabolite 1 was similar to that of kynurenine (Figure S4A), a major product of tryptophan catabolism in mammals (Cervenka et al., 2017, Kolodziej et al., 2011). However, the HR-ESI-QTOF-MS data of 1 (m/z 287.0032 and 289.0013 [relative intensity 1:1]) similarly supported the presence of a bromine atom in its chemical formula (C10H11BrN2O3) (Figure S1 and Table S1), suggesting that the structure of 1 could be a bromo-kynurenine. The chemical formulas of 2, 3, 7, and 8 were deduced as C11H11BrN2O4, C10H9BrNO3, C7H6BrNO2, and C10H9BrNO3, respectively, and UV-visible spectroscopic data suggested that they were additional brominated metabolites processed through the kynurenine pathway: N-formyl-bromo-kynurenine (2), bromo-CKA (carboxyketoalkene derivative of kynurenine) (3), bromo-anthranilic acid (7), and bromo-kynurenine yellow (8) Figures 2B, S5A, S6A, S10A, and S11A and Table S1).
The structural characterization of metabolites 1–3 was achieved through chemical synthesis (Figures S3A and S12–S14, and Table S2). Briefly, a substrate, 6-bromo-L-tryptophan (5), was prepared and meta-chloroperoxybenzoic acid (0.32 g, 1.854 mmol, 10.5 equiv.) was added. The chemical reaction was stirred at room temperature under air atmosphere for 20 h to yield the 8-bromo-N-formyl-L-kynurenine (2). Deformylation with 50% trifluoroacetic acid (TFA) led to the product 8-bromo-L-kynurenine (1), which was deaminated under 70°C to give the 8-bromo-CKA (3) (Figure S3A). Co-injection and Marfey's analysis with synthetic and natural materials unambiguously confirmed the absolute chemical structures of these metabolites (Figures S4–S6). The structure of 7 was determined as 4-bromo-anthranilic acid by comparison of UV spectra and liquid chromatography (LC)-MS co-injection with a commercial standard (Figure S10). Last, we chemically synthesized compound 8 employing a synthetic scheme analogous to a previous report (Higgins et al., 2009) (Figures S3B, S17, and S18 and Table S4), and co-injection studies with the synthetic standard and the natural skin material confirmed the proposed structure of 8 (Figure S11).
Brominated Metabolites in Chain Catshark Skin Tissue
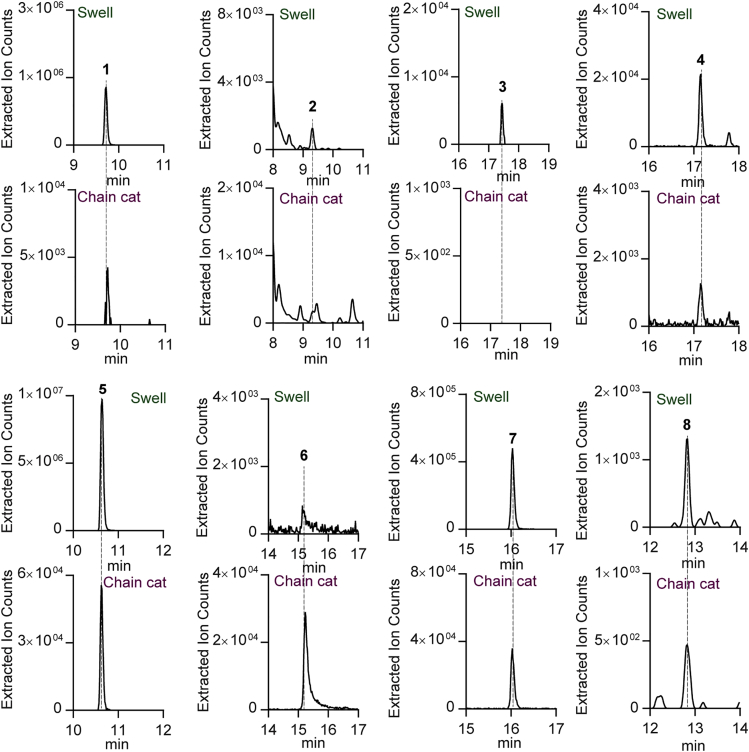
We next examined whether these metabolites existed in the biofluorescent chain catshark, S. retifer. Comparative metabolic analysis revealed that the same brominated metabolites are also present in chain catsharks (Figure 3), although metabolite 3 was below the detection limit under the conditions of our experiments. In addition, we identified major metabolite 6, a brominated variant of indole-3-glyoxylic acid in S. retifer and confirmed its structure by co-injection experiments with an authentic standard (Figure S9). These studies identified a total of eight small molecule metabolites from biofluorescent shark skins.
Figure 3.
Comparative Metabolic Analysis of Skin Extracts from Cephaloscyllium ventriosum (Swell Shark) and Scyliorhinus rotifer (Chain Catshark)
Kynurenine Metabolism
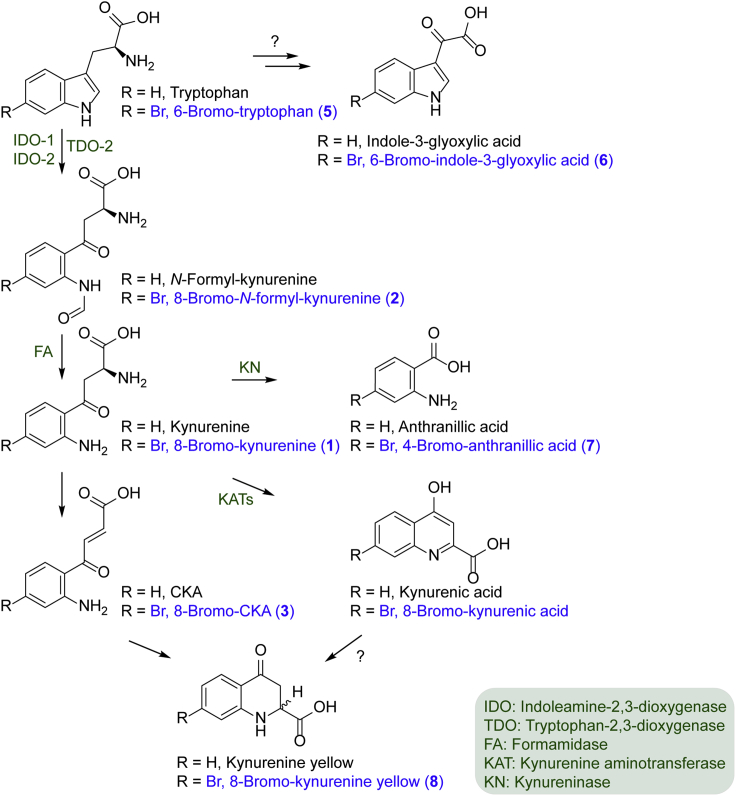
The structures of the brominated metabolites in biofluorescent shark skins suggest that the brominated tryptophan found in both dark and light skin tissues is promiscuously processed through the tryptophan-kynurenine pathway (Figure 4). In humans, kynurenine metabolism is known to regulate important biological processes, including host-microbiome signaling and immune response, and is also linked to the pathogenesis of a variety of diseases such as diabetes, inflammation, neurodegenerative disorders, and cancer (Cervenka et al., 2017, Mazarei and Leavitt, 2015, Van der Leek et al., 2017). As enzymes involved in kynurenine metabolism can also be found in diverse bacteria (Bortolotti et al., 2016, Forouhar et al., 2007, Kurnasov et al., 2003), we tested if the common gut inhabitant E. coli is capable of metabolizing 6-bromo-L-tryptophan to 8-bromo-L-kynurenine. Indeed, E. coli Nissle 1917 biotransformed 6-bromo-L-tryptophan to 8-bromo-L-kynurenine in the ratio of 64:1, as determined by LC-MS (Figure S19), further supporting flexibility in functionalized tryptophan catabolism.
Figure 4.
Overview of the Kynurenine and Bromo-kynurenine Pathway
Bromo-tryptophan appears to be promiscuously processed through the kynurenine pathway to generate the biofluorescent shark metabolites.
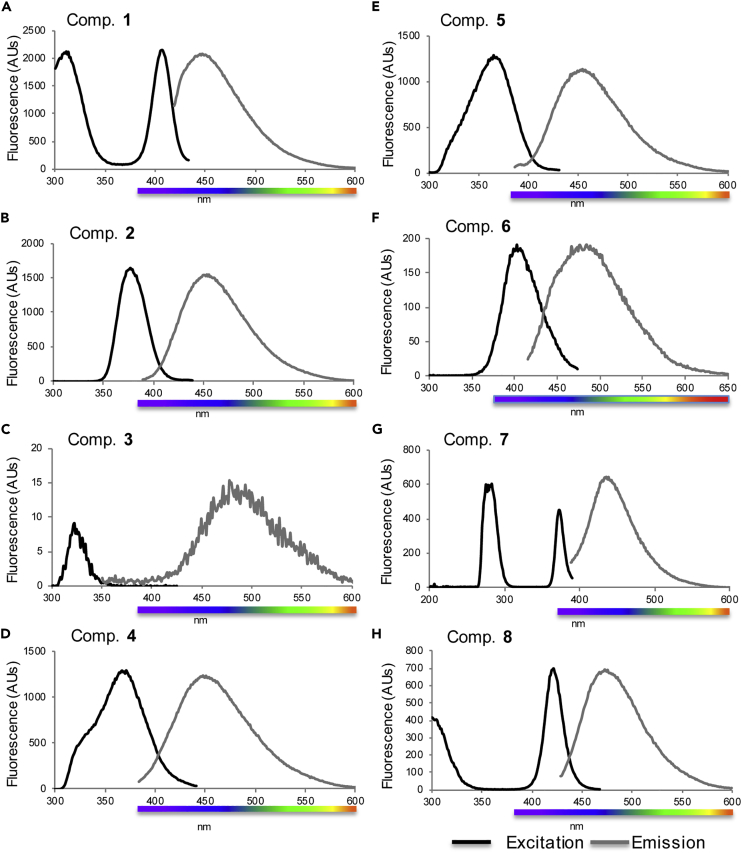
Absorbance and Fluorescence Emission of Metabolites
The absorbance and fluorescence excitation and emission spectra were taken for the characterized compounds. First, absorbance and fluorescence spectra of all compounds were measured at 5 mM concentrations in methanol. Based on the absorbance of the compounds, initial excitations were run using the absorbance values.
Compounds 1 and 7 were found to have similar excitation and emission spectra as L-kynurenine and were found to have two excitation peaks (Figure 5). However, of the two excitation peaks, one was found to cause a much stronger fluorescence emission. Peak absorbance of 1 and 7 was found to be 375 nm, whereas the excitation spectra were found to be maximal at 407 and 374 nm, respectively. Emission spectra for 1 and 7 were found to be in the blue wavelength range at 447 nm (1) and 439 nm (7) (Figures 5A and 5G).
Figure 5.
Absorbance and Fluorescence Emission of Shark Skin Metabolites
(A–H) Fluorescence Spectra of Pure Compounds 1–8 in methanol. See Figure S20 for spectra in PBS.
Compounds 2–5 and 6 were found to have similar excitation and emission spectra as L-tryptophan (Figure 5). Unlike 1 and 7, these compounds were only excited by one peak. Compounds 2, 4, and 5 were found to emit in the blue wavelength with emissions close to 450 nm (453, 448, and 454 nm, respectively) and were excited at 370 nm. Compound 3 was found to exhibit significantly red-shifted fluorescence than the other compounds, which exhibited a blue wavelength emission peak of 477 nm with broad green spectrum tailing. Similarly, compound 6 was found to have a blue wavelength emission, although it trailed into the green spectra with a peak emission of 484 nm. Non-brominated kynurenine yellow is known to exhibit bright green fluorescence with an emission maxima of ∼520 nm (Mizdrak et al., 2007, Zelentsova et al., 2013), whereas our experimental fluorescence spectrum of bromo-kynurenine yellow (8) obtained in methanol revealed a blue wavelength emission maximum of 472 nm (Figure 5H). Given the discrepancy, we further obtained fluorescence spectra of the compounds in a more biologically relevant phosphate buffered saline (PBS) system, and the compounds displayed significant shifts of their fluorescence spectra compared with those in methanol (Table 1 and Figure S20). Specifically, the fluorescence emission spectrum of 8 exhibited a green emission maximum of 507 nm (Table 1 and Figure S20H). Consistent with kynurenine yellow, a large Stokes shift was observed in 8, which is known to be derived from a charge transfer from the amino group to the carbonyl group in the kynurenine yellow scaffold (Zelentsova et al., 2013). Thus, bromo-tryptophan-kynurenine metabolism products in an aqueous environment represent the chemical source of bright green biofluorescence in shark skins.
Table 1.
Fluorescence of Compounds in Methanol and PBS
| Methanol |
PBS |
|||
|---|---|---|---|---|
| Excitation Max (nm) | Emission Max (nm) | Excitation Max (nm) | Emission Max (nm) | |
| Compound 1 | 407 | 447 | 414 | 451 |
| Compound 2 | 370 | 453 | 401 | 481 |
| Compound 3 | 323 | 477 | NAa | NAa |
| Compound 4 | 370 | 448 | 360 | 400 |
| Compound 5 | 370 | 454 | 363 | 465 |
| Compound 6 | 403 | 484 | 388 | 495 |
| Compound 7 | 374 | 439 | 360 | 420 |
| Compound 8 | 422 | 472 | 438 | 507 |
Not applicable due to low solubility.
Antibacterial Activities of the Metabolites
Collectively, we structurally characterized the previously undescribed chemical source of green-dominated biofluorescence in the skin of both sharks (Figure 6). In the marine environment, C. ventriosum is benthic (bottom-dwelling) and predominantly remains in direct contact with underwater sediment. Figures S21 and S22 show the demersal habitat of C. ventriosum from Scripps Canyon, California. As marine sediments have higher concentrations of bacteria than the water column (Karl and Novitsky, 1988), we evaluated the antibacterial activities of metabolites 1–7 against two bacterial pathogens, including a Gram-positive methicillin-resistant Staphylococcus aureus (MRSA) and a Gram-negative Vibrio parahaemolyticus. V. parahaemolyticus was chosen as a candidate as it is a common marine bacterium, often found in sediment. Major compound 4 found in both light and dark skin tissue samples exhibited a half-maximal inhibitory concentration (IC50) value of 66 μM against MRSA, and compound 3 showed growth inhibitory activity against V. parahaemolyticus at an IC50 of 14 μM. The other metabolites showed no significant activity at concentrations up to 100 μM (Figures S23 and S24). Based on these observations, the antibacterial properties of these biofluorescent metabolites may thus also contribute in part to chemical defense against microbial pathogens in the marine environment.
Figure 6.
Cross-Section of Sharks Showing Localization of Fluorescence in Skin Tissue
(A) Scyliorhinus retifer excited with 450–500 nm and imaged through 514nmLP, (B) cross section of skin with white light; (C) fluorescence emission, (D) Cephaloscyllium ventriosum excited with 450–500 nm and imaged through 514nmLP, (E) cross section of skin with white light, (F) fluorescence emission.
Discussion
Although biofluorescence is a fascinating phenomenon that has been reported in an increasing diversity of marine species in recent years, including sharks (Gruber et al., 2016, Sparks et al., 2014) and sea turtles (Gruber and Sparks, 2015), its molecular origins remain largely unexplored. In this study, we describe a family of natural small molecule fluorophores that significantly contribute to shark skin biofluorescence. Interestingly, these small molecule metabolites represent a parallel bromo-tryptophan-kynurenine biosynthetic pathway to that of the established tryptophan-kynurenine pathway widely found in vertebrates (Cervenka et al., 2017). For example, kynurenine and its analogs regulate diverse human biological processes, and accumulation of kynurenine in the brain is tightly associated with mental health disorders such as depression and schizophrenia (Cervenka et al., 2017). Of note, a halogenated kynurenine, L-4-chloro-kynurenine, is a neuro-pharmaceutical prodrug of 7-chlorokynurenic acid, used to treat major depressive disorder (Vécsei et al., 2012). Although potential neurological activities of the brominated variant 1 and its analogs remain an open question, it is intriguing to find brominated variants of mammalian signaling molecules in shark skin. Although the exact source of the halogenase and its bromo-tryptophan product (whether it be of shark, microbial symbiont, or environmental origin) remain unresolved, the observed diversity of bromo-tryptophan catabolic processing in E. coli suggests that similarly flexible metabolic pathways may also be at play in shark skin. As we observed differential regulation of these metabolites in light versus dark skin, future studies aimed at assessing whether tryptophan-kynurenine metabolism genes are similarly regulated, are warranted. Analogous to non-brominated kynurenine molecules functioning as UV radiation filters in the human lens, these brominated kynurenine molecules could, in principle, complement melanin pigments within shark skin to offer photo-protection from low-wavelength light (Sweet et al., 2012, Taylor et al., 2002). Collectively, the discovery of brominated tryptophan-kynurenine metabolism products from marine shark skins in our study not only illuminates the likely source of naturally occurring biofluorescence of shark skins but also raises new questions regarding their potential roles in central nervous system signaling, the resilience of shark skins to microbial infections, and diverse skin pigmentation phenotypes.
Limitations of the Study
A wide range of marine organisms are known to be biofluorescent, but the chemical ecological contributions to naturally occurring biofluorescence phenotypes has not been investigated at the detailed molecular level. As representative marine vertebrate animals, we focused on elucidating the bright green biofluorescence phenotype in swell sharks and in chain catsharks. A bromo-tryptophan precursor and a bromo-diketopiperazine antibiotic were found in both skin types, whereas in light skin, the bromo-tryptophan precursor was processed through the kynurenine metabolic pathway, generating a collection of brominated metabolites with varying spectral properties. Although we focused on the characterization of small molecule sources involved in shark biofluorescence phenotypes in this study, underlying biosynthesis of these metabolites still remains to be explored. As aqueous sodium hydroxide was the solvent used for extraction of shark skin materials in our system, we cannot rule out the possibility that the small molecule fluorophores derive from base-catalyzed ester hydrolysis of currently uncharacterized parent molecules. However, pure bromo-tryptophan as a standard solubilized under basic conditions and precipitated under acidic conditions (Figure S25), consistent with our small molecule findings.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Our work was supported by the Camille & Henry Dreyfus Foundation (TC-17–011 to J.M.C), Yale University, the National Geographic Society (grant # RNG-2018-01 to D.F.G) and the US National Science Foundation (grant # MCB-1652731 to J.P.G.). We thank Chelsea Gardiner for preparing shark skin materials for initial metabolic screening, Tom Ferrante for microscopy assistance, Aida Verdes for genomic screening, and John Sparks for discussions on shark biofluorescence.
Author Contributions
H.B.P., D.F.G., and J.M.C conceived and designed the experiments. H.B.P and Y.C.L. carried out the analytical isolation and analysis, chemical synthesis, and structural characterization of metabolites. J.P.G. and S.R.K. obtained the fluorescence spectra of metabolites. J.C.W. performed fluorescent microscopy of sharks. D.F.G. performed in situ underwater fluorescent imagery of sharks. V.P. designed or engineered underwater fluorescent lamps. R.H. isolated bromo-kynurenine. H.B.P, J.P.G., D.F.G., and J.M.C wrote the manuscript. All authors reviewed and edited the manuscript.
Declaration of Interests
The authors declare that no competing interests.
Published: August 8, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.019.
Contributor Information
David F. Gruber, Email: david.gruber@baruch.cuny.edu.
Jason M. Crawford, Email: jason.crawford@yale.edu.
Supplemental Information
References
- Bortolotti P., Hennart B., Thieffry C., Jausions G., Faure E., Grandjean T., Thepaut M., Dessein R., Allorge D., Guery B.P. Tryptophan catabolism in Pseudomonas aeruginosa and potential for inter-kingdom relationship. BMC Microbiol. 2016;16:137. doi: 10.1186/s12866-016-0756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J.I. Oxford University Press; 2011. The Sharks of North America. [Google Scholar]
- Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W.W., Prasher D.C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Claes J.M., Dean M.N., Nilsson D.E., Hart N.S., Mallefet J. A deepwater fish with 'lightsabers'–dorsal spine-associated luminescence in a counterilluminating lanternshark. Sci. Rep. 2013;3:1308. doi: 10.1038/srep01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno L.J.V. Vol. 4. Food and Agriculture Organization of the United Nations; 1984. FAO species catalogue; pp. 251–655. (Sharks of the world: an annotated and illustrated catalogue of shark species known to date. FAO Fish Synop 125). [Google Scholar]
- Deheyn D.D., Kubokawa K., McCarthy J.K., Murakami A., Porrachia M., Rouse G.W., Holland N.D. Endogenous green fluorescent protein (GFP) in amphioxus. Biol. Bull. 2007;213:95–100. doi: 10.2307/25066625. [DOI] [PubMed] [Google Scholar]
- Forouhar F., Anderson J.L.R., Mowat C.G., Vorobiev S.M., Hussain A., Abashidze M., Bruckmann C., Thackray S.J., Seetharaman J., Tucker T. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. U S A. 2007;104:473–478. doi: 10.1073/pnas.0610007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber D.F., Gaffney J.P., Mehr S., DeSalle R., Sparks J.S., Platisa J., Pieribone V.A. Adaptive evolution of eel fluorescent proteins from fatty acid binding proteins produces bright fluorescence in the marine environment. PLoS One. 2015;10:e0140972. doi: 10.1371/journal.pone.0140972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber D.F., Kao H.-T., Janoschka S., Tsai J., Pieribone V.A. Patterns of fluorescent protein expression in scleractinian corals. Biol. Bull. 2008;215:143–154. doi: 10.2307/25470695. [DOI] [PubMed] [Google Scholar]
- Gruber D.F., Loew E.R., Deheyn D.D., Akkaynak D., Gaffney J.P., Smith W.L., Davis M.P., Stern J.H., Pieribone V.A., Sparks J.S. Biofluorescence in catsharks (Scyliorhinidae): Fundamental description and relevance for elasmobranch visual ecology. Sci. Rep. 2016;6:24751. doi: 10.1038/srep24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber D.F., Pieribone V.A. Belknap Press of Harvard University Press; 2006. Aglow in the Dark: The Revolutionary Science of Biofluorescence; p. 288. [Google Scholar]
- Gruber D.F., Sparks J.S. First observation of fluorescence in marine turtles. Am. Mus. Novit. 2015;3845:1–8. [Google Scholar]
- Hayashi S., Toda Y. A novel fluorescent protein purified from eel muscle. Fish. Sci. 2009;75:1461. [Google Scholar]
- Higgins R.C., Townsend N.O., Jackson Y.A. Benzylic oxidation of N-acyl-1,2,3,4-tetrahydroquinolines. Heterocycles. 2009;78:3011–3021. [Google Scholar]
- Karl D.M., Novitsky J.A. Dynamics of microbial growth in surface layers of a coastal marine sediment ecosystem. Mar. Ecol. Prog. Ser. 1988;50:169–176. [Google Scholar]
- Kolodziej L.R., Paleolog E.M., Williams R.O. Kynurenine metabolism in health and disease. Amino Acids. 2011;41:1173–1183. doi: 10.1007/s00726-010-0787-9. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Ando R., Miyatake H., Greimel P., Kobayashi T., Hirabayashi Y., Shimogori T., Miyawaki A. A bilirubin-inducible fluorescent protein from eel muscle. Cell. 2013;153:1602–1611. doi: 10.1016/j.cell.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Kurnasov O., Jablonski L., Polanuyer B., Dorrestein P., Begley T., Osterman A. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiol. Lett. 2003;227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- Matz M.V., Fradkov A.F., Labas Y.A., Savitsky A.P., Zaraisky A.G., Markelov M.L., Lukyanov S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999;17:969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- Mazarei G., Leavitt B.R. Indoleamine 2,3 dioxygenase as a potential therapeutic target in Huntington's disease. J. Huntingtons Dis. 2015;4:109–118. doi: 10.3233/JHD-159003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers A., Fradkov A.F., Shagin D.A., Widder E.A., Barsova E.V., Nunez J.M., Ugalde J.A., Lukyanov K.A., Matz M.V., Lukyanov S.A. GFP-like proteins as ubiquitous metazoan superfamily: evolution of functional features and structural complexity. Mol. Biol. Evol. 2004;21:841–850. doi: 10.1093/molbev/msh079. [DOI] [PubMed] [Google Scholar]
- Mizdrak J., Hains P.G., Kalinowski D., Truscott R.J.W., Davies M.J., Jamie J.F. Novel human lens metabolites from normal and cataractous human lenses. Tetrahedron. 2007;63:4990–4999. [Google Scholar]
- Schnepel C., Minges H., Frese M., Sewald N. A high-throughput fluorescence assay to determine the activity of tryptophan halogenases. Angew. Chem. Int. Ed. 2016;55:14159–14163. doi: 10.1002/anie.201605635. [DOI] [PubMed] [Google Scholar]
- Shagin D.A., Barsova E.V., Yanushevich Y.G., Fradkov A.F., Lukyanov K.A., Labas Y.A., Semenova T.N., Ugalde J.A., Meyers A., Nunez J.M. GFP-like proteins as ubiquitous metazoan superfamily: evolution of functional features and structural complexity. Mol. Biol. Evol. 2004;21:841–850. doi: 10.1093/molbev/msh079. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F.H., Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Sparks J.S., Schelly R.C., Smith W.L., Davis M.P., Tchernov D., Pieribone V.A., Gruber D.F. The covert world of fish biofluorescence: a phylogenetically widespread and phenotypically variable phenomenon. PLoS One. 2014;9:e83259. doi: 10.1371/journal.pone.0083259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet M., Kirkham N., Bendall M., Currey L., Bythell J., Heupel M. Evidence of melanoma in wild marine fish populations. PLoS One. 2012;7:e41989. doi: 10.1371/journal.pone.0041989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L.M., Andrew Aquilina J., Jamie J.F., Truscott R.J.W. UV filter instability: consequences for the human lens. Exp. Eye Res. 2002;75:165–175. doi: 10.1006/exer.2002.2012. [DOI] [PubMed] [Google Scholar]
- Tsien R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Van der Leek A.P., Yanishevsky Y., Kozyrskyj A.L. The kynurenine pathway as a novel link between allergy and the gut microbiome. Front. Immunol. 2017;8:1374. doi: 10.3389/fimmu.2017.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vécsei L., Szalárdy L., Fülöp F., Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discov. 2012;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- Zelentsova E.A., Sherin P.S., Snytnikova O.A., Kaptein R., Vauthey E., Tsentalovich Y.P. Photochemistry of aqueous solutions of kynurenic acid and kynurenine yellow. Photochem. Photobiol. Sci. 2013;12:546–558. doi: 10.1039/c2pp25357g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.