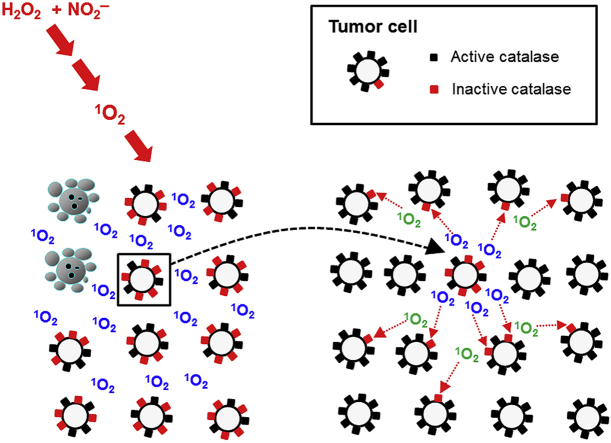
Abstract
Treatment of tumor cells with H2O2 and nitrite, two long-lived species derived from cold atmospheric plasma, induces a complex autoamplificatory, singlet oxygen-mediated process, which leads to catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Experimental dissection and quantification of this process is described in this study. When tumor cells were pretreated with H2O2 and nitrite, and then were added to untreated tumor cells, they propaged singlet oxygen mediated catalase inactivation and generation of singlet oxygen to the untreated cell population. This bystander effect allowed to analyze the biochemical requirements of a) induction of the bystander effect-inducing potential, b) transmission of the bystander effect to untreated neighbouring cells, and c) the biochemical consequences of these signaling events. The induction of bystander effect-inducing potential requires the generation of “primary singlet oxygen” through the reactions following the interaction between nitrite and H2O2, followed by local inactivation of a few catalase molecules. This primary effect seems to be very rare, but is efficiently enhanced by the generation of "secondary singlet oxygen" through the interaction between H2O2 and peroxynitrite at the site of inactivated catalase. Transmission of bystander signaling between pretreated and untreated tumor cells depends on the generation of secondary singlet oxygen by the pretreated cells and singlet oxygen-mediated catalase inactivation of the untreated recipient cells. This induces autoamplificatory propagation of secondary singlet oxygen generation in the population. This experimental approach allowed to quantify the efficiencies of primary and secondary singlet oxgen generation after CAP and PAM action, to dissect the system and to study the underlying chemical biology in detail. Our data confirm that CAP and PAM-derived components are merely the trigger for the activation of autoamplificatory mechanisms of tumor cells, whereas the tumor cells efficiently propagate their cell death through their own ROS/RNS signaling potential.
Keywords: Cold atmospheric plasma, Hydrogen peroxide, Singlet oxygen, Catalase, Apoptosis, Bystander effect
Graphical abstract
Highlights
-
•
Primary 1O2 generated by H2O2 and NO2─ induces in tumor cells the potential for bystander signaling.
-
•
Bystander signaling depends on inactivation of membrane-associated catalase.
-
•
It is propagated by secondary singlet oxgen generated by cell-derived H2O2 and peroxynitrite.
-
•
The action of primary singlet oxygen is a rare effect.
-
•
Secondary singlet oxygen is generated in a sustained mode and acts efficiently.
1. Introduction
1.1. Interactions between H2O2 and nitrite with redox-related elements on the surface of tumor cells
Hydrogen peroxide and nitrite are two long-lived and far-ranging species from cold atmospheric plasma (CAP) and plasma-activated medium (PAM) [[1], [2], [3], [4]]. In a preceding manuscript [5], we reported that their combined action in a defined concentration range is sufficient to establish selective apoptosis induction in tumor cells in vitro. This might explain the mechanism of an analogous effect of CAP and PAM on tumors in vivo [[1], [2], [3],[6], [7], [8], [9], [10], [11], [12], [13]].
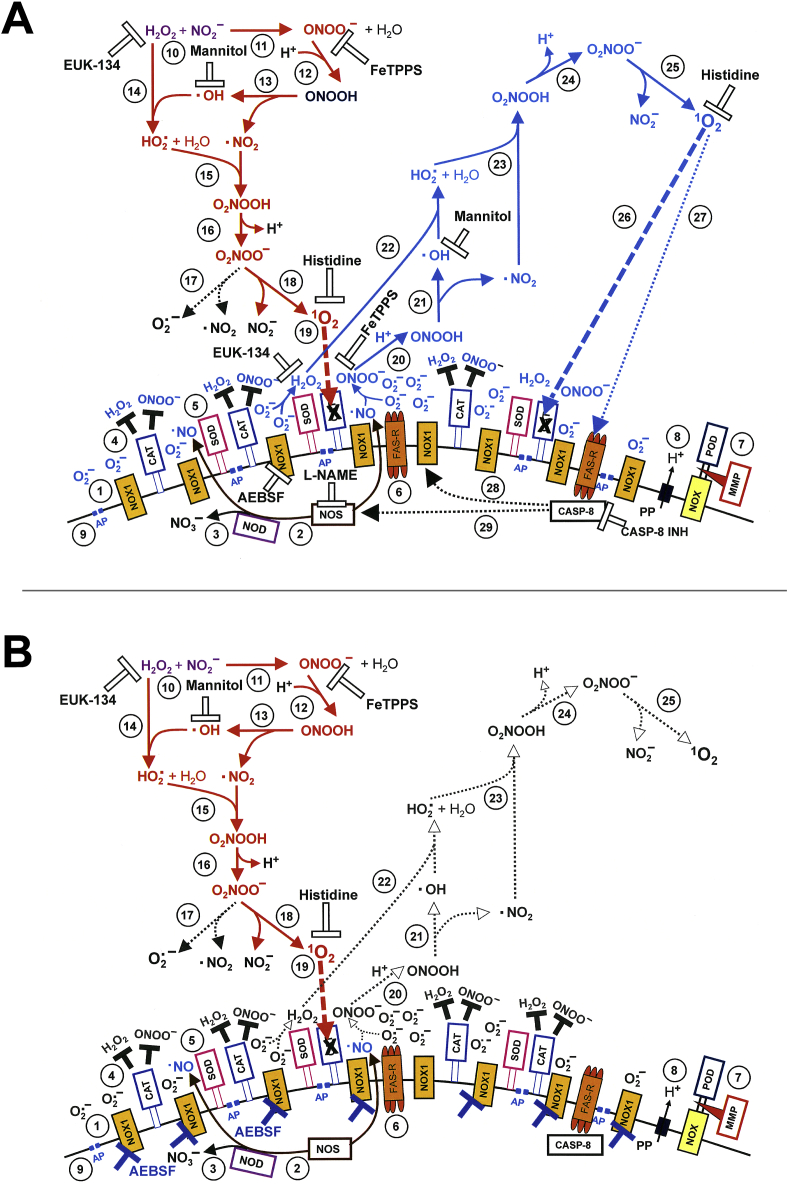
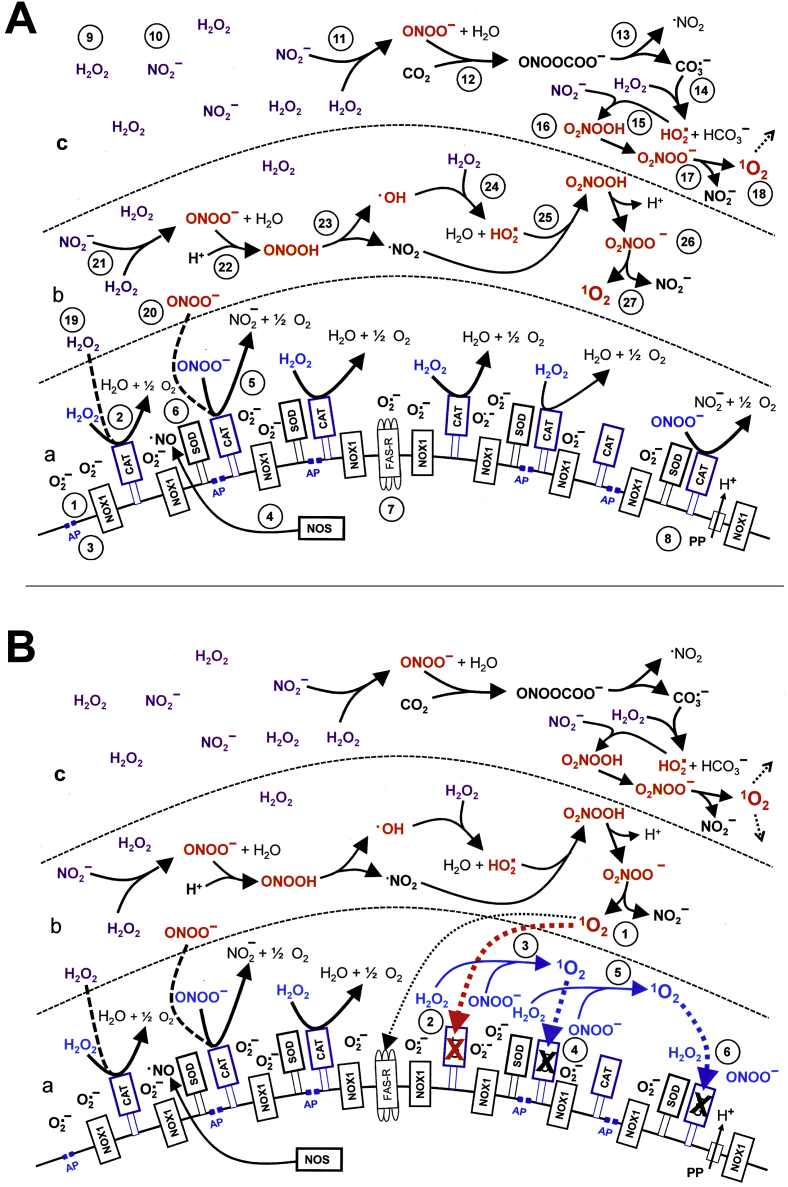
The specific redox-related composition of the surface of tumor cells composed of NOX1, catalase, SOD, aquaporins, proton pumps, FAS receptor [[14], [15], [16], [17], [18], [19], [20], [21], [22]] thereby represented the “molecular switchboard” that was triggered by H2O2/nitrite interaction to react in an autoamplificatory mode. (Please find details on the composition of the membrane and on its interactions in the preceding manuscript [5] and in Fig. 14, Fig. 15 of this manuscript.)
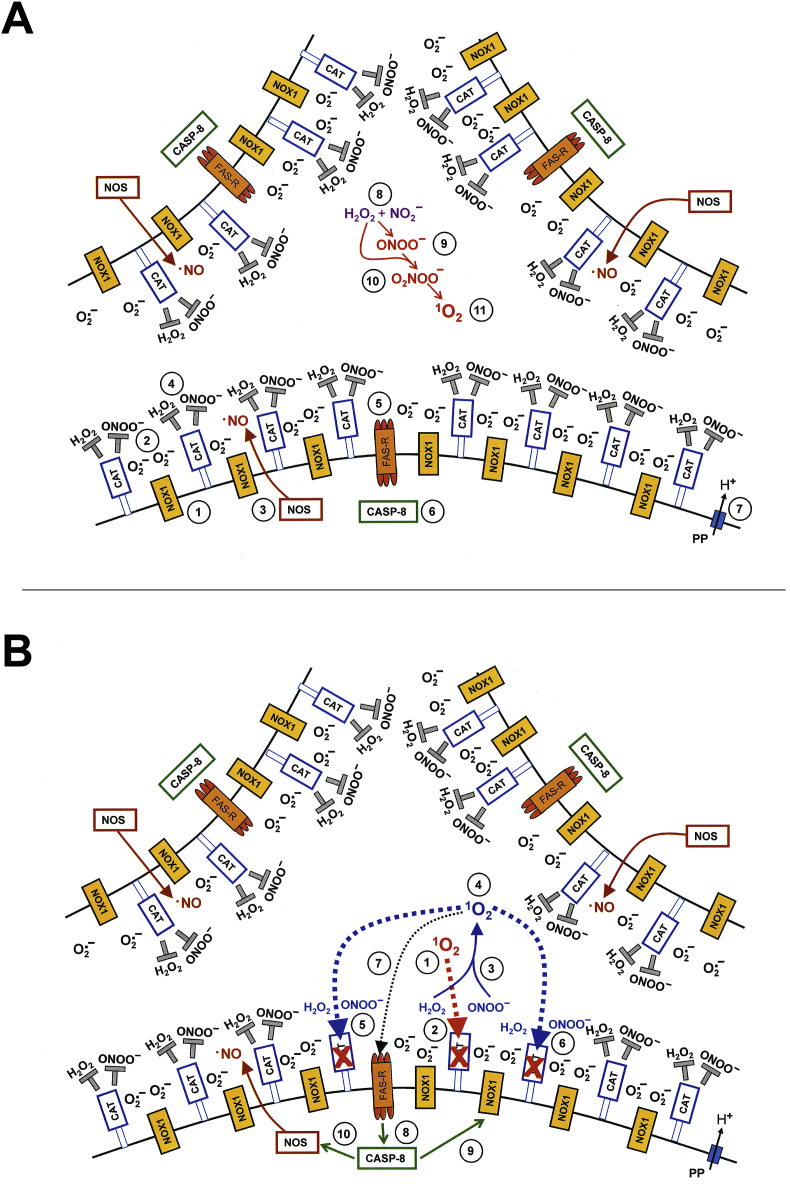
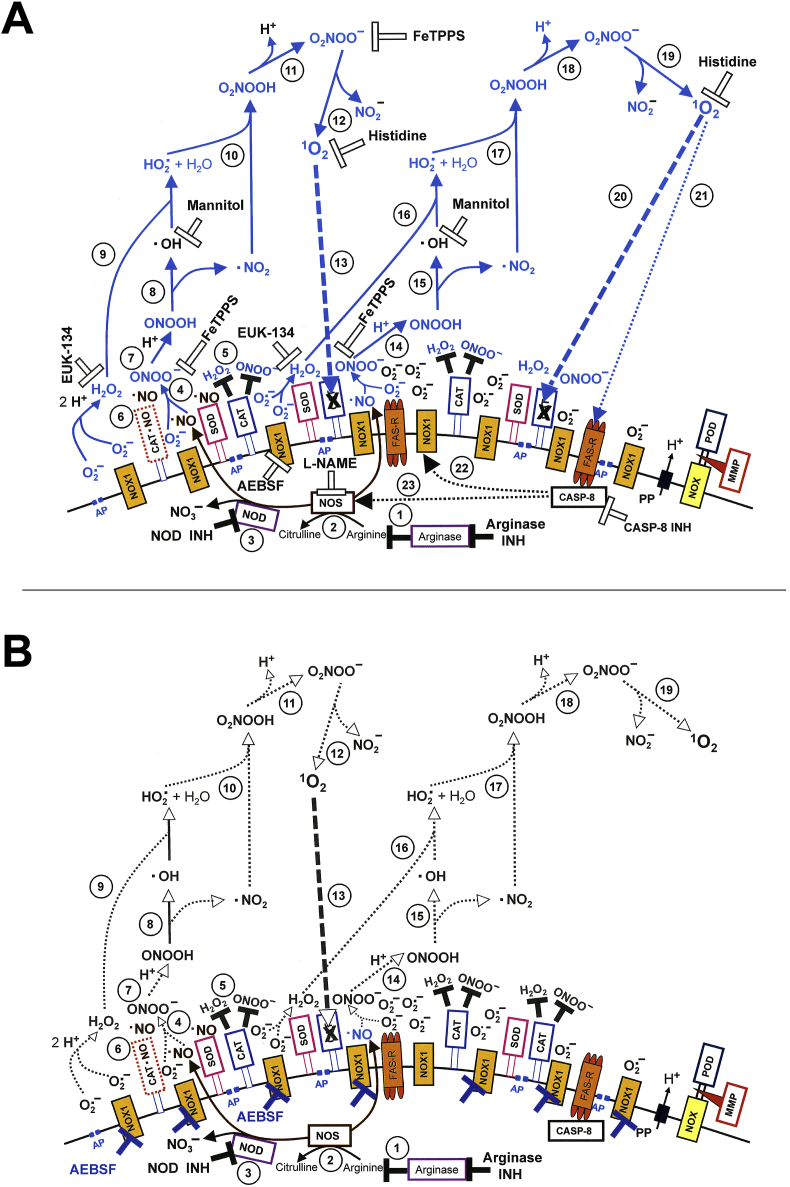
Fig. 14.
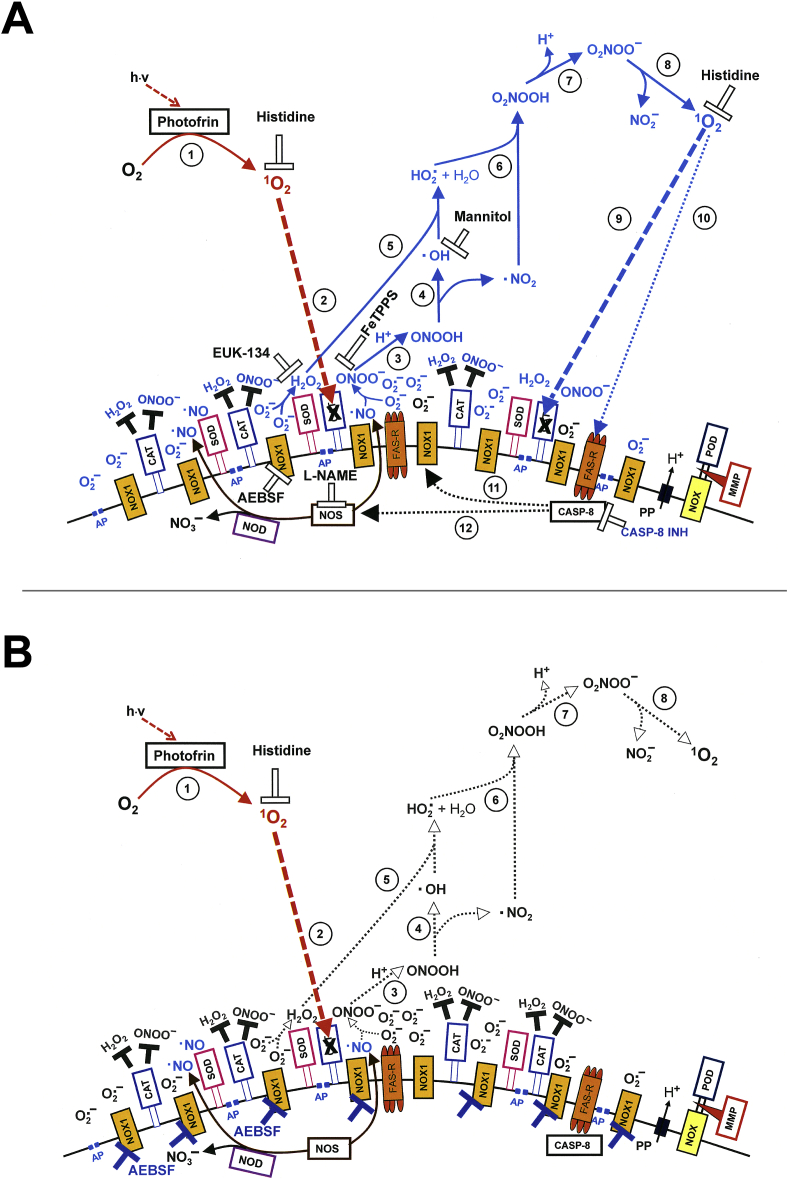
Mechanism of bystander signaling of tumor cells after treatment with H2O2 and nitrite. First steps. A. The membrane of tumor cells carries active NADPH oxidase-1 (NOX1) (#1) that generates extracellular superoxide anions (#2). NO synthase (NOS) (#3) generates NO that passes through the membrane. Membrane-associated catalase (#4) protects the tumor cells towards HOCl and NO/peroxynitrite signaling through decomposition of H2O2 and peroxynitrite. Oxidation of NO by catalase as well as the comodulatory activity of membrane-associated SOD that prevents superoxide anion-dependent inhibition of catalase is not shown in the Figure for simplicity. The figure shows the FAS receptor (#5), caspase-8 (#6) and proton pumps (#7). Long-lived species H2O2 and nitrite from CAP or PAM (#8) interact and generate primary singlet oxygen (#9 - #11) (simplified scheme, please see Fig. 16 for more details). B. Primary singlet oxygen (#1) causes local inactivation of catalase (#2). As a result, cell-derived H2O2 and peroxynitrite are not decomposed at that site and may form secondary singlet oxygen (#3, #4). The full complexity of reaction #3 is shown in Fig. 16. Secondary singlet oxygen inactivates further catalase molecules (#5, #6) or activates the FAS receptor (#7). This leads to the activation of caspase-8 (#8) and subsequent activation of NOX1 (#9) and enhancement of NOS expression (#10).
Fig. 15.
Mechanism of bystander signaling of tumor cells after treatment with H2O2 and nitrite. Continuation. A. Secondary singlet oxygen (#1, #4) causes inactivation of catalase on the original cell (# 2, #5) or on neighbouring cells (#3, #7), or activates the FAS receptor on neighbouring cells (#6). As a consequence, the generation of secondary singlet oxygen is activated within the cell population (#8 - #10) in an autoamplificatory mode. B. After sufficient inactivation of catalase in the cell population (#1) H2O2 generated through dismutation of NOX1-derived superoxide anions (#2) is no longer decomposed and is used as substrate by peroxidase (POD) (#3) for the generation of HOCl (#4). The reaction between HOCl and superoxide anions (#5) yields hydroxyl radicals (#6) in close vicinity to the membrane. This results in lipid peroxidation (#7) and the subsequent induction of the mitochondrial pathway of apoptosis (#8). For simplicity, it is not shown that apoptosis induction by lipid peroxidation requires a preceding influx of H2O2 through aquaporins that lowers the intracellular gluatathione level.
Kinetic analysis and experimental dissection of the biological system in vitro, combined with differential addition of inhibitors and scavengers, allowed to define three essential steps in this scenario. The first step comprises a) primary singlet oxygen generation initiated by nitrite/H2O2 interaction, b) local inactivation of membrane-associated catalase by primary singlet oxygen, c) subsequent sustained generation of secondary singlet oxygen in an autoamplificatory mode. This causes substantial inactivation of membrane-associated catalase. Despite its complexity, this step is completed within 30 min in a population of tumor cells at sufficient density in vitro.
The second step was characterized by H2O2 influx through aquaporins that were no longer gated by membrane-associated catalase. This seems to lead to depletion of intracellular glutathione and thus abrogates the potential of glutathione peroxidase-4/glutathione to antagonize the effects of lipid peroxidation by extracellular ROS. In vitro, step two seems to be completed within 1 h. Keidar's group were the first to recognize the central importance of aquaporins for the control of CAP and PAM action [23,24], but did not consider the hampering effect of membrane-associated catalase on the flux of H2O2 through aquaporins [19].
During the third step, which depends on inactivated catalase and requires several hours for completion, intercellular HOCl signaling causes lipid peroxidation and the activation of the mitochondrial pathway of apoptosis. The third step is only successful after completion of steps 1 and 2.
● Therefore, ROS/RNS-dependent apoptosis induction in CAP/PAM-treated tumor cells seems to be controlled by two separate key events that are mechanistically linked and have to interact in a precise kinetic mode to finally allow apoptosis induction: catalase inactivation and aquaporin-mediated intracellular glutathione depletion. As both of these control steps are dependent on active NOX1, and as the resultant HOCl signaling itself is also driven by NOX1 at two distinct biochemical sites (generation of H2O2 and HOCl/superoxide anion interaction) [20], an impressively high degree of selectivity with respect to the malignant phenotype with its hallmark "active NOX1" is warranted.
Inhibitor studies and the elaborated chemical biology of ROS/RNS allowed to establish a model [5] in which the interaction between nitrite and H2O2 first caused the formation of peroxynitrite. Proton pump-derived protons then allowed formation of peroxynitrous acid in close vicinity of the cell membrane [18], competing well with the faster reaction between peroxynitrite and CO2 distant of the membrane [[24], [25], [26], [27]]. It was suggested that this was followed by the decomposition of peroxynitrous acid into NO2 and hydroxyl radicals. Interaction between hydroxyl radicals and H2O2 caused the generation of hydroperoxyl radicals that combined with NO2, and yielded peroxynitric acid (O2NOOH). Deprotonation of peroxynitric acid led to the formation of peroxynitrate that yielded singlet oxygen after decomposition [28]. Singlet oxygen then caused local inactivation of membrane-associated catalase through reaction with histidine at the active center of catalase [29,30].
The inhibitor studies of step one, i. e. the interaction between exogenous H2O2/nitrite with tumor cells already indicated that singlet oxygen generated solely by CAP- or PAM-derived H2O2 and nitrite cannot explain the extent of catalase inactivation measured. Rather, NOX1-derived superoxide anions and NOS-derived NO seemed to play a dominant role in addition. This allowed to formulate the concept of generation of secondary singlet oxygen at the site of inactivated catalase, through interaction between H2O2 and peroxynitrite that are generated in the extracellular space of tumor cells. These two species are no longer decomposed at the site of inactivated catalase. Their interaction leads to singlet oxygen, following the reactions that have also been suggested for the final part of nitrite/H2O2 interaction. This process can be predicted to inherit a strong potential for autoamplificatory enhancement, leading to the inactivation of neighbouring catalase molecules on the outside of the originally triggered cell, as well as on neighbouring cells. It was predictable, that this process should be accompanied and based on constantly increasing generation of secondary singlet oxygen, fuelled by the sustained activities of NOX and NOS. The generation of secondary singlet oxygen by tumor cells has also been described for tumor cells triggered with catalase-inactivating singlet oxygen generated by a photosensitizer [31], after modulation of the endogenous NO level [18,32] and after application of high concentrations of H2O2 [33]. It therefore seems to represent a mechanism that can be reactived in tumor cells by different selective antitumor regimens.
1.2. Strategy of this study
The following experiments aimed at the elucidation of the mechanisms of the dynamic and autoamplificatory processes triggered by the addition of H2O2 and nitrite to tumor cells. Thereby the differentiation between primary and secondary singlet oxygen generation and the underlying dynamics of these processes are particularly in the focus of this experimental approach. This experimental approach is essentially based on the concept that tumor cells that had been pretreated with singlet oxygen-generating H2O2/nitrite, therefore had inactivated membrane-associated catalase and generated secondary singlet oxygen. Their transfer into an untreated tumor cell population should therefore transfer catalase inactivation and singlet oxygen generation in a bystander-like mechanism. This process should be accessible to strict biochemical analysis.
2. Material and methods
2.1. Materials
Photofrin (a product of Axcan, Canada) was obtained from Meduna Arzneimittel GmbH (Aschaffenburg, Germany). Nω-Hyroxy-nor-l-arginine.acetate (NOR-NOHA) was obtained from Axxora/Enzo Life Sciences, Lörrach, Germany. Taxol was obtained from Sigma Aldrich (Schnelldorf, Germany). All other materials are described in the preceding manuscript [5].
2.2. Methods
Cells, methods of cell culture, apoptosis induction, quantitation of apoptotic cells, siRNA-mediated knockdown of NOX1 and statistical methods used in this paper have been described in detail in the preceding paper [5].
The experimental setup to study bystander signaling is described in the respective figures.
3. Results
3.1. Basic principles of bystander signaling
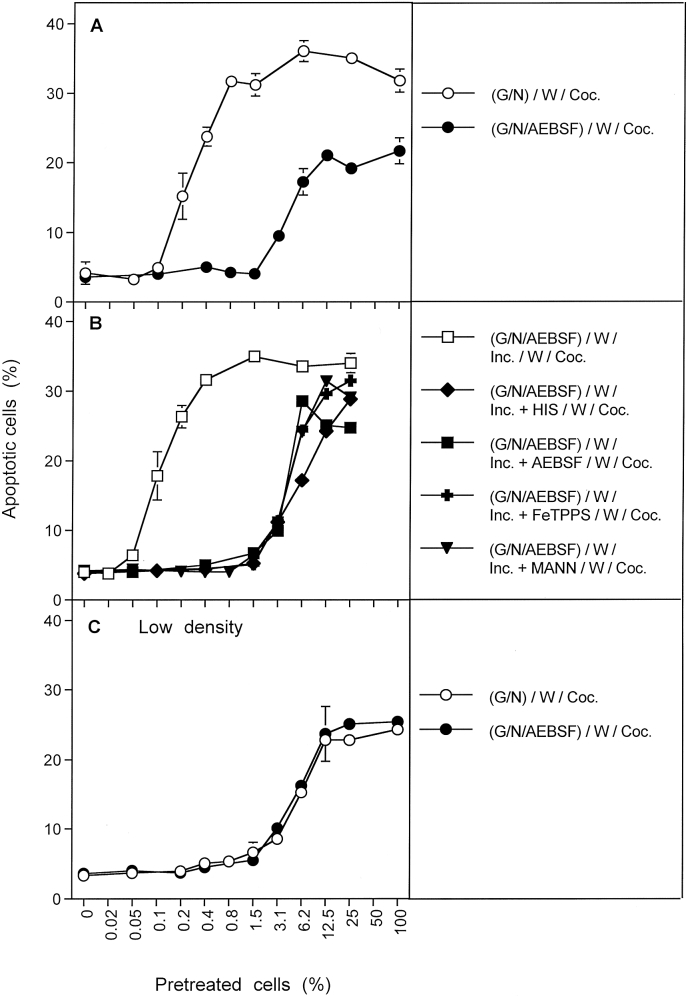
MKN-45 human gastric carcinoma cells were pretreated with H2O2-generating glucose oxidase (GOX) and nitrite for 25 min, washed, and were then added at increasing percentages to untreated MKN-45 cells. Thereby the lowest percentage of pretreated cells was zero, i. e. it reflected exclusively the population of untreated cells. The highest percentage of pretreated cells was 100%, allowing to assess specifically apoptosis induction of pretreated cells. The mixtures with increasing percentages of pretreated cells in between aimed at the analysis of potential bystander signaling processes. The term “bystander effect” has been originally developed in radiation biology and can be used in a wider sense whenever initial targeting a minority of cells has a subsequent impact on the majority of nontargeted cells in the surrounding [34].
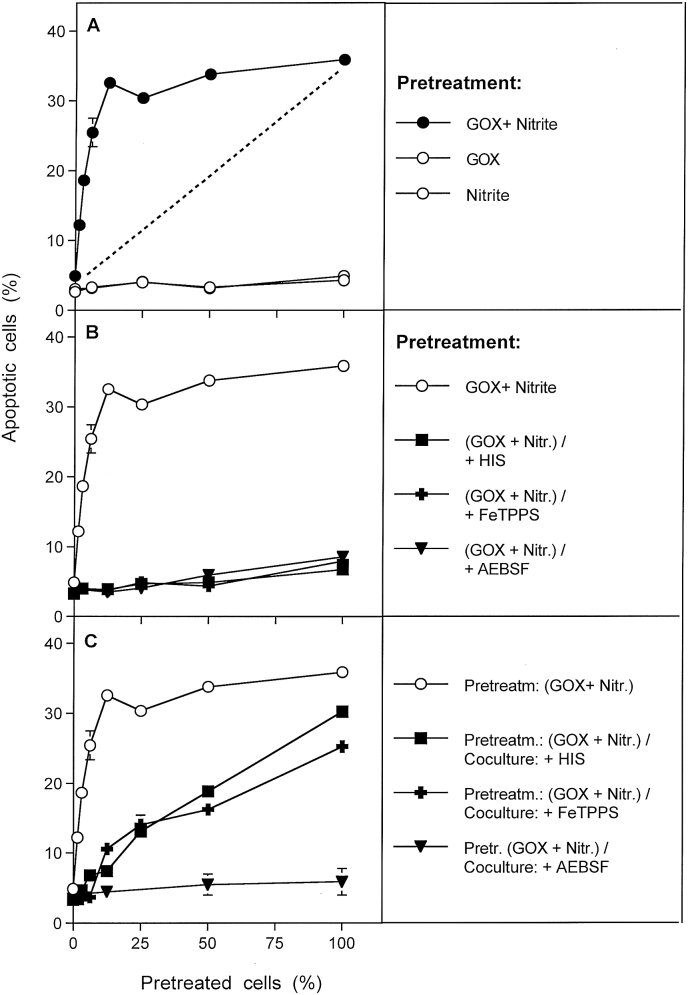
Apoptosis induction in mixtures of GOX/nitrite-pretreated and untreated tumor cells after coculture for 4 h was much higher than to be expected from the percentage of pretreated cells being present in the assays (Fig. 1 A). This finding shows that tumor cells pretreated with GOX/nitrite must have transferred catalase inactivation and the potential for reactivation for apoptosis induction by intercellular ROS/RNS signaling to their untreated neighbouring cells. This biochemical transfer occured efficiently, despite the removel of the original triggering compounds nitrite and H2O2. This interaction might be characterized by the term “intercellular bystander signaling between pretreated and untreated tumor cells” and will be called “intercellular bystander signaling” throughout this manuscript for convenience. Treatment of tumor cells with either GOX or nitrite alone was not sufficient to allow induction of bystander signaling. It also did not sensitize pretreated cells to apoptosis induction by intercellular ROS signaling. This points to the necessary action of one or more reaction partners derived from the interaction between nitrite and H2O2, in order to sensitize tumor cells for intercellular apoptosis-inducing signaling after catalase inactivation.
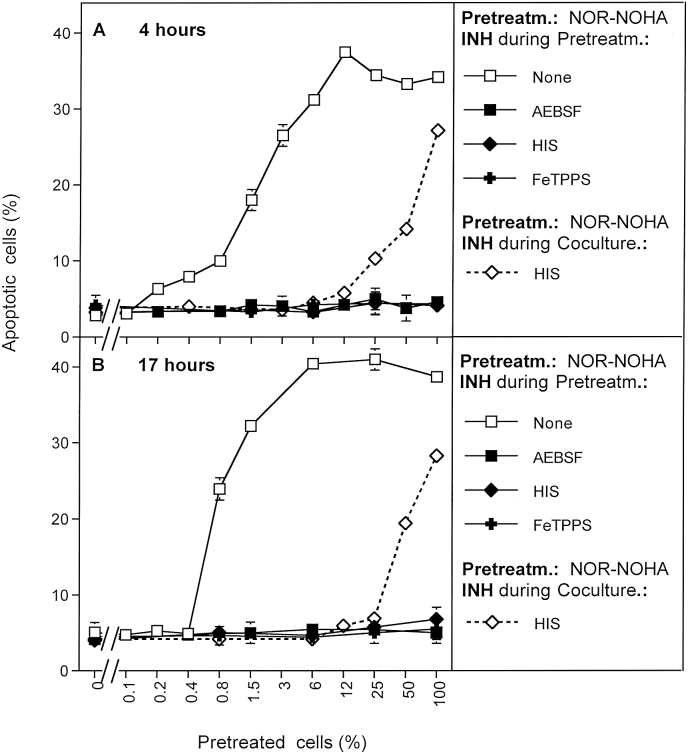
Fig. 1.
Basic principles of bystander signaling between tumor cells pretreated with H2O2/nitrite and untreated tumor cells. A. MKN-45 cells were pretreated for 25 min either with 0.05 mU/ml GOX, or 1 mM nitrite, or a combination of GOX and nitrite. After pretreatment, the cells were subjected to three cycles of washing and were added at increasing concentrations to untreated MKN-45 cells. The percentages of apoptotic cells were determined after 5 h. B. The experiment was performed as described under A, with the modification that pretreatment of tumor cells with 0.05 mU/ml GOX and 1 mM nitrite was also performed in the presence of 2 mM histidine, or 25 μM FeTPPS or 100 μM AEBSF. C. The experiment was performed as described under A, with the modification that histidine, or FeTPPS or AEBSF were added during coculture of GOX/nitrite-pretreated cells and untreated cells. The results show that pretreatment of tumor cells with H2O2 and nitrite, but not with H2O2 or nitrite alone, induces their potential to transmit sensitization for intercellular apoptosis inducing signaling to neighbouring cells that had not been pretreated. This bystander effect becomes visible when a mixed population of pretreated and untreated cells shows more apoptosis induction than to be expected from the percentage of pretreated cells (marked by the dashed line) in the population (A). These data also show a) that the acquisition of the potential to transmit bystander signaling is dependent on singlet oxygen, peroxynitrite and superoxide anions, b) that transmission of bystander signaling requires singlet oxygen and peroxynitrite. Statistical analysis: A: The addition of GOX/nitrite-pretreated tumor cells to untreated tumor cells causes highly significant apoptosis induction (p < 0.001). The percentage of apoptosis induction measured is different from the value theoretically expected (dashed line) in a highly significant mode (p < 0.001), except for zero percent and 100% pretreated cells. B. Inhibition of apoptosis induction by histidine, FeTPPS or AEBSF present during pretreatment of the cells with GOX/nitrite is highly significant (p < 0.001). C: The curves of apoptosis induction obtained for the presence of histidine or FeTPPS during coculture are different from the control curve, as well as from the curve obtained for AEBSF during coculture, in a highly significant way (p < 0.001). The curves obained for histidine or FeTPPS during coculture are not significantly different from the values indicated in the dashed line in Fig. 1A.
Pretreatment of tumor cells with GOX and nitrite, in the presence of the singlet oxygen scavenger histidine, the peroxynitrite decomposition catalyst FeTPPs or the NOX inhibitor AEBSF prevented subsequent apoptosis induction in the pretreated cells, as well as their induction of bystander signaling in neighbouring untreated cells (Fig. 1 B). The presence of histidine or FeTPPS during coculture between GOX/nitrite-pretreated and control cells prevented bystander signaling, but it did not prevent apoptosis induction in the subpopulation of cells within the coculture that had been pretreated with GOX/nitrite (Fig. 1C). As a consequence, apoptosis induction in the presence of histidine or FeTPPS correlated well with the percentage of pretreated cells present in the mixture under these conditions. When AEBSF had been present during coculture, no apoptosis induction was observed. This finding is explained by the dual role of NOX-derived superoxide anions, i. e. a) for the generation of secondary singlet oxygen that is required for bystander signaling, and b) for subsequent intercellular apoptosis-inducing signaling.
The experimental concept shown in Fig. 1 was used throughout the experimental analysis described in this manuscript. When combined with additional inhibitors that were either added during pretreatment or during coculture of pretreated cells and nontreated tumor cells, this approach should allow to elucidate the role of defined ROS and RNS at the various steps in this biochemical scenario. In addition, data obtained from experimental dissection as presented in the preceding manuscript [5] were used for detailed interpretation and counter-control.
3.2. The dynamics of bystander signaling
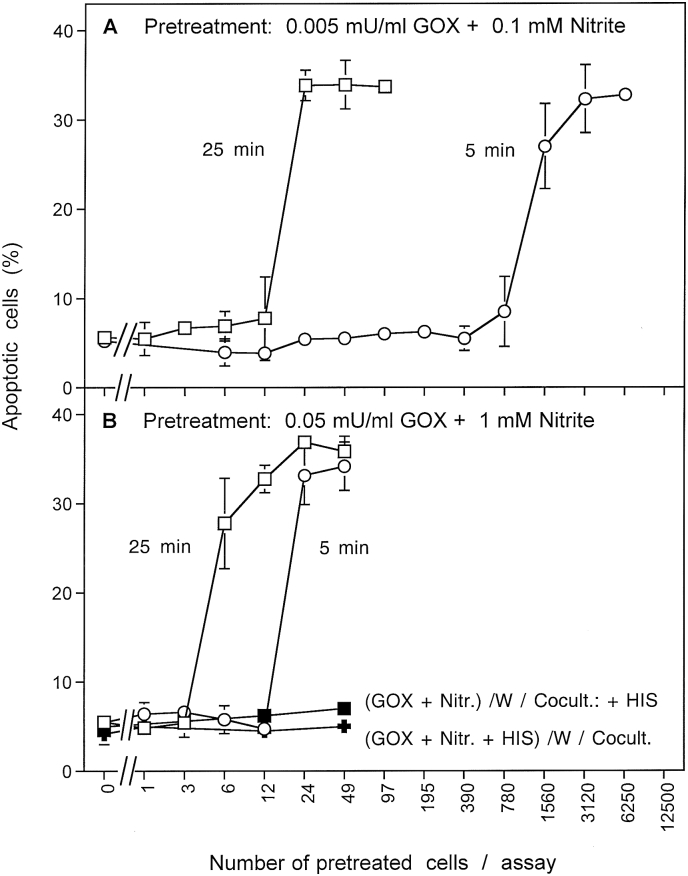
Pretreatment of tumor cells with ten-fold differences in the concentrations of GOX and nitrite, as well as for varying length of time, showed that the induction of the potential to trigger bystander signaling was dependent on the concentration of GOX/nitrite and on time (Fig. 2). Induction of this potential seemed to be a rare effect, which increased with the time or pretreatment. Out of a tumor cell population pretreated with 0.005 mU/ml GOX and 0.1 mM nitrite for 5 min, more than 1500 cells were required to induce detectable bystander signaling in an untreated cell population (Fig. 2 A). When smaller samples of pretreated cells were transfered, there was no chance to communicate the bystander effect, even after long incubation times. This finding indicates that the interaction between nitrite/H2O2 and tumor cells must have lead to a “signature” in pretreated cells that enabled them to transmit bystander signaling to untreated cells. Induction of this signature seemed to be a very rare effect that comprised only a very small subpopulation of cells. When tumor cells were pretreated with 0.005 mU/ml GOX and 0.1 mM nitrite for 25 min, a sample of 24 pretreated cells was sufficient to transmit the bystander effect. This finding shows that a five-fold increase in the time of pretreatment had caused a more than 60 fold increase in the efficiency to transmit the bystander effect. This finding indicates that the obviously rare effect induced by H2O2 and nitrite must have been supplemented by a fast and efficient secondary process that is exponential rather than linear with response to time. For an increase of efficiency of the initial processes, the concentrations of GOX and nitrite were increased to 0.05 mU/ml GOX/1 mM nitrite. 24 cells out of the cell population that had been pretreated with this tenfold higher concentrations of GOX/nitrite for 5 min were then sufficient for induction of bystander signaling in a population of 12 500 untreated cells. This number was reduced to 6–12 cells when pretreatment had been extended to 25 min.
Fig. 2.
Bystander signaling is highly dynamic. MKN-45 cells were either pretreated with 0.005 mU/ml GOX plus 0.1 mM nitrite (A) or with 0.05 mU/ml GOX plus 1 mM nitrite (B) for 5 or 25 min. After three cycles of washing, the cells were mixed in increasing numbers with untreated tumor cells. The percentages of apoptotic cells were determined after 4 h. The results show that the proportion of cells within a population that can induce bystander signaling can be determined in a quantitative way. It is dependent on the concentrations of GOX and nitrite and on time of GOX/nitrite treatment. Statistical analysis: Apoptosis induction by all four modes of treatment is highly significant (p < 0.001), as are the differences in the dependencies of number of pretreated cells and apoptosis induction.
These findings indicate that the initial effects initiated by H2O2 and nitrite are rare, but that spreading from sensitized cells to their neighbours must be a fast process. They also show conclusively, that very few cells with the signature for bystander signaling are sufficient to efficiently transmit their effect into an untreated cell population. This dynamic process is exciting and experimentally challenging.
3.3. The role of NADPH oxidase-1 (NOX1) for bystander signaling
For the elucidation of the role of NOX1 for bystander signaling, as well as apoptosis induction of tumor cells, MKN-45 carcinoma cells were pretreated either with irrelevant control siRNA (“siCo”) or with siRNA directed towards NOX1 (“siNOX1”). Knockdown was complete after 24 h, as determined by control experiments in which extracellular superoxide anion production was titrated by SOD (see Materials and Methods).
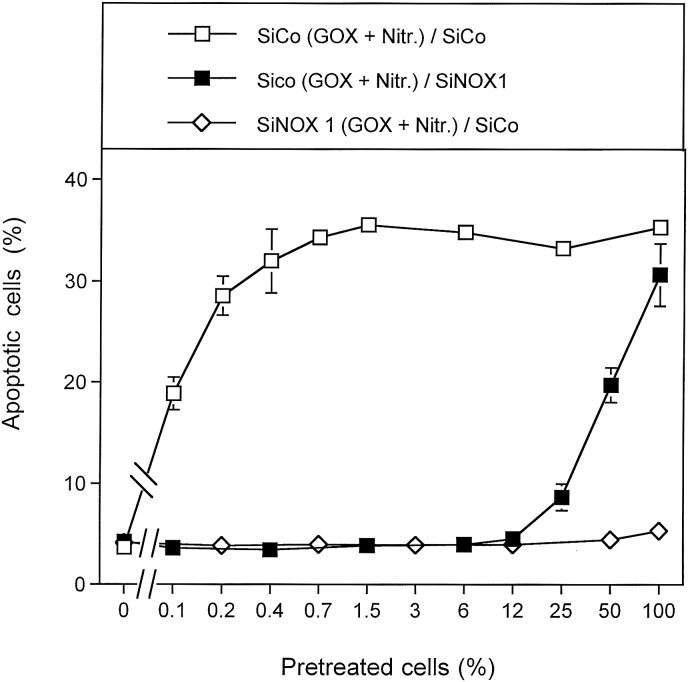
“SiCO” as well as “siNOX1” cells were pretreated with GOX plus nitrite, and were then mixed at increasing concentrations to either “siCo” or “siNOX1” cells that had not been pretreated. Fig. 3 shows that pretreatment of siCo cells with GOX and nitrite, and subsequent mixing with untreated siCo cells, resulted in massive bystander signaling. This can be deduced from the strong increase in apoptosis induction in mixtures between untreated cells and very low concentrations of pretreated cells. SiNOX1 cells that had been pretreated with GOX and nitrite did not transmit bystander signaling. They also showed no apoptosis induction themselves. This finding confirms that establishment of the bystander inducing potential, as well as intercellular ROS-dependent apoptosis-inducing signaling depend on superoxide anions generated by NOX1. When siCo cells had been pretreated with GOX and nitrite and were then mixed with siNOX cells, no response to bystander signaling was observed. However the population of pretreated cells (with functional NOX1) showed apoptosis induction, as the percentages of apoptotic cells correlated directly to the percentages of pretreated cells in the population This finding indicates that NOX1-deficient cells cannot respond to bystander signaling.
Fig. 3.
The stringent requirement of active NOX1 for bystander signaling. MKN-45 cells were transfected with 24 nM control siRNA (siCo) or siRNA directed towards NOX1 (siNOX1) for 24 h and the knockdown of NOX1 was confirmed. SiCo-transfected cells were treated with 0.05 mU/ml GOX/1 mM nitrite for 25 min and then washed by three cycles. They were added either to untreated siCO cells (open squares) or to untreated siNOX1 cells (closed squares). In addition, GOX/nitrite-treated SiNOX1 cells were added to untreated siNOX1 cells (open diamonds). The percentages of apoptotic cells were determined after 4.5 h. The results show that reception as well as induction of bystander signaling requires the expression of NOX1. Statistical analysis: The differences between the three curves (percentage of apoptotic cells induced and dependencies on the percentage of pretreated cells) are highly significant (p < 0.001).
3.4. Elucidation of the mechanisms underlying bystander signaling through defined inhibitors and scavengers
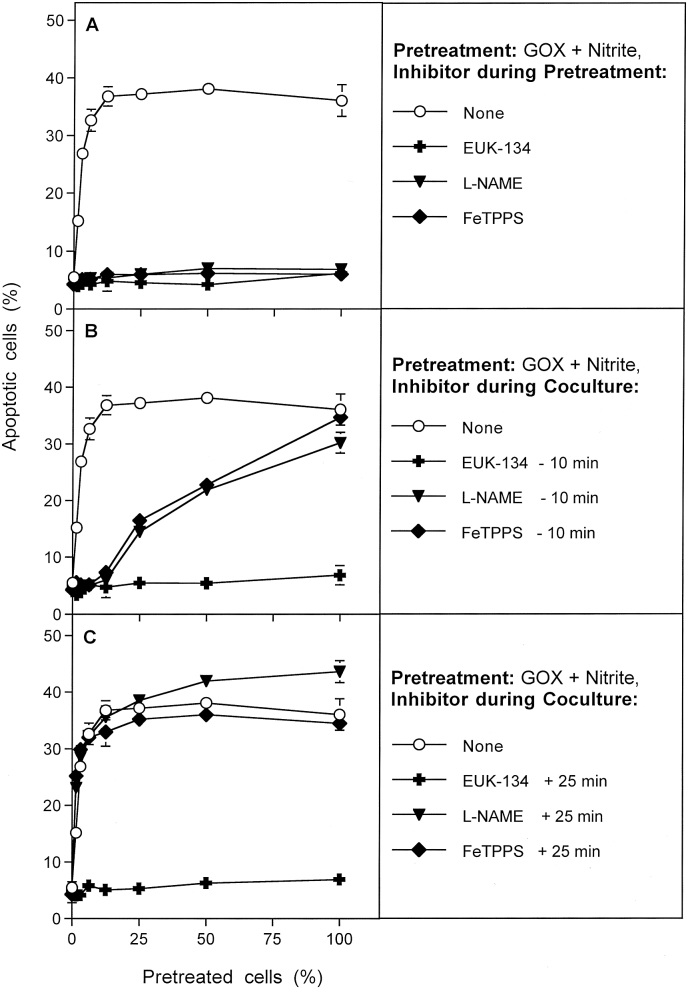
Pretreatment of MKN-45 cells with GOX and nitrite in the presence of either the catalase mimetic EUK-134, the NOS inhibitor l-NAME or the peroxynitrite decomposition catalyst FeTPPS prevented apoptosis induction in the cells. It also prevented their potential to transmit sensitizing bystander signaling to cells that had not been pretreated (Fig. 4 A). When tumor cells had been pretreated with GOX and nitrite in the absence of inhibitors, and then the induction of bystander signaling was tested in cocultures, removal of H2O2 by EUK-134 during coculture completely prevented a subsequent apoptotic response (Fig. 4B). Even if EUK-134 had been added 25 min after beginning of the coculture (Fig. 4C), this strong negative effect was still maintained. This indicates that H2O2 was also involved in a late signaling step (Fig. 4C). l-NAME and FeTPPS added at the beginning of coculture prevented transmission of the bystander effect, but allowed apoptosis induction in the pretreated cells (Fig. 4 B). This finding demonstrates that NO and peroxynitrite were required for transmission of bystander signaling, but are not required for intercellular apoptosis-inducing signaling under these conditions. As l-NAME and FeTPPS had no more inhibitory effect when they were added 25 min after beginning of coculture (Fig. 4C), the process of bystander transmission in which NO and peroxynitrite are involved seems to be fast and completed within this short time span.
Fig. 4.
The role of H2O2, NO and peroxynitrite for bystander signaling. MKN-45 cells were pretreated with 0.05 mU/ml GOX and 1 mM nitrite for 25 min, subjected to three cycles of washing and then resuspended in fresh medium. Pretreated cells were added at increasing percentages to untreated tumor cells. A. During pretreatment with GOX/nitrite, additional assays received either 20 μM EUK-134, or 2.4 mM l-NAME or 25 μM FeTPPS. B. During coculture of GOX/nitrite-pretreated cells with untreated cells, EUK-134, or l-NAME or FeTPPS was present. The inhibitors had been added to the cells 10 min before mixing the populations. C. The experiment was performed as described under B, with the modification that the inhibitors were added 25 min after the beginning of coculture. In all assays, the percentages of apoptotic cells were determined at 4.5 h. The results show that acquisition of bystander effect-inducing potential through treatment with GOX/nitrite involves H2O2, NOS-derived NO and peroxynitrite. The data also show that NO and peroxynitrite play a dominant role for bystander signaling during the first 25 min, but not later. H2O2 plays a role at all times, as it is involved in several steps, including late HOCl signaling. Statistical analysis: A. The effects of all three inhibitors are highly significant (p < 0.001). B. The effects of all three inhibitors, as well as the difference between inhibition by EUK-134 versus inhibition by l-NAME or FeTPPS are highly significant (p < 0.001). C. Only the inhibitory effect of EUK-134 is highly significant (p < 0.001).
The HOCl scavenger taurine did not prevent induction of the bystander-inducing potential of GOX/nitrite pretreated cells (Fig. 5 A), but completely prevented apoptosis induction when it was present during the coculture phase (Fig. 5 B). Thereby the complete inhibitory effect was visible, even if taurine had been added 25 min after the beginning of coculture (Fig. 5 C). The latter finding indicates that HOCl is crucial for the reactivated intercellular apoptosis-inducing signaling. As the presence of taurine specifically during the pretreatment phase had not effect on subsequent apoptosis induction (Fig. 5 A), complete removal through the washing step was assured.
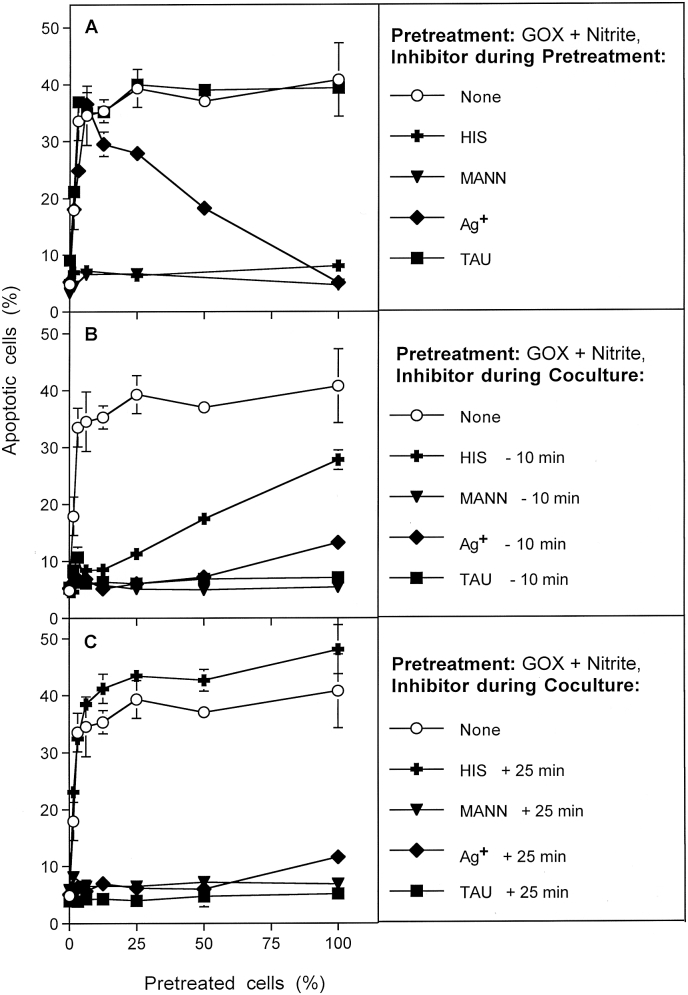
Fig. 5.
Determination of the potential roles of singlet oxygen, hydroxyl radicals, aquaporins and HOCl for bystander signaling. MKN-45 cells were pretreated with 0.05 mU/ml GOX and 1 mM nitrite for 25 min, subjected to three cycles of washing and then resuspended in fresh medium. Pretreated cells were added at increasing percentages to untreated tumor cells. A. During pretreatment with GOX/nitrite, additional assays received 2 mM histidine, 20 mM mannitol, 5 μM Ag+ or 50 mM taurine. B. During coculture of GOX/nitrite-pretreated cells with untreated cells, histidine, mannitol, Ag+ or FeTPPS were also present. The inhibitors had been added to the cells 10 min before mixing the populations. C. The experiment was performed as described under B, with the modification that the inhibitors had been added 25 min after the beginning of coculture. In all assays, the percentages of apoptotic cells were determined at 4 h. These results show that singlet oxygen plays a central role for activating the bystander effect-inducing cells and within the first 25 min of coculture for transmission of bystander signaling. Hydroxyl radicals seem to play a role in early and late steps, in line with multiple roles in this scenario. Aquaporins play no role for activation and transmission bystander signaling, but seem to play a role for allowance of intercellular apoptosis-inducing signaling. HOCl plays no role for activating bystander signaling and its transmission, but are of central importance for apoptosis inducing signaling. Statistical analysis: A: Inhibition by histidine and mannitol is highly significant (p < 0.001), whereas taurine causes no significant inhibition. Ag+ does not cause significant inhibition up to 25% pretreated cells, but causes highly significant inhibition (p < 0.001) at higher percentages of pretreated cells. B: All inhibitors caused highly significant inhibition (p < 0.001). The differences seen for the histidine-mediated inhibition curve compared to the curves obtained with the other inhibitors are highly significant (p < 0.001). C. All inhibitors cause highly significant inhibition (p < 0.001), except for histidine.
The patterns of inhibition and the assessment of action are more difficult for irreversible inhibitors. The aquaporine inhibitor Ag+ causes irreversible inhibition of aquaporins and thus prevented apoptosis induction through intercellular signaling. Therefore, 100% of cells pretreated with GOX and nitrite in the presence of the Ag+ (Fig. 5 A) did not undergo apoptosis induction. With decreasing concentrations of GOX/nitrite/Ag+-pretreated cells in the population, this negative effect was decreased as well. It was no longer observed when less than 10% of pretreated cells had been added. However, this low concentration of pretreated cells still caused a strong bystander effect that was not distinguishable from the control curve for bystander induction. This finding clearly shows, that aquaporins are not required for sensitization of tumor cells for transmission of bystander signaling and for the transmission process itself. However, they are necessary for the final intercellular apoptosis-inducing signaling. Therefore, the presence of Ag+ during the coculture phase (Fig. 5 B, C) completely prevented apoptosis induction.
The hydroxyl radical scavenger mannitol blocked both the sensitizing step of GOX/nitrite-pretreated cells, as well as the effects occuring early and late during coculture (Fig. 5A–C). The understanding of this complex action of mannitol at several steps requires to memorize that mannitol inhibits catalase inactivation as well as intercellular apoptosis-inducing HOCl signaling [5]. In contrast, the singlet oxygen scavenger histidine inhibited sensitization during pretreatment as well as the fast initial step during coculture, but was not inhibitory when added 25 min after beginning of the coculture.
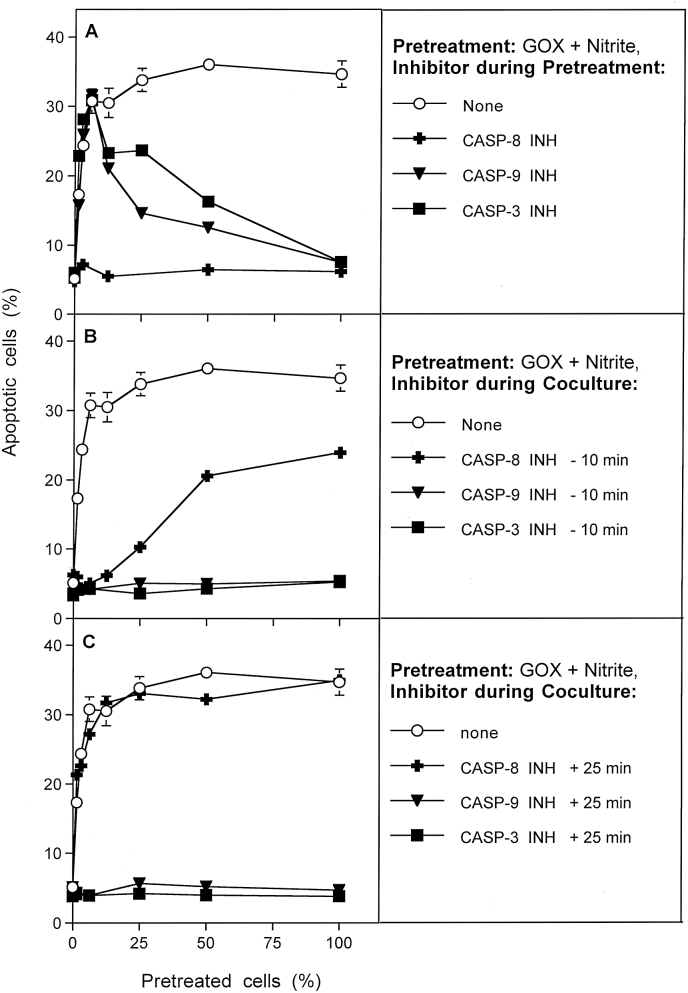
Caspase inhibitors cause irreversible inhibition of their respective targets. Therefore, the inhibition profile of inhibitors for caspase-3 and caspase-9 shown in Fig. 6 is analogous to the profile shown for aquaporin inhibitor Ag+ in the preceding Figure. This indicates that caspases-3 and -9 are not required for sensitization of GOX/nitrite-pretreated cells and for transmission of the bystander effect, but are required for the processes that occur during the late stage of coculture. This picture is contrasted by the inhibitor profile of caspase-8 inhibitor (Fig. 6A–C). Caspase-8 seems to be strictly required for sensitization of pretreated cells and for transmission of bystander signaling between pretreated and untreated cells, but not for later execution of apoptosis.
Fig. 6.
Determination of the potential roles of caspases-3, -8, -9 for bystander signaling. MKN-45 cells were pretreated with 0.05 mU/ml GOX and 1 mM nitrite for 25 min, subjected to three cycles of washing and then resuspended in fresh medium. Pretreated cells were added at increasing percentages to untreated tumor cells. In all assays, the percentages of apoptotic cells were determined at 4 h . A. During pretreatment with GOX/nitrite, additional assays received 50 μM caspase-3 inhibitor, 25 μM caspase-8 inhibitor or 25 μM caspase-9 inhibitor. B. During coculture of GOX/nitrite-pretreated cells with untreated cells, the indicated caspase inhibitors were also present. The inhibitors had been added to the cells 10
min before mixing the populations. C. The experiment was performed as described under B, with the modification that the caspase inhibitors had been added 25 min after the beginning of coculture. In all assays, the percentages of apoptotic cells were determined at 4 h. The results show that caspase-8 is required for activating bystander-inducing signaling and during the first 25 min of coculture. Caspase-8 is not involved in apoptosis signaling itself, whereas the role of caspases-3 and -9 seems to be restricted to the execution of apoptosis. Statistical analysis: A. Inhibition by caspase-8 inhibitor is highly significant (p < 0.001). Inhibition by caspase-9 and caspase-3 inhibitor is highly significant (p < 0.001) when more than 25% pretreated cells had been applied. B: The effect of all inhibitors, as well as the differences between the effect of caspase-8 inhibitors and the other two inhibitors are highly significant (p < 0.001). C. The effects of caspase-3 and caspase-9 inhibitor are highly significant (p < 0.001), whereas caspase-8 inhibitor does not cause a significant effect.
The inhibitor data presented in the preceding manuscript [5], as well as the data obtained during the analysis of bystander signaling in this manuscript, are best explained by a sequence of biochemical events consisting of.
-
a)
initial singlet oxygen generation through the interaction between H2O2 and nitrite from CAP or PAM,
-
b)
local inactivation of membrane-associated catalase,
-
c)
multiple subsequent autoamplificatory rounds of singlet oxygen generation through the reaction of tumor cell-derived H2O2 and peroxynitrite that are no longer under the control by membrane-associated catalase.
-
d)
reactivation of intercellular ROS-mediated apoptosis inducing signaling after sufficient inactivation of catalase and after aquaporin-mediated intrusion of H2O2 into the cells.
This mechanistic model was fully confirmed through experiments in which a portable air plasma ‘corona pen’ plasma source (developed in the laboratory of Dr. Z. Machala, Comenius University, Bratislava, Slovakia) or plasma-activated medium obtained through application of this source had been applied (Bauer et al., manuscript submitted).
3.5. Differentiation between the effects of primary and secondary singlet oxygen
However, the analysis so far had no potential to unequivocally dissect between the obviously rare effect of singlet oxygen generation by PAM and CAP and the dominating subsequent and continuous singlet oxygen generation by the target tumor cells themselves. This is due to the fact that both processes seem to follow the same reactions in their final part, but start with different sources for H2O2 and peroxynitrite. A closer look at the biochemical scenario (please see Fig. 16) opens a chance for discrimination of the two reactions, despite their massive difference with respect to abundance: Singlet oxygen generation by PAM or CAP starts with H2O2 from the plasma source that reacts with nitrite and generates peroxynitrite [35]. The interaction between H2O2 and peroxynitrite then allows for singlet oxygen generation. In this scenario for singlet oxygen generation, NOX1-derived superoxide anions are not required. In contrast, secondary singlet oxygen generation by the tumor cells is based on the generation of H2O2 and peroxynitrite by two processes, which are driven by NOX1-derived superoxide anions. These are dismutation of superoxide anions to H2O2 and formation of peroxynitrite through the reaction of superoxide anions with NOS-derived NO. Therefore, in the presence of the NOX1 inhibitor AEBSF, generation of secondary singlet oxygen should be completely prevented and the effect of primary singlet oxygen derived from CAP or PAM should be exclusively manifested in a corresponding signature of the cells.
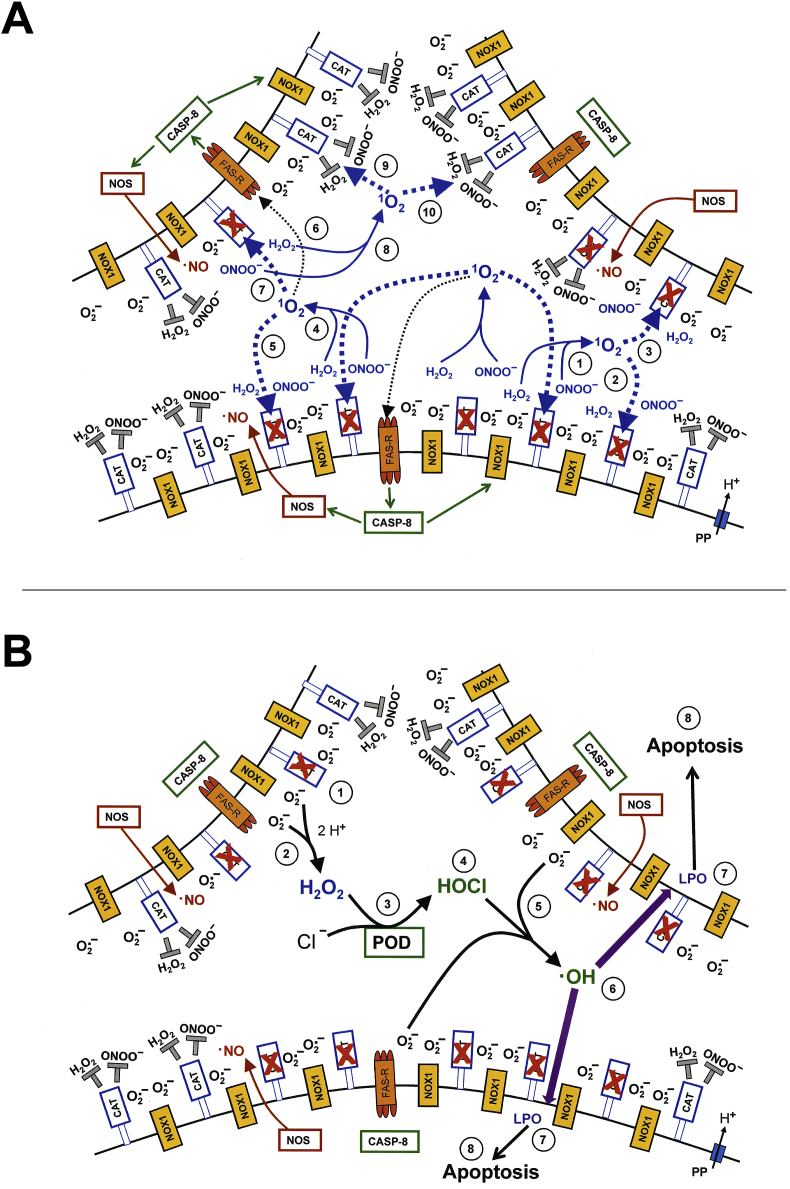
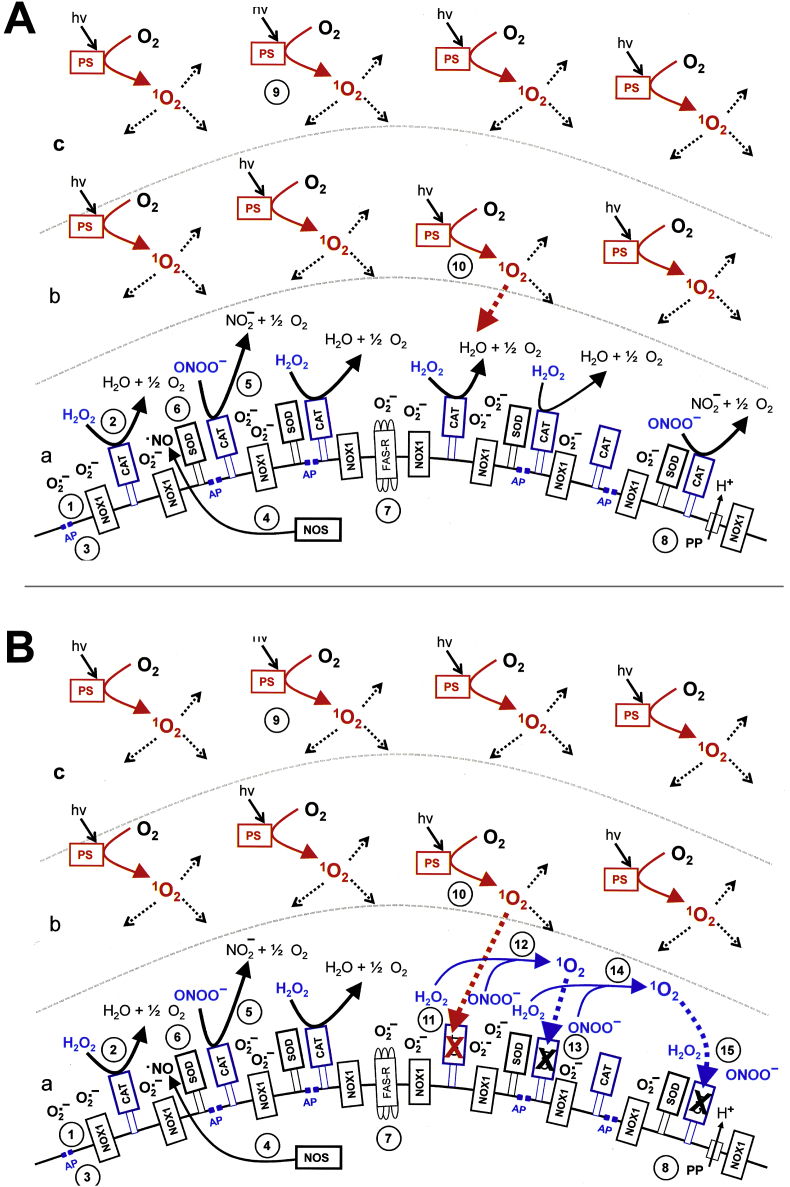
Fig. 16.
Primary and secondary singlet oxygen triggered by H2O2 and nitrite in contact with tumor cells. A. NADPH oxidase 1 (NOX1) is expressed in the membrane of malignant cells and generates extracellular superoxide anions (#1). NO synthase (NOS) (#2) generates NO which can be either oxidated by NO dioxygenase (NOD) (#3) or pass through the cell membrane. Membrane-associated catalase (#4) protects tumor cells towards intercellular ROS/RNS-mediated signaling through decomposition of H2O2 and peroxynitrite, as well as oxidation of NO (not shown in the figure). Comodulatory SOD (#5) is required to prevent superoxide anion-mediated inhibition of catalase. Further important elements in the membrane are the FAS receptor (#6), Dual oxidase (DUOX) (#7), from which a peroxidase (POD) domain is split through matrix metalloprotease (MMP), proton pumps (#8) and aquaporins (#9). The interaction between H2O2 and nitrite (#10) leads to the generation of primary singlet oxygen (#11 - #19). Primary singlet oxygen (#19) causes local inactivation of catalase. As H2O2 and peroxynitrite are not decomposed at the site of inactivated catalase, these two species interact and generate secondary singlet oxygen (reactions # 20- #25). Secondary singlet oxygen can inactivate further catalase molecules (#26) and in this way promote autoamplificatory generation of secondary singlet oxygen/catalase inactivation, or activate the FAS receptor (#27), which leads to an enhancement of the activities of NOX1 and NOS (#28, 29). This enhancement is necessary for efficient generation of secondary singlet oxygen. B. Inhibition of NOX1 by AEBSF prevents the generation of secondary singlet oxygen and thus allows to study the generation of primary singlet oxygen and the induction of its signature specifically.
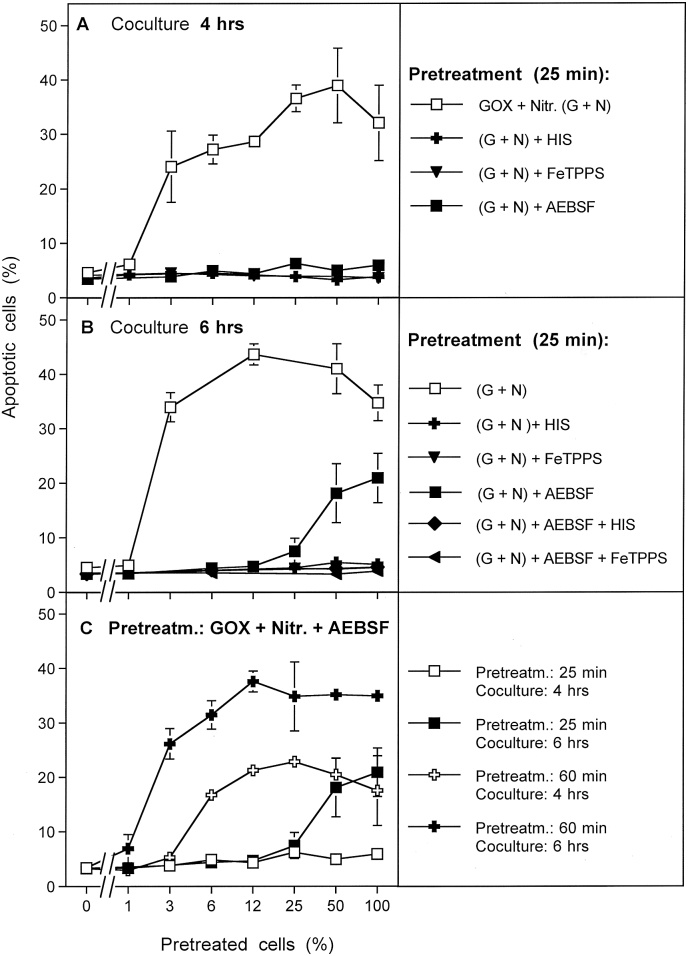
Fig. 7A shows that the bystander effect-inducing potential of MKN-45 cells that had been pretreated with GOX and nitrite for 25 min was completely abolished when the pretreatment had been performed in the presence of AEBSF, histidine or FeTPPS. Inhibition by histidine and FeTPPS are indicative for singlet oxygen generated by H2O2 and nitrite, with peroxynitrite as intermediate. The complete inhibition by AEBSF might either indicate that PAM or CAP-derived primary singlet oxygen does not cause a stable induction of bystander inducing potential in the absence of NOX1 activity, or that, for kinetic reasons, the effect is too low to be detected after 4 h of coculture. The latter assumption seems to be true, as analysis of apoptosis induction in the coculture at 6 h (Fig. 7 B) rather than the previous 4 h allowed to detect a discrete, though significant effect of apoptosis induction in assays that contained 100 or 50% of cells that had been pretreated with GOX/nitrite in the presence of AEBSF. This effect was completely inhibited when either histidine or FeTPPS had been present during pretreatment in parallel to AEBSF. The inhibition by histidine confirmed that singlet oxygen was involved in this process. The inhibition by FeTPPS indicated the intermediate role of peroxynitrite in singlet oxygen generation. As AEBSF prevented cellular peroxynitrite formation through the interaction between NOX-derived superoxide anions and NOS-derived NO, peroxynitrite involved in singlet oxygen generation in the presence of AEBSF must have been generated through interaction between nitrite and GOX-derived H2O2.
Fig. 7.
The signature for bystander signaling induced by primary singlet oxygen. A, B. MKN-45 cells were treated with 0.05 mU/ml GOX and 1 mM nitrite for 25 min in the absence of inhibitors or in the presence of 2 mM histidine, 25 μM FeTPPs, 100 μM AEBSF or the indicated combinations. After three cycles of washing, the cells were added at increasing percentages to untreated tumor cells. The percentages of apoptotic cells were determined after 4 h (A) or 6 h (B) of coculture. C. MKN-45 cells were pretreated with 0.05 mU/ml GOX and 1 mM nitrite either for 25 min or 60 min. The cells were subjected to three cycles of washing and were added at increasing percentages to untreated tumor cells. The percentages of apoptotic cells were determined after 4 h or 6 h, as indicated in the legend. These results show that activation of bystander effect-inducing potential is mostly due to the signature imprinted to cells by secondary singlet oxygen. However, the signature of primary singlet oxygen can be determined when secondary singlet oxygen generation is blocked by AEBSF-mediated inhibition of NOX1, and when the time of coculture and/or the time of pretreatment are extended. Statistical analysis: A. Apoptosis induction in the control assay and the effects of all inhibitors are highly significant (p < 0.001). B. Apoptosis induction in the control assay (G+N) and the control assay plus AEBSF “(G+ N) + AEBSF” is highly significant (p < 0.001). All inhibitors cause highly significant inhibition (p < 0.001). C. The differences between the curves are highly significant (p < 0.001).
Fig. 7C shows that extension of the time of pretreatment of cells with GOX/nitrite in the presence of AEBSF from 25 to 60 min enhanced the induction of bystander effect-inducing potential of primary singlet oxygen. Extension of the time point of analysis thereby improved the detectability of bystander signaling.
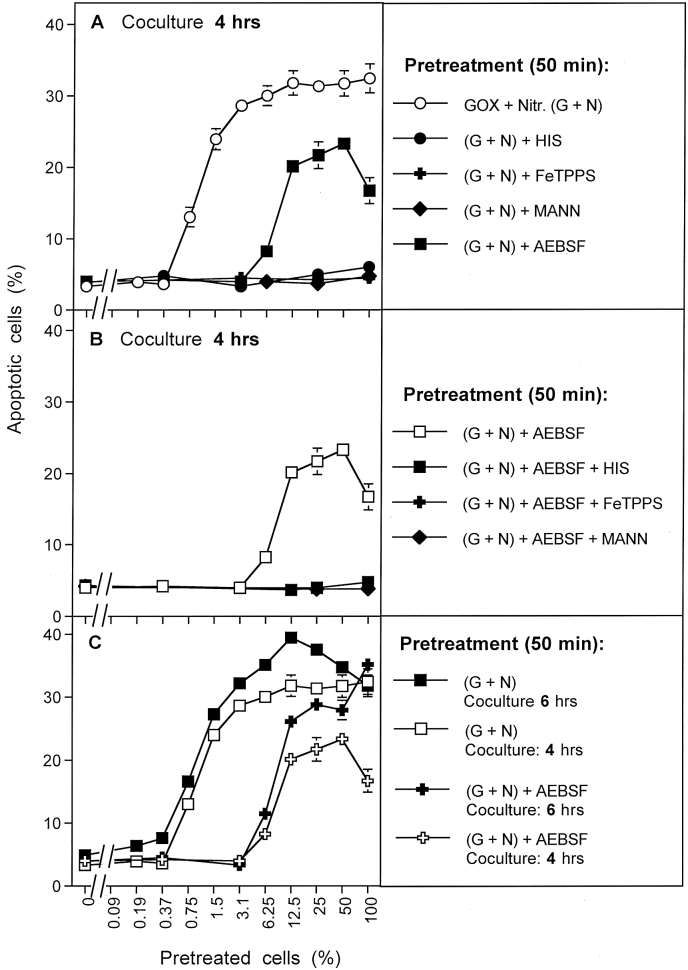
The induction of bystander effect-inducing potential of pretreatment with GOX and nitrite in the presence and absence of AEBSF was studied in more detail in the next experiment. When tumor cells had been pretreated with GOX and nitrite for 50 min and cocultured at varying percentages, a strong bystander effect was induced (Fig. 8 A). 0.75% of pretreated cells in coculture with untreated cells already caused a significant bystander-inducing effect. This bystander signaling was completely prevented when pretreatment had been performed in the presence of histidine, FeTPPS or mannitol, pointing to the essential roles of singlet oxygen, peroxynitrite and hydroxyl radicals. The NOX inhibitor AEBSF caused a rightward shift of the curve, thus demonstrating that the cell population pretreated with GOX and nitrite in the presence of AEBSF, i. e. under conditions where secondary singlet oxygen generation is blocked, contained much less bystander effect-inducing cells than the control without inhibitor.
Fig. 8.
Primary singlet oxygen generation depends on H2O2, peroxynitrite and hydroxyl radicals. MKN-45 tumor cells were pretreated with 0.05 mU/ml GOX and 1 mM nitrite for 50 min either without additional inhibitors or in the presence of 2 mM histidine, 25 μM FeTPPS, 20 mM mannitol, 100 μM AEBSF or a combination of AEBSF with either histidine, FeTPPS or mannitol. After three cycles of washing, increasing percentages of pretreated cells were added to untreated cells. The percentages of apoptotic cells were determined after 4 h (A, B) or 4 h and 6 h (C). The results show that prevention of secondary singlet oxygen generation through the presence of AEBSF during pretreatment has a remarkable negative effect on bystander inducing activity of the tumor cells. Nevertheless, 50 min pretreatment allowed to clearly demonstrate the effect of the signature of primary singlet oxygen, i. e. the bystander inducing effect of the cells that had been pretreated in the presence of AEBSF. Establishment of the signature was dependent on singlet oxygen, peroxynitrite, hydroxyl radicals, H2O2 and nitrite. Statistical analysis: A, B: All inhibitor effects are highly significant (p < 0.001). C: The effect of AEBSF is highly significant (p < 0.001).
The cells that show bystander-inducing effect despite the presence of AEBSF during pretreatment carry the specific signature of action of primary singlet oxygen that must have been generated through the interaction of exogenous nitrite/H2O2 without additional enhancement by tumor cell specific NOX1. As shown in Fig. 8 B, the establishment of this signature also required the action of singlet oxygen, peroxynitrite and hydroxyl radicals, as the bystander inducing effect achieved independently of NOX1 (i. e. in the presence of AEBSF) was completely inhibited by histidine, FeTPPS and mannitol. These data show that initial effect of singlet oxygen generated by CAP or PAM and the subsequent enhancing effects of tumor cell derived singlet oxygen can be differentiated through inhibition of NOX. Importantly, the generation of primary as well as secondary singlet oxygen seems to require peroxynitrite and hydroxyl radicals as intermediates, whereas NOX1-derived superoxide anions are only required for generation of secondary singlet oxygen. Finally, Fig. 8C shows that an increase in the incubation time of coculture with a preceding constant time of pretreatment did not increase the bystander effect-inducing potential of tumor cells pretreated in the absence or presence of AEBSF, but caused an increase in overall apoptosis induction. This effect is simply based on kinetics and may facilitate quantitation of apoptosis under certain conditions.
When MKN-45 cells were pretreated with GOX and nitrite in the presence of AEBSF for 40 min, a bystander effect-inducing cell population with the signature of primary singlet oxygen action was generated, indicated by the rightward shift compared to the control induction curve (Fig. 9 A). When the cell population that had been pretreated with GOX, nitrite and AEBSF for 40 min was washed and then further incubated for 20 min in the absence of AEBSF, GOX and nitrite, it subsequently showed a strongly enhanced potential to induce bystander effects when it had been fractionated and added to untreated tumor cells (Fig. 9B). This enhancement can be attributed to the generation and spread of secondary singlet oxygen after removal of AEBSF. This secondary singlet oxygen was generated through peroxynitrite/H2O2 that depended on NOX1 activity and that also involved hydroxyl radicals, as it was inhibited by AEBSF, FeTPPS, histidine and mannitol.
Fig. 9.
The signature of primary singlet oxygen allows for generation of secondary singlet oxygen after release of NOX1 inhibition. A. MKN-45 cells were pretreated with 0.05 mU/ml GOX and 1 mM nitrite, in the absence or presence of 100 μM AEBSF for 40 min. After three washing cycles, the cells were added at increasing percentages to untreated MKN-45 cells. B. MKN-45 cells were pretreated with GOX and nitrite for 40 min in the presence of 100 μM AEBSF. The cells were washed in three cycles and then resuspended in fresh medium. Further incubation for 25 min was performed either in the absence of inhibitors (open square), 2 mM histidine (closed diamond), 100 μM AEBSF (closed square), 25 μM FeTPPS (closed cross) or 20 mM mannitol (closed triangle). The cells were washed in three cycles and added at increasing concentrations to untreated cells. C. The experiment was performed as described under A, with the exception that pretreatment with GOX/nitrite was carried out with cells at a density of 3000 cells/100 μl instead of 12 500 cells/100 μl. The percentages of apoptotic cells were determined at 4.5 h after beginning of the coculture. The results shown under A confirm that inhibition of NOX1 allows the signature of primary singlet oxygen. The cells imprinted by primary singlet oxygen alone show a strongly reduced potential to induce bystander signaling compared to a control population that also allows secondary singlet generation. The result shown in B demonstrates that tumor cells with the specific signature by primary singlet oxygen acquire a much higher bystander effect inducing potential after the inhibition of NOX1 is abrogated and the cells are allowed to interact for 25 min. This interaction results in the generation of secondary singlet oxygen, with superoxide anions, peroxynitrite and hydroxyl radicals as intermediates. Part C shows that the signature of primary singlet oxygen is independent of cell density, whereas subsequent secondary singlet oxygen generation is dependent. Statistical analysis: A: Apoptosis induction and the differences between the curves are highly significant (p < 0.001). B:The effects of all four inhibitors is highly significant (p < 0.001) up to a percentage of pretreated cells of 3.1%. There is no significant difference between the effects of individual inhibitors. C. Apoptosis induction is highly significant (p < 0.001), but AEBSF does not cause significant inhibition.
When pretreatment of tumor cells with GOX and nitrite was performed at a cell density that was fourfold lower than the standard cell density, subsequent coculture of these cells with untreated tumor cells at standard density caused bystander signaling that was much lower than that observed under standard conditions (Fig. 9C). 6.2% of pretreated cells (i. e. 775 cells) were necessary for induction of significant bystander signaling under these conditions, in contrast to 0.2% (i. e. 25 cells) under standard conditions. Importantly, the effect of pretreatment at low cell density was not affected by the presence of AEBSF. This indicates that treatment of cells with GOX/nitrite at low cell density does not allow the generation of secondary singlet oxygen and therefore merely reflects the effects derived from the action of primary singlet oxygen that is derived from the interaction of the long-lived species of CAP and PAM. Taken together, these findings show that the action of primary singlet oxygen derived from the interaction between nitrite and H2O2 seems to be independent of the cell density, wherease the generation and spread of secondary singlet oxygen is strictly dependent on optimal cell density.
3.6. Bystander signaling induced by a defined source of singlet oxygen and by modulators of the NO metabolism
In order to determine the validity of the method underlying this analysis and to determine its robustness, it was tested whether it is applicable to other protocols for induction of secondary generation of singlet oxygen by tumor cells. Application of a direct singlet oxygen generator [31] as well as application of modulators of NO metabolism [18,32] were chosen for these control experiments.
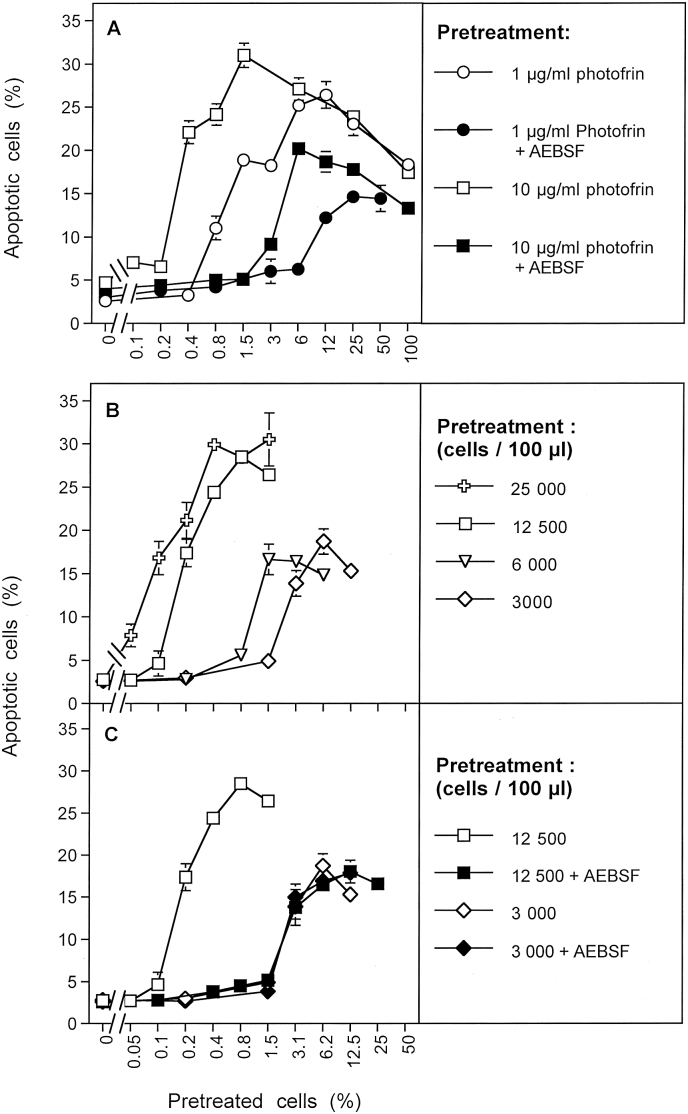
Following a previous study [31], application of a direct singlet oxygen generator was predicted to cause secondary singlet oxygen generation like the application of the CAP or PAM-derived species H2O2 and nitrite, and thus finally led to the reactivation of apoptosis-inducing ROS signaling. However, in the case of the singlet oxygen generator the generation of primary singlet oxygen should be completely independent of H2O2 and peroxynitrite (please see Fig. 17 for details). When MKN-45 cells were illuminated in the presence of different concentrations of the established singlet oxygen generator photofrin, bystander effect-inducing potential was detectable in subsequent mixing experiments (Fig. 10 A). The strength of the effect was dependent on the concentration of photofrin applied. When the treatment with photofrin was performed in the presence of the NOX1 inhibitor AEBSF, the effect was strongly reduced. This finding confirms that photofrin-derived singlet oxygen can cause stable induction of bystander inducing signaling under conditions where NOX1-driven generation of secondary singlet oxygen is prevented. However, the full effect of treatment with the photosensitizer depends on the additional generation of secondary singlet oxygen. When photofrin treatment was applied to MKN-45 cells at different densities, the bystander inducing potential of the treated cell population was dependent on the cell density (Fig. 10 B). Treatment of tumor cells at a density of 12 500 cells/100 μl assay or 3000 cells/100 μl assay, both in the absence and presence of AEBSF showed that the bystander inducing potential of the cells treated at low density was not affected by the presence of AEBSF, whereas that of the higher cell population was strongly affected (Fig. 10C). These findings indicate that cells at low density can receive the bystander inducing signature from an exogenous singlet oxygen source, but do not increase their inducing potential through generation of secondary singlet oxygen, due to lack of cell density-dependent effects. In contrast, in a cell population of higher density, the primary effect of singlet oxygen derived from the photosensitizer is enhanced by the generation of secondary singlet oxygen.
Fig. 17.
Primary and secondary singlet oxygen triggered by the singlet oxygen generator photofrin in contact with tumor cells. A. Illuminated photofrin generates primary singlet oxygen (#1) that causes local inactivation of catalase (#2). This allows for the generation of secondary singlet oxygen through reactions # 3 - #8, followed by autoamplification of secondary singlet oxygen and catalase inactivation. B. The action and signature of primary singlet oxygen can be specifically studied when the generation of secondary singlet oxygen is inhibited by AEBSF. Please note that only histidine can inhibit the signature of primary singlet oxygen from photofrin, whereas the signature of primary singlet oxygen generated through interaction between H2O2 and nitrite (Fig. 16) can be inhibited by histidine, FeTPPS, EUK-134 and mannitol.
Fig. 10.
The signature of primary singlet oxygen directly generated by a photosensitizer. A. MKN-45 tumor cells received 1 μg/ml or 10 μg/ml photofrin, in the absence or presence of 100 μM AEBSF. The cells were illuminated with visible light for 20 min and were then subjected to three cycles of washing. Increasing percentages of pretreated cells were added to untreated cells and further incubated. The percentages of apoptotic cells were determined 4 h after the beginning of the coculture. B, C. Cells at the indicated densities were pretreated with 10 μg/ml photofrin, 20 min light, in the absence or presence of 100 μM AEBSF, washed in three cycles and added at increasing percentages to untreated cells. All assays had a density of 12 500 cells/100 μl assays during coculture. The percentages of apoptotic cells were determined after 5 h. The results from A show that the bystander inducing potential of directly applied singlet oxygen from a photosensitizer depends on the signature by the primary singlet oxygen plus secondary singlet oxygen. The signature of secondary singlet oxygen can be prevented by AEBSF. Part B and C shows that imprinting the signature of primary singlet oxygen is independent of cell density, whereas the induction of bystander effect inducing potential by secondary singlet oxygen requires a high cell density. Statistical analysis: A: The differences between the groups with respect to dependence on the percentage of pretreated cells are highly significant (p < 0.001). B: The differences between the two groups of curves are highly significant (p < 0.001). C: The differences between the assays containing 12 500 cells and the other three assays are highly significant (p < 0.001).
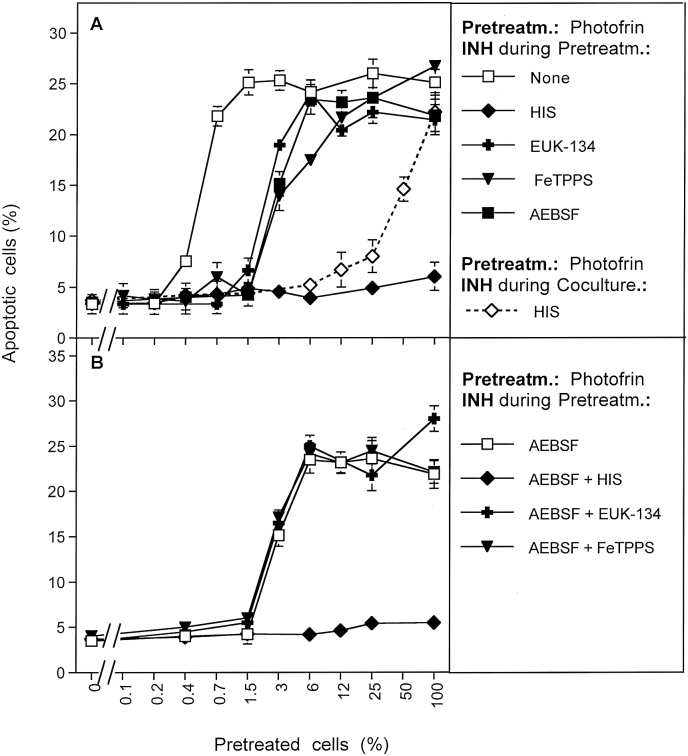
The use of further inhibitors in this analytical system showed that induction of bystander effect-inducing potential by photofrin was completely inhibited by the singlet oxygen scavenger histidine, thus confirming the central role of singlet oxygen as well as the scavenging potential of histidine (Fig. 11 A). Scavenging peroxynitrite (by FeTPPS) or H2O2 (by the catalase mimetic EUK-134) caused the same degree of inhibition of bystander signaling as application of the NOX inhibitor AEBSF (Fig. 11 A). This finding is in agreement with the conclusion that the initial effect of primary singlet oxygen on bystander signaling was independent of cell-derived superoxide anions, H2O2 and peroxynitrite, whereas the generation of secondary singlet oxygen required these compounds. As shown in Fig. 11 B, the inhibitory effect of AEBSF was not further enhanced by the parallel presence of either EUK-134 or FeTPPS. This indicates that each of these inhibitor caused optimal inhibition of the same process, i. e. the generation of secondary singlet oxygen. The residual bystander effect-inducing potential in the presence of AEBSF, EUK-134 and FeTPPS reflects the direct effect of photofrin-derived singlet oxygen, which is independent on H2O2 and peroxynitrite, but inhibited by the singlet oxygen scavenger histidine.
Fig. 11.
Imprinting the signature of primary singlet oxygen from a photosensitizer is independent of peroxynitrite and H2O2. MKN-45 cells were treated with 5 μg/ml photofrin plus light for 20 min in the absence of inhibitors or in the presence of 2 mM histidine, 25 μM EUK-134, 25 μM FeTPPS, 100 μM AEBSF or a combination of AEBSF + histidine, AEBSF + EUK-134, AEBSF + FeTPPS. The cells were subjected to three cycles of washing and added to untreated tumor cells at increasing percentages. The percentages of apoptotic cells were determined 4 h after beginning of the coculture. In addition, cells that had been pretreated with photofrin in the absence of inhibitors were added to untreated cells and the coculture was performed in the presence of histidine (dashed lind with diamond). This result confirms the differentiation between the action of primary and secondary singlet oxygen after treatment with the singlet oxygen generator photofrin. It also shows that imprinting the signature of primary singlet oxygen can only be inhibited by histidine, but not by EUK-134 or FeTPPS. This finding is expected and confirms the significance of the inhibitors used in our studies. This finding is contrasted by imprinting the signature of primary singlet oxygen generated through the interaction between H2O2 and nitrite, as shown in preceding figures. These reactions are dependent on H2O2 and peroxynitrite. Statistical analysis: A: The inhibitory effects of EUK-134, FeTPPS and AEBSF are highly significant (p < 0.001) and without significant variation among themselves. Inhibition by this group of inhibitors is highly significantly (p < 0.001) different from inhibition by histidine, which is highly significant by itself (p < 0.001). B: Only AEBSF causes highly significant inhibition (p < 0.001).
An increase in the concentration of tumor cell-generated NO has been recently shown to trigger autoamplification of singlet oxygen generation, catalase inactivation and reactivation of intercellular ROS/RNS-driven apoptosis-inducing signaling [18,31,32]. This NO-driven process is starting with NO-dependent, reversible inhibition of catalase. Local NO-mediated inhibition of catalase is then the basis for the generation of tumor cell-derived singlet oxygen through the interaction between free cell-derived H2O2 and peroxynitrite (please see Fig. 18 for detail). The generation of both compounds requires active NOX1. Therefore, singlet oxygen-dependent processes after modulation of the NO concentrations should start with secondary singlet oxygen generated by free H2O2 and peroxynitrite of the tumor cells. As NO-mediated inhibition of catalase is reversible and therefore cannot trigger bystander inducing potential per se, it is predictable, that induction of bystander inducing potential after an increase in the NO concentration should be strictly dependent on singlet oxygen. The generation of this singlet oxygen should be based on NOX1-generated superoxide anions, H2O2 and peroxynitrite. Fig. 12 shows that pretreatment of tumor cells with the arginase inhibitor NOR-NOHA caused strong induction of bystander effect-inducing potential. This effect was completely blocked when either AEBSF, histidine or FeTPPS had been present during pretreatment of the cells. Even if the assays were monitored after 17 h of coculture between NOR-NOHA-pretreated and untreated cells, rather than 4 h after beginning of coculture, all of these inhibitory effects were still complete. This finding demonstrates that the increase of the NO concentration alone is not sufficient to induce the signature for detectable bystander effect inducing potential. Rather the generation of tumor cell-derived secondary singlet oxygen, based on NOX1-driven generation of tumor cell derived H2O2 and peroxynitrite seems to be necessary. In comparison with the results obtained for the long-lived species from CAP and PAM, as well as the photosensitizer photofrin, this analysis shows that these different treatments are triggering the same effect, i.e. autoamplification of cell-derived singlet oxygen, but differ significantly in their initial biochemical effects. For the control of the bystander effect promoting effect, cocultures of NOR-HOHA pretreated and untreated cells were cocultured in the presence of histidine. Under these conditions, the degree of apoptosis induction correlated with the percentage of pretreated cells, indicating that transmission of bystander signaling had been prevented. These can be well differentiated by the method of bystander signaling analysis. Importantly, the strong and endurant effect of AEBSF in the case of treatment with NOR-NOHA shows that AEBSF can maintain stringent control of NOX1 over long time. Therefore, it is excluded that the positive effects measured for photofrin and CAP/PAM in the presence of AEBSF were artificially due to abortive control by AEBSF.
Fig. 18.
Autoamplificatory cell-dependent singlet oxygen generation after NO-mediated inhibition of catalase. A. The generation of NO is controlled by arginase (#1), NO synthase (NOS) (#2) and NO dioxygenase (NOD) (#3). The reaction between NO and superoxide anions results in the formation of peroxynitrite (#4), which is decomposed by membrane-associated catalase in analogy to H2O2 (#5). Catalase is also oxidating NO, but a local increase in NO may lead to reversible inhibition of catalase (#6). This opens the path for the generation of cell-derived singlet oxygen (“secondary singlet oxygen”) through reactions # 7 - #12 and further inactivation of catalase (#13). In this way, autoamplificatory singlet oxygen generation and catalase inactivation is established. B. When NOX1 is inhibited by AEBSF, singlet oxygen generation is completely blocked. As NO-mediated inhibition of catalase is reversible, treatment of tumor cells with modulators of NO metabolism in the presence of AEBSF does not lead to the induction of signature for bystander inducing potential.
Fig. 12.
Apoptosis induction in tumor cells after modulation of the NO concentration: generation of secondary singlet oxygen without involvement of primary singlet oxygen. MKN-45 cells were pretreated with 50 μM of the arginase inhibitor NOR-NOHA for 50 min, either in the absence of further inhibitors or in the presence of 100 μM AEBSF, 2 mM histidine or 25 μM FeTPPS. The cells were subjected to three cycles of washing and were then added to untreated cells at increasing percentages. The cocultures were cultivated for 4 h or 17 h before the percentages of apoptotic cells were determined. In some assays (dashed line, diamond) cells that had been pretreated with NOR-NOHA in the absence of further inhibitors were cocultivated with untreated cells in the presence of histidine. These results show that tumor cells pretreated with the arginase inhibitor showed impressive bystander inducing potential through a singlet oxygen-dependent process. The activation of the bystander inducing potential of the cell population through modulation of the NO concentration was solely dependent on the generation of secondary singlet oxygen generated by the cells. Effects of primary singlet oxygen were not observed and also not expected, as initial local inhibition of catalase is mediated by NO in this experimental approach. Statistical analysis: Apoptosis induction in the control curves and the action of all inhibitors were highly significant (p < 0.001).
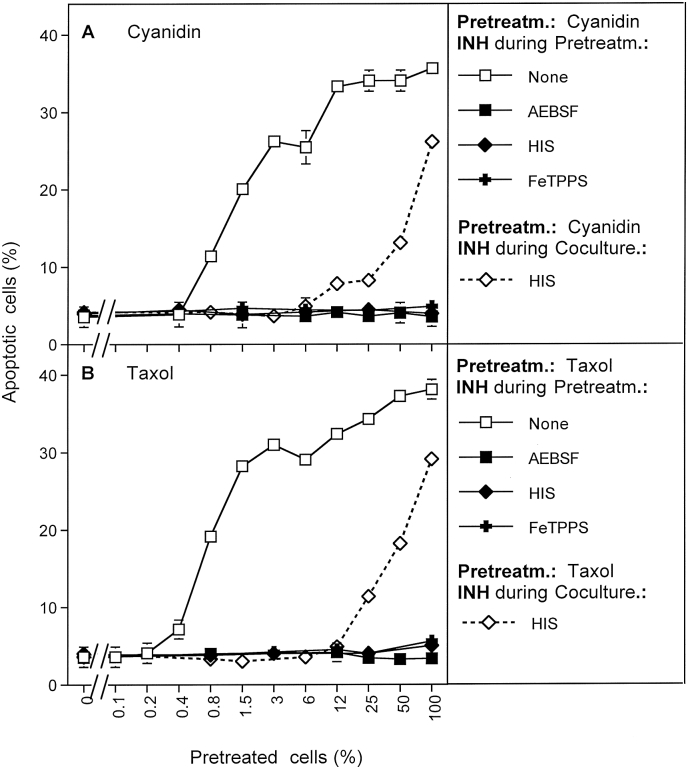
An increase in the endogenous NO concentration of tumor cells with its strong impact on initial catalase inhibition, followed by the generation of singlet oxygen and further catalase inhibition can also be achieved by inhibition of NO dioxygenase (NOD). If our concept and its experimental analysis are right, the treatment of tumor cells with NOD inhibitors should result in analogous bystander inducing effect as shown for the arginase inhibitor NOR-NOHA. To falsify or verify this prediction, MKN-45 cells were pretreated with the NOD inhibitors cyanidin (Fig. 13 A) or taxol (Fig. 13 B) and the bystander inducing potential was analyzed in the absence or presence of inhibitors. The obtained results show that in both cases, establishment of bystander effect-inducing potential stringently required superoxide anions, singlet oxygen, and peroxynitrite, indicating that singlet oxygen generation was completely dependent on NOX1 of tumor cells. This was in perfect agreement with the results established for the arginase inhibitor NOR-NOHA. For a final check of conclusiveness of the data obtained with this analytical system, MKN-45 cells pretreated with, cyanidin or taxol were added at increasing percentages to untreated tumor cells in the presence of histidine. As shown in Fig. 13 A, B, transmission of the bystander effect was dependent on singlet oxygen. Therefore, in the presence of histidine, only the pretreated cells seemed to contribute to apoptosis induction, without any indication of transmission of bystander signaling.
Fig. 13.
Apoptosis induction through modulation of the NO concentration: the use of inhibitors of NO dioxygenase (NOD). The experiment was performed in exact analogy to the experiment described in Fig. 12, with the exception that the cellular NO concentration was modulated by either 1 μg/ml cyanidin or 1 μg/ml taxol instead of NOR-NOHA. Cyanidin and taxol inhibit NO dioxygenase, and thus raise the NO level of tumor cells [18,32]. The results confirm the validity of methods for the discrimination of primary and secondary singlet oxygen signatures. Both NO-dependent approaches did not indicate the action of primary singlet oxygen, but demonstrated the strong potential of cell-derived secondary singlet oxygen. Statistical analysis: Apoptosis induction in the control curves and the action of all inhibitors were highly significant (p < 0.001).
4. Discussion
H2O2 and nitrite are two long-lived species derived from CAP and found in PAM. The study described in the preceding manuscript [5] allows to conclude that their interaction leads to the generation of primary singlet oxygen which causes local inactivation of membrane-associated catalase of tumor cells. This supposedly relatively rare effect triggers a sustained generation of secondary singlet oxygen by the tumor cells, based on the activity of their membrane-associated NOX1 and intracellular NOS, as well as on the lack of decomposition of H2O2 and peroxynitrite at the site of the inactivated catalase molecule. As a result, membrane-associated catalase is inactivated by secondary singlet oxygen to a degree that allows for HOCl synthesis and lipid peroxidation through the HOCl signaling pathway. This is followed by execution of the mitochondrial pathway of apoposis.
This manuscript extends this concept and presents data that show that tumor cells that are treated with H2O2 and nitrite also have the potential to transmit singlet oxygen generation and catalase inactivation to untreated neighbouring tumor cells, but not to nonmalignant cells. This potential was predictable from the previously established model [5]. Therefore its direct demonstration here adds further evidence to the validity of our model, which explains the selective antitumor action of long-lived ROS/RNS from plasma and plasma-activated medium. It also may be concluded that bystander inducing signaling towards neighbouring tumor cells mimics the biochemical effects that are relevant for spreading of singlet oxygen-mediated catalase inactivation on the membrane of tumor cells that are originally hit by singlet oxygen derived from CAP or PAM.
The strength of the analytical method presented in this paper is to dissect primary and secondary singlet oxygen generation precisely and in an quantitative mode. This is an essential improvement of the previous evaluation of the underlying processes.
This transmission between pretreated and untreated tumor cells occurs in a “bystander signaling”-like intercellular signaling mechanism. The induction of the potential to transmit bystander signaling is shown to be identical to the mechanism of catalase inactivation, as it is dependent on the combined action of H2O2 and nitrite, is mediated by 1O2, and shows the same inhibitor profile as H2O2/nitrite-mediated inactivation of membrane-associated catalase. These are histidine, AEBSF, l-NAME, FeTPPS, mannitol and caspase-8 inhibitor, pointing to the roles of singlet oxygen, NOX1-derived superoxide anions, NOS-derived NO, peroxynitrite, hydroxyl radicals and caspase-8. HOCl signaling, aquaporine function or the activity of caspase-3 and -9 are not essential for this particular primary step. These data show that induction of the potential to transmit bystander signaling in an optimal mode already requires the action of primary and secondary singlet oxygen on the same cell. Catalase inactivation is required for this step, as free H2O2 and peroxynitrite are necessary to drive singlet oxygen generation.
In line with these conclusions, tumor cells with siRNA-mediated knockdown of their membrane-associated catalase have previously been shown to efficiently transmit singlet oxygen-dependent bystander signaling to control tumor cells [36]. Furthermore, bystander signaling by tumor cells that had been pretreated with the singlet oxygen donor photofrin caused bystander signaling towards their untreated neighbouring tumor cells that resulted in catalase inactivation within the total cell population and was driven by the generation of secondary singlet oxygen [31]. This previous approach was repeated in this study as positive control system. It allowed to monitor the relatively low efficiency of action of primary singlet oxygen, followed by the high efficiency of secondary singlet oxygen generation.
Induction of the bystander inducing potential is a fast process that is completed within less than half an hour in vitro, provided that secondary singlet oxygen generation is allowed to follow the action of primary singlet oxygen. This is in good agreement with completion of catalase inactivation within the same period of time.
Therefore it is not unexpected, that the subsequent transmission of bystander signaling in the total cell population is a fast process as well. It is based on secondary singlet oxygen whose generation is based on NOX1-derived superoxide anions, NOS-derived NO, and the resultant peroxynitrite, as well as hydroxyl radicals as intermediates, according to the reaction schemes previously established. Thereby, cells that induce bystander signaling as well as recipient cells for bystander signaling are required to express active NOX1. The high speed of bystander transmission is demonstrated by the finding that this process can only be inhibited by the singlet oxygen scavenger histidine or the peroxnitrite decomposition catalyst FeTPPS when these compounds are present during the first 25 min after mixing pretreated with untreated tumor cells. This fast transmission of bystander signaling is followed by HOCl signaling that leads to cell death that is mediatedthrough the mitochondrial pathway of apoptosis and executed by caspases-3 and 9.
Fig. 14, Fig. 15 summarize the essential steps of bystander signaling after treatment of tumor cells with H2O2/nitrite. This summary is based on the data from the studies presented in this and the preceding manuscript [5]. The remarkable points deduced from this scenario are the following:
-
●
The potential to transmit bystander signaling as well as the potential to respond to it is strictly connected to the transformed state, characterized by the strong expression of membrane-asociated NOX1 and generation of extracellular superoxide anions. Therefore, bystander signaling is the essential basis of the selective action of H2O2/nitrite-initiated apoptosis induction in malignant cells.
-
●
Membrane-associated catalase, the hallmark of tumor cells [16,17], is the central target for primary and secondary singlet oxygen. Inactivation of catalase is the basis for the sustained generation of secondary singlet oxygen, which is driven by NOX1 and NOS.
-
●
The generation of primary singlet oxygen through the interaction between nitrite and H2O2 is a relatively inefficient effect and only hits a minority of cells within a population of tumor cells. Further propagation of the biological effect seems to require the generation of secondary singlet oxygen on the originally hit cells, followed by bystander signaling within the cell population. This ensures spreading or secondary singlet oxygen generation, catalase inactivation and apoptosis-inducing HOCl signaling in the whole cell population.
4.1. Rare action of primary singlet oxygen
Despite the partial mechanistic overlap between the generation of primary and secondary singlet oxygen, the action specifically induced by primary singlet oxygen can be determined when the generation of secondary singlet oxygen is blocked by the NOX inhibitor AEBSF or the NOS inhibitor l-NAME (Fig. 16). Under standard conditions of the experiments as presented in the preceding manuscript [5], the effect of primary singlet oxygen on catalase inactivation (as determined by induction of sensitivity towards an exogenous challenge with peroxynitrite) where not detectable.
The approach of analysis of bystander signaling, as worked out in this manuscript, however, allowed to detect the biochemical effects of primary singlet oxygen, though the effects seemed to be very rare. When tumor cells were pretreated with H2O2/nitrite in the presence of AEBSF, the generation of secondary singlet oxygen was prevented and only singlet oxygen generation through H2O2/nitrite interaction was possible. Under these conditions, a very large proportion of the pretreated cell population had to be transfered to untreated cells in order to establish bystander signaling. In contrast, allowance of generation of secondary singlet oxygen after H2O2/nitrite application established bystander inducing potential very efficiently. Under these conditions about 10 cells out of the pretreated population were sufficient to induce bystander signaling in an untreated population. This comparison determines that imprinting the signature of primary singlet oxygen is a very rare process, whereas spreading within the cell population by secondary singlet oxygen is highly efficient.
Experimental modifications allowed to increase the effects of primary singlet oxygen and to focus on this particular aspect. Treatment of tumor cells with H2O2/nitrite in the presence of AEBSF and for longer times than under standard conditions increased the bystander effect inducing signature of the cells. This approach also allowed to confirm that imprinting this signature was based on singlet oxygen generation initiated by H2O2/nitrite interaction.
This finding confirms that the initial effect of CAP/PAM-derived species is only to trigger the biochemical switchboard of tumor cells to generate secondary singlet oxygen in a sustained mode and thus to lead to catalase inactivation and reactivation of intercellular apoptosis-inducing HOCl signaling.
Substitution of H2O2/nitrite-dependent generation of primary singlet oxygen by direct application of singlet oxygen (generated by an illuminated photosensitizer) or by a pulse of NO, allowed to study the analogous generation of secondary singlet oxygen after these initial treatments.
When the singlet oxygen generator photofrin was applied to tumor cells, the effects of the primary singlet oxygen derived from the source also required the induction of secondary singlet oxygen by the tumor cells in order to establish the full bystander effect, leading to tumor cell apoptosis (Fig. 17). This approach confirms the role of primary singlet oxygen as a trigger, and also ensures the validity of the inhibitor data, as the photofrin-mediated effect in the presence of AEBSF was not dependent on H2O2 and peroxynitrite, in contrast to the generation of primary singlet oxygen through interaction between nitrite and H2O2 (Fig. 16).
Finally, the general applicability of bystander signaling of tumor cells was shown for tumor cells after enhanced NO availability (Fig. 18). In this case, no primary singlet oxygen was required to trigger the generation of secondary singlet oxygen, as the process was initiated by the reversible inhibition of catalase by NO.
Importantly, secondary singlet oxygen generation seemed to be the driving force for CAP/PAM-, photosensitizer- and NO-dependent antitumor effects in vitro.
This finding implies that primary singlet oxygen at concentrations that do not harm nonmalignant tissue will provoke a strong apoptotic response when it meets responsive target cells, i. e. tumor cells with high expression of NOX1 and catalase.
These tumor cells do the major job for their selfdestruction through the generation of secondary singlet oxygen, driven by NOX1 and NOS, leading to glutathione depletion, driven by NOX1 and aquaporins and finally establishment of ROS signaling, driven by NOX1 and peroxidase or NOX1 and NOS. The understanding of these processes in vitro and in vivo provides a unique chance to determine potential synergistic effects that are based on established chemical biology of ROS/RNS and that may possible improve therapeutic applications of CAP and PAM.
The relatively rare effects of primary singlet oxygen derived from a photosensitizer or generated through the interaction between nitrite and H2O2 is only astonishing on the first sight. A closer look to the steric situation in the vicinity of tumor cells explains the background and the specific needs of the action of primary singlet oxygen.
As shown in Fig. 19, singlet oxygen generated by a photosensitizer most likely reacts with compounds in the supernatant of the cells, due to the high reactivity of singlet oxygen and the extreme short free diffusion path length and life time associated with that. Therefore, only few molecules will actually hit catalase on the surface of tumor cells and in this way trigger a sustained effect through induction of secondary singlet oxygen generation.
Fig. 19.
Singlet oxygen interaction with tumor cells. This figure analyzes redox reactions in close vicinity of the membrane of tumor cells (zone a: low nanometer range), in the high nanometer/low micrometer range (zone b) and in the medium and high micrometer range (zone c). A. NADPH oxidase (NOX1) in the membrane of tumor cells generates extracellular superoxide anions (#1). The resultant H2O2 is decomposed by membrane-associated catalase (#2) and thus the influx of H2O2 into the cells through aquaporins (AP) is prevented (#3). NO synthase (NOS) (#4) generates NO that passes the cell membrane. Peroxynitrite resulting from NO/superoxide anion interaction is decomposed by catalase (#5). Membrane-associated SOD prevents superoxide anion-dependent inhibition of catalase (#6). The FAS receptor (#7) and proton pumps (PP) (#8) are other essential membrane-associated elements. Illumination of a photosensitizer (PS) in the supernatant of tumor cells leads to considerable concentrations of singlet oxygen (#9). However, due to the high reactivity of singlet oxygen and its short life time and diffusion path length, only rare cases of direct interaction of primary singlet oxygen with the cells can be expected. B. At the few sites where catalase is inactivated by primary singlet oxygen (#11), the interaction between free H2O2 and peroxynitrite can now generate secondary singlet oxygen (#12), which induces further rounds of autoamplification of secondary singlet oxygen generation and catalase inactivation. The full complexity of H2O2/peroxynitrite interaction is shown in Fig. 16.
The action of the combination of H2O2 and nitrite is even more complex (Fig. 20).
Fig. 20.
Generation and action of primary singlet oxygen derived from the interaction between H2O2 and nitrite: rare effects with high triggering potential. This figure analyzes redox reactions in close vicinity of the membrane of tumor cells (zone a: low nanometer range), in the high nanometer/low micrometer range (zone b) and in the medium and high micrometer range (zone c). A. NADPH oxidase (NOX1) in the membrane of tumor cells generates extracellular superoxide anions (#1). The resultant H2O2 is decomposed by membrane-associated catalase (#2) and thus the influx of H2O2 into the cells through aquaporins (AP) is prevented (#3). NO synthase (NOS) (#4) generates NO that passes the cell membrane. Peroxynitrite resulting from NO/superoxide anion interaction is decomposed by catalase (#5). Membrane-associated SOD prevents superoxide anion-dependent inhibition of catalase (#6). The FAS receptor (#7) and proton pumps (PP) (#8) are other essential membrane-associated elements. CAP or PAM-derived H2O2 and nitrite distant of the cell membrane (#9, #10) have a good chance to generate peroxnitrite (#11) that has a high chance to react with CO2 (#12). The subsequent reactions #13 - #17 may generate singlet oxygen (#18). However, singlet oxygen generated at that distance from the cells has no chance to reach the cells. H2O2 and peroxynitrite generated from CAP/PAM (#19, #20) that come close to zone a may be decomposed by tumor cell catalase. When the reaction between H2O2 and nitrite (#21), resulting in the formation of peroxynitrite is sufficiently distant of the membrane to avoid decomposition of H2O2 and peroxynitrite, but sufficiently close to allow protonation of peroxynitrite (#22) by proton pump-derived protons, reactions #23-#26 have a chance to generate primary singlet oxygen (#27). B. Singlet oxygen (#1) generated in zone a has a chance to inactivate catalase (#2) and to trigger autoamplification of secondary singlet oxygen generation and catalase inactivation (#3 - # 6). It is obvious that the inactivation of catalase by primary singlet oxygen must be a very rare effect, though with a high triggering potential for secondary singlet oxygen generation.
H2O2 derived from CAP and PAM, as well as peroxynitrite generated through H2O2/nitrite interaction will be decomposed by membrane-associated catalase of tumor cells, as soon as these molecules approach the cell membrane. This will lead to a constant decrease in their concentration. Peroxynitrite, generated distant of the membrane has a much higher chance to interact with CO2 (reaction # 4) than to get protonated. Though there is a theoretical chance to subsequently generate singlet oxygen through reactions # 5–10, this singlet oxygen has a much higher chance to react with medium compounds rather than to reach catalase on the membrane of tumor cells. Peroxynitrite that is generated in closer vicinity to the cell membrane has a chance to get decomposed by catalase or to get protonated through the activity of membrane-associated proton pumps (reaction # 14). If singlet oxygen generated through reactions # 15–19 actually reaches catalase, it may cause inactivation of one molecule of catalase (Fig. 20 B, reaction #1). As a result, a sustained generation of secondary singlet oxygen, driven by NOX1 and NOS will cause amplification of catalase inactivation on the same cell and on neighbouring cells through the bystander signaling described in this manuscript. In this way a rare effect of CAP/PAM constituents will selectively trigger a process that maintains selectivity for tumor cells, shows autoamplificatory potential and finaly leads to apoptosis induction through HOCl signaling which also is selectively acting on malignant cells.
The analysis of bystander signaling allowed for specific analysis of primary and secondary singlet oxygen generation and offered the chance to focus on details of this signaling system with higher analytical sensitivity. The mechanistic overlap in the pathways leading to primary and seconary singlet oxygen was a great challenge for the initial study of CAP and PAM action. The present analysis, utilizing the transfer of bystander effect inducing potential allowed to clearly confirm that the central and final part of the mechanism of generation of primary and secondary singlet oxygen utilizes the same key elements, i. e. H2O2, peroxynitrite and hydroxyl radicals. The results from Fig. 8 B confirms that these key molecules are involved in the generation of primary singlet oxygen, whereas Fig. 9 B ensures that the generation of secondary singlet oxygen by cells that had been imprinted with the action of primary singlet oxygen before, requires exactly the same key compounds. These findings are in good agreement with the conclusion that both pathways follow the scheme summarized in Fig. 16 A.
The efficiency and speed of bystander signaling in a tumor cell population after treatment with the long-lived species H2O2 and nitrite offers a conclusive explanation for the frequently made observation that treatment of tumors with plasma results in biological effects also at sites that had not been directly hit by initial plasma treatment [[37], [38], [39], [40]]. This spread of a specific CAP or PAM-mediated biological effect can be expected to be further maintained and enhanced by subsequent immunological antitumor effects that are triggered by the immunogenic cell death of plasma-treated cells [19,[41], [42], [43], [44], [45], [46]].
The results in this manuscript confirm that singlet oxygen, generated through the reaction between the long-lived CAP- and PAM-derived components nitrite and H2O2, is merely the trigger for the activation of autoamplificatory mechanisms of tumor cells, whereas the tumor cells efficiently propagate their cell death through their own ROS/RNS signaling potential [5]. They also allow to quantify the involvement of defined species at defined steps in this process and thus to demonstrate the highly dynamic nature of this process.
The combination of nitrite and H2O2 in a defined concentration range is sufficient for selective apoptosis induction in tumor cells, characterizing a prominent signaling pathway for PAM- and CAP-mediated antitumor effects. These findings do, however, not exclude that treatment with PAM or CAP, instead of mere application of nitrite and H2O2, might have beneficial effects on tumor treatment.
Based on a quantitative comparison of the effects induced by PAM with those mediated by a combination of nitrite and H2O2, Kurake et al. [2] already concluded that PAM should contain additional effectors to induce tumor cell death. These effectors have not yet been defined, so far. As PAM does no longer contain short-lived species derived from CAP, the reaction products between certain biomolecules (particularly lipids) and certain short-lived CAP-derived ROS/RNS might inherit direct signaling functions or interact synergistically with the signaling pathway established by nitrite and H2O2. These hypothetical signaling molecules might affect apoptosis induction and/or the quality of immunogenic cell death beyond the effects caused by singlet oxygen derived from nitrite/H2O2 interaction. These aspects deserve further experimental analysis.
CAP contains molecular species that may cause direct inactivation of tumor cell catalase, such as singlet oxygen [[29], [30], [31],36] and ozone [47,48]. Furthermore, CAP-derived ozone might generate singlet oxygen after its reaction with amino acids [49,50]. The conceivable reaction of the short-lived CAP-derived species NO2 with CAP-derived superoxide anions or hydroperoxyl radicals may lead to the generation of peroxynitrate and peroxynitric acid. These interaction may finally result in singlet oxygen generation through decomposition of peroxynitrate [19,36]. Furthermore, peroxynitrite directly derived from CAP might enhance the formation of singlet oxygen generation through its interaction with H2O2, thereby avoiding the relatively slow formation of peroxynitrite through the interaction between H2O2 and nitrite. The role of chloride-dependent species in Cap also deserves attention, as discussed in Ref. [5]. The reason, why all these appealing potential CAP effects on tumor cells seem to much less effective than the relatively rare effects based on the interaction of nitrite and H2O2 [Bauer et al., submitted for publication] is very simple: the high reactivity of the compounds involved seems to prevent that they reach their target cells through a layer of medium in vitro and potentially also through a layer of biological material in vivo. It is conceivable that efforts to modulate the composition of CAP (e. g. increase its content in singlet oxygen or other defined compounds), combined with biophysical research aiming at an increased accessibility of tumor cells by CAP in vivo, may further increase the efficiency of tumor treatment with plasma. These approaches have a good chance to be more effective than direct application of the minimally required chemicals, e. g. nitrite and H2O2. Theoretical considerations, based on experimental data [19,36], suggest that the proposed “optimized direct CAP action” as outlined above, may most likely be also mediated by the singlet oxygen-dependent autoamplification process, with a major contribution by the tumor cells themselves, as described here after application of the pure chemicals. The knowledge about the underlying signaling chemistry, based on our studies, might then allow to rationally define the right dosage that causes optimal elimination of tumor cells, while sparing nonmalignant tissue. It also might become the basis for the establishment of valuable synergistic effects for enhancement of antitumor effects at low doses of CAP or PAM.
Acknowledgements
Intellectual support by many colleagues during IWPCT 2016, 2017, 2018, ICPM-7 and the Gordon Research Conference on Plasma Processing Science 2018 was of central importance for this work and is gratefully acknowledged.
I thank J. Brandel (Freiburg) for technical support.
Financial support by the University of Freiburg (Germany), Medical Faculty, is acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101301.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yan A., Talbot N., Nourmokammadi X., Cheng J., Canady J., Sherman J.H., Keidar M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015;5 doi: 10.1038/srep18339. 1833901-18339017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurake N., Tanaka H., Ishikawa K., Kondo T., Sekine M., Nakamura K., Kajiyama H., Kikkawa F., Mizuno M., Hori M. Cell survival of glioblastoma grown in medium containing hydrogen peroxide and/or nitrite, or in plasma-activated medium. Arch. Biochem. Biophys. 2016;605:102–108. doi: 10.1016/j.abb.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Girard P.-M., Arbabian A., Fleury M., Bauville G., PuechV, Dutreix M., Sousa J.S. Synergistic effect of H2O2 and NO2─ in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016;6:29098. doi: 10.1038/srep29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchida G., Nakajima A., Takenaka K., Kawasaki T., Koja K., Shiratani M., Setsuhara Y. Effects of nonthermal plasma jet irradiation on the selective production of H2O2 and NO2─ in liquid water. J. Appl. Phys. 2016;120:201102. [Google Scholar]
- 5.Bauer G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019;26:101291. doi: 10.1016/j.redox.2019.101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka H., Ishikawa K., Nakamura K., Kajiyama H., Komo H., Kikkawa T., Hori M. Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Med. 2011;1:265–277. [Google Scholar]
- 7.Utsumi F., Kajiyama H., Nakamura K., Tanaka H., Mizuno M., Ishikawa K., Kondo H., Kano H., Hori M., Kikkawa F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemoresistant ovarian cancer cells in vitro and in vivo. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081576. e8157601-e815760110. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan D., Sherman J.H., Cheng X., Ratovitski E., Canady J., Keidar M. Controlling plasma stimulated media in cancer treatment application. Appl. Phys. Lett. 2014;105:22410101–22410104. [Google Scholar]
- 9.Adachi T., Tanaka H., Nonomura S., Hara H., Kondo S.-I., Hori M. Plasma-activated medium induces A459 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015;79:28–44. doi: 10.1016/j.freeradbiomed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Mohades S., Laroussi M., Sears J., Barekzi N., Razavi H. Evaluation of the effects of a plasma-activated medium on cancer cells. Phys. Plasmas. 2015;22:122001. [Google Scholar]
- 11.Kumar N., Park J.H., Jeon S.N., Park B.S., Chori E.H., Attri P. The action of microsecond -pulsed plasma-activated media on the inactivation of human lung cancer cells. J. Phys. D Appl. Phys. 2016;49:11540101–11540109. [Google Scholar]
- 12.Yan D., Nourmohammadi N., Bian K., Murad F., Sherman J.H., Keidar M. Stabilizing the cold plasma-stimulated medium by regulating medium's composition. Sci. Rep. 2016;6:2602. doi: 10.1038/srep26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koensgen D., Besic I., Gumbel D., Kaul A., Weiss M., Diesing K., Kramer A., Bekeschus S., Mustea A., Stope M.B. Cold atmospheric plasma (CAP) and CAP-stimulated cell culture media suppress ovarian cancer cell growth – a putative treatment option in ovarian cancer therapy. Anticancer Res. 2017;37:6739–6744. doi: 10.21873/anticanres.12133. [DOI] [PubMed] [Google Scholar]
- 14.Heinzelmann S., Bauer G. Multiple protective functions of catalase against intercellular apoptosis-inducing ROS signaling of human tumor cells. Biol. Chem. 2010;391:675–693. doi: 10.1515/BC.2010.068. [DOI] [PubMed] [Google Scholar]
- 15.Böhm B., Heinzelmann S., Motz M., Bauer G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biol. Chem. 2015;396:1339–1356. doi: 10.1515/hsz-2014-0234. [DOI] [PubMed] [Google Scholar]
- 16.Bauer G. Tumor cell protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 2012;32:2599–2624. [PubMed] [Google Scholar]
- 17.Bauer G. Targeting extracellular ROS signaling of tumor cells. Anticancer Res. 2014;34:1467–1482. [PubMed] [Google Scholar]
- 18.Bauer G. Increasing the endogenous NO level causes catalase inactivation and reactivation of intercellular apoptosis signaling specifically in tumor cells. Redox Biol. 2015;6:353–371. doi: 10.1016/j.redox.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer G. Signal amplification by tumor cells: clue to the understanding of the antitumor effects of cold atmospheric plasma and plasma-activated medium. IEEE Trans. Radiat. Plasma. Med. Sci. 2018;2:87–98. [Google Scholar]
- 20.Bauer G. HOCl and the control of oncogenesis. J. Inorg. Biochem. 2018 b;179:10–23. doi: 10.1016/j.jinorgbio.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Bauer G. Targeting the protective catalase of tumor cells with cold atmospheric plasma-treated medium (PAM) Anti Cancer Agents Med. Chem. 2018;18:784–804. doi: 10.2174/1871520617666170801103708. [DOI] [PubMed] [Google Scholar]
- 22.Bauer G. Cold atmospheric plasma and plasma-activated medium: antitumor cell effects with inherent synergistic potential. Plasma Med. 2019 [Google Scholar]
- 23.Yan D.Y., Talbot A., Nourmohammadi N., Sherman J.H., Cheng X.Q., Keidar M. Toward understanding the selective anticancer capacity of cold atmospheric plasma- A model based on aquaporins. Biointerphases. 2015;10 doi: 10.1116/1.4938020. 040801. [DOI] [PubMed] [Google Scholar]
- 24.Yan D., Xiao H., Zhu W., Nourmohammadi N., Zhang L.G., Bian K., Keidar M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D Appl. Phys. 2017;50 055401. [Google Scholar]
- 25.Denicola A.B.A., Freeman B.A., Trujillo M., Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated reactions. Arch. Biochem. Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein S., Czapski G. Formation of peroxynitrate from the reaction of peroxynitrite with CO2: evidence for carbonate radical production. J. Am. Chem. Soc. 1998;120:3458–3463. [Google Scholar]
- 27.Squadrito G.L., Pryor W.A. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto S., Ronsein G.E., Correa T.C., Martinez G.R., Medeiros M.H.G., Di Mascio P.- Direct evidence of singlet molecular oxygen generation form peroxynitrate, a decomposition product of peroxynitrite- Dalton Trans. 2009;29:5720–5729. doi: 10.1039/b905560f. 2009. [DOI] [PubMed] [Google Scholar]
- 29.Escobar J.A., Rubio A., Lissi E.A. SOD and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 1996;20:285–290. doi: 10.1016/0891-5849(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.K., Kwon O.J., Park J.-W. Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie. 2001;83:437–444. doi: 10.1016/s0300-9084(01)01258-5. [DOI] [PubMed] [Google Scholar]
- 31.Riethmüller M., Burger N., Bauer G. Singlet oxygen treatment of tumor cells triggers extracellular singlet oxygen generation, catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Redox Biol. 2015;6:157–168. doi: 10.1016/j.redox.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheit K., Bauer G. Direct and indirect inactivation of tumor cell protective catalase by salicylic acid and anthocyanidins reactivates intercellular ROS signaling and allows for synergistic effects. Carcinogenesis. 2015;36:400–411. doi: 10.1093/carcin/bgv010. [DOI] [PubMed] [Google Scholar]
- 33.Bauer G. Autoamplificatory singlet oxygen generation sensitizes tumor cells for intercellular apoptosis-inducing signaling. Mech. Ageing Dev. 2018;172:59–77. doi: 10.1016/j.mad.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int. J. Radiat. Biol. 2007;83:887–902. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- 35.Lukes P., Dolezalova E., Sisrova I., Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014;23 015019. [Google Scholar]
- 36.Bauer G., Graves D.B. Mechanisms of selective antitumor action of cold atmospheric plasma-derived reactive oxygen and nitrogen species. Plasma Process. Polym. 2016;13:1157–1178. [Google Scholar]
- 37.Graves D.B. Reactive species from cold atmospheric plasma: implications for cancer therapy. Plasma Process. Polym. 2014;11:1120–1127. [Google Scholar]
- 38.Graves D.B. Oxy-nitroso shielding burst model of cold atmospheric plasma therapeutics. Clin. Plasma. Med. 2014;2:38–49. [Google Scholar]
- 39.Keidar M., Walk R., Shashurin A., Srinivasan P., Sandler P., Sandler A., Dasgupta S., Ravi R., Guerrero-Preston R., Trink B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Canc. 2011;105:1295–1301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratovitski E.A., Heng X., Yan D., Sherman J.H., Canady J., Trink B., Keidar M. Anti-Cancer Therapies of 21st century: novel approach to treat human cancers using cold atmospheric plasma. Plasma Process. Polym. 2014;11:1128–1137. [Google Scholar]
- 41.Lin A., Truong B., Patel S., Kaushik N., Choi E.H., Fridman G., Fridman A., Miller V. Nanosecond-pulsed DBD plasma-generated reactive oxygen species trigger immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017;18:966. doi: 10.3390/ijms18050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin A., Truong B., Fridman G., Fridman A., Miller V. Immune cells enhance selectivity of nanosecond-pulsed DBD plasma against tumor cells. Plasma Med. 2017;7:85–96. [Google Scholar]
- 43.Lin A.G., Xiang B., Merlino D.J., Baybutt T.R., Sahu J., Fridman A., Snook A.E., Miller V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2018.1484978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller V., Lin A., Fridman A. Why target immune cells for plasma treatment of cancer. Plasma Chem. Plasma Process. 2016;36:259–268. [Google Scholar]
- 45.Mizuno K., Yonetamari Y., Shirakawa Y., Akiyama T., Ono R. Anti-tumor immune response induced by nanosecond pulsed streamer discharge in mice. J. Phys. D Appl. Phys. 2017;50:12LT01. [Google Scholar]
- 46.Bekeschus S., Mueller A., Miller V., Gaipl U., Weltmann K.-D. Physical plasma elicits immunogenic cancer cell death and mitochondrial singlet oxygen. IEE trans. Radiat. Plasma. Med. Sci. 2018;2:138–147. [Google Scholar]
- 47.Whiteside C., Hassan H.M. Role of oxyradicals in the inactivation of catalase by ozone. Free Radic. Biol. Med. 1988;5:305–312. doi: 10.1016/0891-5849(88)90101-3. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y.-K., Kim S.M.K., Hand S. Ozone-induced inactivation of antioxidant enzymes. Biochimie. 2003;85:947–952. doi: 10.1016/j.biochi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Kanofsky J.R., Sima P. Singlet oxygen production from the reactions of ozone with biological molecules. J. Biol. Chem. 1991;266:9039–9042. [PubMed] [Google Scholar]
- 50.Adam W., Kazakov D.V., Kazakov V.P. Singlet-oxygen chemiluminescence in peroxide reactions. Chem. Rev. 2005;105:3371–3387. doi: 10.1021/cr0300035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.