Abstract
Objective
Myocardial infarction (MI) is a leading cause of mortality and morbidity worldwide and new treatment strategies are highly sought-after. Paradoxically, reperfusion of the ischemic myocardium, as achieved with early percutaneous intervention, results in substantial damage to the heart (ischemia/reperfusion injury) caused by cell death due to aggravated inflammatory and oxidative stress responses. Chronic therapy with vitamin E is not effective in reducing the cardiovascular event rate, presumably through failing to reduce atherosclerotic plaque instability. Notably, acute treatment with vitamin E in patients suffering a MI has not been systematically investigated.
Methods and results
We applied alpha-tocopherol (α-TOH), the strongest anti-oxidant form of vitamin E, in murine cardiac ischemia/reperfusion injury induced by ligation of the left anterior descending coronary artery for 60 min. α-TOH significantly reduced infarct size, restored cardiac function as measured by ejection fraction, fractional shortening, cardiac output, and stroke volume, and prevented pathological changes as assessed by state-of-the-art strain and strain-rate analysis. Cardioprotective mechanisms identified, include a decreased infiltration of neutrophils into cardiac tissue and a systemic anti-inflammatory shift from Ly6Chigh to Ly6Clow monocytes. Furthermore, we found a reduction in myeloperoxidase expression and activity, as well as a decrease in reactive oxygen species and the lipid peroxidation markers phosphatidylcholine (PC) (16:0)-9-hydroxyoctadecadienoic acid (HODE) and PC(16:0)-13-HODE) within the infarcted tissue.
Conclusion
Overall, α-TOH inhibits ischemia/reperfusion injury-induced oxidative and inflammatory responses, and ultimately preserves cardiac function. Therefore, our study provides a strong incentive to test vitamin E as an acute therapy in patients suffering a MI.
Keywords: Oxidative stress, Tocopherol, ROS-Sensitive nanoprobe, Myocardial infarction, Ischemia/reperfusion injury
Graphical abstract
Highlights
-
•
α-TOH reduces cardiac I/R injury and preserves cardiac function in male C57BL/6 mice.
-
•
α-TOH reduces neutrophil and monocyte infiltration in the infarcted cardiac tissue.
-
•
Expression of inflammatory and oxidative markers is down-regulated by α-TOH.
-
•
α-TOH decreases the production of reactive oxygen species and peroxidized lipids.
1. Introduction
Myocardial infarction (MI) is the single most frequent cause of death worldwide [1]. Substantial progress in the treatment of MI has been achieved by early reperfusion strategies based either on pharmacological thrombolysis or percutaneous coronary intervention resulting in reperfusion of the ischemic myocardium. However, reperfusion itself causes additional damage to the myocardium, damage which has been estimated to contribute to about 50% of the overall functional loss of the infarcted heart [2]. This ischemia/reperfusion (I/R) injury is characterized by necrosis of myocardial tissue which is caused by a combination of extensive inflammatory and oxidative stress [3]. Infiltration of immune cells, particularly neutrophils [4] and monocytes [5], followed by the production and release of chemokines and cytokines, is a central component of I/R-induced inflammation.
One of the enzymes that contributes to oxidative stress in I/R injury is myeloperoxidase (MPO). MPO is stored in leukocyte granules, and is released by leukocyte activation during inflammatory reactions and oxidative stress [6]. MPO is elevated in patients with a MI compared to healthy subjects [7], and has therefore been discussed as a potential circulating biomarker for MI [8]. In addition, MPO causes endothelial dysfunction, and affects the function and distribution of cholesterol [9], lipid peroxidation, and oxidation of lipoproteins [10]. Oxidized lipids are excessively taken up by macrophages via non-feedback-regulated pathways, which in turn cause foam cell formation, apoptosis, and further release of these lipids. Therefore, accumulation of lipids and lipid peroxidation during ischemia and reperfusion is an initiator of lipotoxicity, which causes apoptotic cell death, cardiac dysfunction, remodeling, and ultimately heart failure [11].
One of the most effective anti-oxidant and anti-inflammatory agents is vitamin E and its derivatives. Vitamin E has eight derivative forms, which differ in the methylation of the chromanol ring and the saturation of the side chain. Within this group, α-Tocopherol (α-TOH) is known to be the most active anti-oxidant [12]. Besides protection against H2O2-induced lipid peroxidation due to increased anti-oxidative enzyme systems such as glutathione and catalase [13], α-TOH reduces oxidative stress-induced apoptosis [14]. In addition to its anti-oxidative capacities, α-TOH acts as a regulator of genes involved in lipid metabolism and homeostasis, inflammation [15] and the immune defense system, the latter demonstrated by boosting resistance against pneumococcal infection [16]. Indeed, infection-induced transepithelial migration of neutrophils in the lung is reduced by α-TOH [17]. Furthermore, α-TOH prevents macrophage foam cell formation [18,19], lipotoxicity in macrophages [20], and the release of pro-inflammatory cytokines [21].
A recent study of Huang et al. reported that a higher α-TOH serum concentration correlates with decreased all-cause mortality and disease-specific mortality, such as cardiovascular disease and heart disease [22]. As the plasma levels of vitamin E decrease in patients within the first 48 h after MI [[23], [24], [25]], and as I/R injury is associated with excessive oxidative stress, increased consumption of this anti-oxidant in the ischemic and reperfused myocardium has been postulated [26,27]. Supplementation of vitamin E as a strong anti-oxidant may thus represent a therapeutic option for anti-oxidative protection of the myocardium and ultimately for patients suffering a MI. Vitamin E supplementation did not fulfil its original promise in several large-scale trials aimed at assessing its potential in primary and secondary prevention of cardiovascular events [28]. However, mechanistically these trials tested for vitamin E's capacity to provide plaque stabilization in a chronic setting, but not its potential to preserve cardiac function in the event of an acute MI. There is only very limited data available that addresses the question of the potential benefits of vitamin E in the acute setting of a MI [29,30]. Nevertheless, the limited data available shows potential benefits of vitamin E in models of ischemia/reperfusion settings of various organs, including a few early studies on cardiac ischemia/reperfusion.
In our study, we systematically address this clinically important question in a mouse model of cardiac I/R using a 60 min ligation of the left anterior descending (LAD) coronary artery. Using extensive echocardiographic and histological assessment to determine cardiac function and injury, in addition to thorough molecular and mechanistic studies, we demonstrate a cardioprotective effect of vitamin E supplementation in cardiac ischemia/reperfusion injury.
2. Materials and methods
2.1. Animals
C57BL/6 mice were acquired from Jackson Laboratories and bred by the Alfred Medical Research and Education Precinct (AMREP) Animal Services in Melbourne, VIC. All experimental work was performed in accordance with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and the Australian code for the care and use of animals for scientific purposes and was approved by the AMREP Animal Ethics Committee (E/1779/2018/B).
2.2. Myocardial ischemia/reperfusion injury in mice
Eight-week-old male C57BL/6 mice underwent open-chest surgery to induce left coronary artery occlusion (CAO) for 60 min, followed by reperfusion as previously described [31]. Beforehand, mice were anesthetized using a combination of ketamine HCl (100 mg/kg BW; Lyppard), xylazine HCl (5 mg/kg BW; Lyppard), and atropine (1 mg/kg BW; Pfizer) via a single intraperitoneal (IP) injection. Randomized mice were intraperitoneally (IP) injected with either a vehicle (PBS with 0.8% DMSO) or α-TOH (2.5 mg/kg BW in 0.8% DMSO; Sigma-Aldrich) 2 h prior to surgery, immediately after reperfusion, and twice per day for three consecutive days. Following surgery, mice were culled at three different time points for respective analysis, using a ketamine HCl (100 mg/kg BW; Lyppard)/xylazine HCl (20 mg/kg BW; Lyppard) overdose IP injection followed by cervical dislocation. More details are given in the online-only Data Supplement.
2.3. Histology and immunofluorescence
Hearts were harvested, fresh-frozen in OCT Tissue Tec (Sakura® Finetek), and cut into 6 μm sections (Microm HM 525 Cryostat, Thermo Fisher Scientific). Cardiac sections were stained to detect neutral lipid content using Oil Red O (ORO, Sigma-Aldrich). To analyze neutrophils, tissue sections were stained using rat anti-mouse Ly6G (Gr-1) monoclonal antibody (ebioscience), followed by secondary Alexa Fluor 546–labeled anti-rat antibody (Life Technologies), and Hoechst 33342 dye counterstaining (Thermo Fisher Scientific). More details are given in the online-only Data Supplement.
2.4. Flow cytometry
Antibodies were purchased from BD Bioscience if not otherwise indicated. Blood samples were taken in 0.5 M anti-coagulant ethylenediaminetetraacetic acid (EDTA) by cardiac puncture. Blood was centrifuged (300×g, 10 min, RT) to separate the plasma. Within 1 h of collection, cells from the blood were isolated and stained for flow cytometric analysis. Neutrophil and monocyte populations in the blood were analyzed using a FACS Canto II (BD Biosciences) and BD FACS DIVA software version 8.0.1. The total monocyte/macrophage population was detected using fluorescent anti-CD11b-FITC and anti-CD115-PE-Cy7 antibodies (Biolegend). For separating pro- and anti-inflammatory monocyte sub-populations, Ly6C-PB staining was performed in parallel. Neutrophils were gated using Ly6G (Gr-1)-PE staining.
2.5. Ribonucleic acid (RNA) isolation and PCR arrays for inflammatory cytokines and oxidative stress
The apex of each heart was collected, and three samples were pooled and used for RNA isolation. Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and converted to cDNA using the RT2 First Strand Kit (Qiagen) according to the manufacturer's instructions. Inflammatory cytokine and oxidative stress gene expression profiles were analyzed using the 384 well RT2 Profiler™ PCR Array Mouse Inflammatory Cytokines & Receptors and the RT2 Profiler™ PCR Array Mouse Oxidative Stress and Antioxidant Defense (Qiagen), respectively. The arrays were performed using QuantStudio 6k Flex (Applied Biosystems), and the GeneGlobe Data Analysis Centre (Qiagen) was used for data analysis.
2.6. Lipid measurement
Blood was collected in EDTA as described above. Total serum cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (TG) were measured with commercial enzymatic kits using a COBAS Integra 400 Plus blood chemistry analyzer (Roche Diagnostics). The instrument was calibrated on the day of use according to the manufacturer's instructions.
2.7. Lipid extraction
Prior to lipid extraction, samples were randomized and blinded. Lipids were isolated from the infarct area of the cardiac tissue samples (36–50 mg) using a single-phase chloroform/methanol extraction as previously described [32]. Lipid analysis was performed by liquid chromatography electrospray ionization-tandem mass spectrometry using an Agilent 1290 HPLC coupled to an Agilent 6490 triple-quadrupole mass spectrometer. Lipid extracts were injected and separated under gradient conditions. The oxidized lipid species phosphatidylcholine (PC) (16:0)-9-hydroxyoctadecadienoic acid (HODE)) and PC(16:0–13-HODE) were measured using dynamic multiple-reaction monitoring (dMRM) and analyzed using Mass Hunter Quantitative analysis version B.07. Relative lipid abundances were calculated by relating each area under the peak for each lipid species (Avanti Polar Lipids, Alabaster, US) to the corresponding internal standard. Correction factors were applied to adjust for different response factors, where these were known. Results are expressed as pmol/mg of heart tissue. Values for each lipid class were calculated as the sum of the individual lipid species. More details are given in the online-only Data Supplement.
2.8. MPO activity assay
The infarct area of cardiac tissue samples (36–50 mg) were collected in ice-cold MPO assay buffer (50 μl/10 mg). After mechanical disruption, cardiac tissues were homogenized three times for 30 s at 30/s using a TissueLyser II (Qiagen). Immediately after homogenization, undiluted fresh samples were used in duplicate for MPO activity measurement using an MPO Fluorometric Activity Assay Kit (Sigma-Aldrich) according to the manufacturer's instructions. Released fluorescein was measured every 15 min and respective MPO activity was calculated using a fluorescein standard curve.
2.9. In vivo reactive oxygen species (ROS) quantification using a fluorescent nanoprobe
Three mice per treatment group underwent I/R injury by CAO for 60 min followed by reperfusion for another 24 h. Recently described [33] ROS-sensitive nanoparticles (5 μg/g BW) were intravenously injected 20 min before mice were euthanized and perfused with PBS; the hearts and blood were then collected. Each heart was cut into four transverse sections and imaged from both sides using an IVIS Lumina XRMS system (PerkinElmer). The 2D scans were performed using the following settings: filter passband = excitation at 420 nm and emission at 670 nm. Results are expressed as total radiance [p/s]/[μW/cm2] levels per g of tissue/blood. Cardiac sections were stained with 1% triphenyltetrazolium chloride (TTC) for 10 min at 37 °C in darkness and scanned using a high-resolution scanner (Epson Perfection Photo Scanner V370) to detect the infarct areas.
2.10. Statistical analysis
Data were statistically analyzed using one- or two-way repeated-measures ANOVA. As a post hoc test, Bonferroni's multiple comparisons test was used. P values of less than 0.05 were considered statistically significant. Results are expressed as means ± standard deviations (SD).
3. Results
3.1. Protection of cardiac function and reduction of infarct size after α-TOH treatment
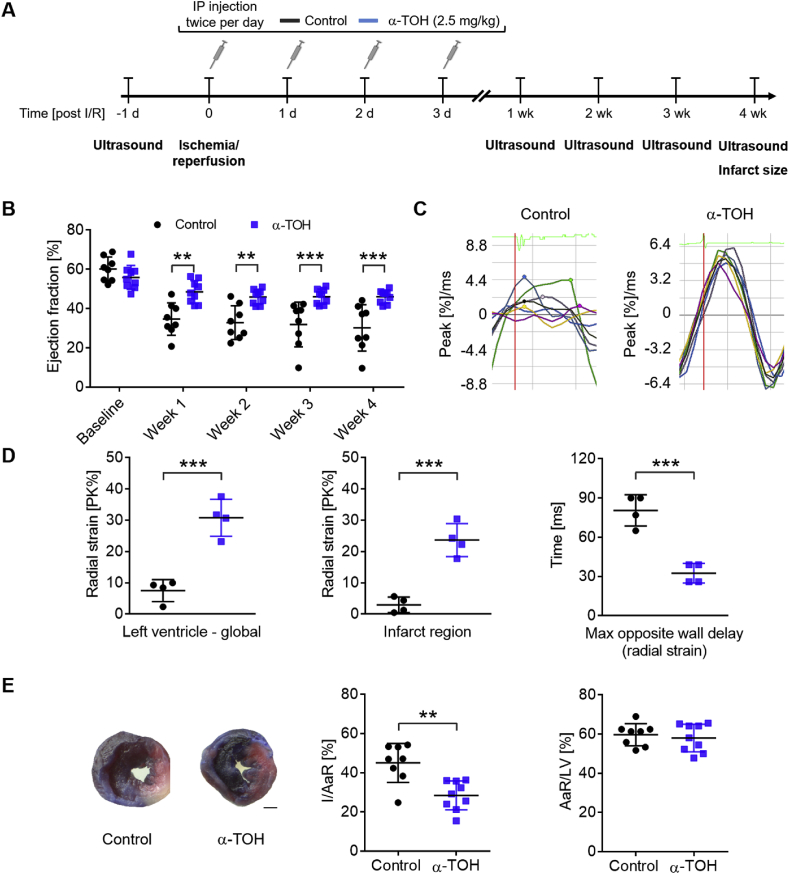
Mice were subjected to cardiac I/R injury to assess the cardioprotective effects of α-TOH as a potential treatment for MI (Fig. 1A). Application of 2.5 mg α-TOH/kg BW twice per day for three consecutive days significantly increased systemic concentration of α-TOH (Supplemental Fig. S1). Weekly echocardiography was performed to assess changes in cardiac function. For precise echocardiographic measurements of left ventricular (LV) function, we used both parasternal long-axis and parasternal short-axis views. At baseline, conventional echocardiographic measures showed similar ejection fractions (EF) for both treatment groups (control: 60.1 ± 6.2 versus [vs] α-TOH: 55.9 ± 6.1% EF, mean ± SD, NS; Fig. 1B). The cardioprotective effect of α-TOH treatment compared to controls was already significant at week 1 post-I/R injury (34.6 ± 8.3 vs 48.5 ± 5.7; **p < 0.01). Similar results were obtained at week 2 (32.8 ± 8.6 vs 45.9 ± 3.7; **p < 0.01), week 3 (31.9 ± 11.4 vs 46.1 ± 3.8; ***p < 0.001), and week 4 (30.2 ± 11.8 vs 46.1 ± 3.3; ***p < 0.001). The measurements of fractional shortening, cardiac output, and stroke volume also showed significant cardioprotective effects of α-TOH (Supplemental Fig. S2A).
Fig. 1.
Treatment with α-TOH protects cardiac systolic function and reduces infarct size in a mouse model of I/R injury. A) Design of long-term study (28 days post-I/R injury). B) α-TOH treatment preserves cardiac function assessed by ejection fraction from week 1 to week 4 after I/R injury; n=8–9, **p < 0.01 and ***p < 0.001, one-way Anova with multiple comparison. C) Representative images of radial strain curves obtained from VevoStrain analysis software shows strain measures over time. Colored lines represent 6 standard myocardial regions; 7th black line calculates average (global) strain at each time point. D) Bar charts show significant decrease in radial strain for control animals, as compared to α-TOH-treated animals, both globally and in infarct area (anterior apex). Maximum opposite-wall delay shows significant increases in time for control animals, as compared to α-TOH-treated animals; n=4, ***p < 0.001, Student's t-Test. E) Representative images of Evans blue/TTC staining 28 days post-I/R injury (scale bar: 1 mm) and quantitative analysis of infarct size (I) per area at risk (AaR), which illustrates a significant decrease in infarct size in mice treated with α-TOH as compared to control animals, while the primarily affected area presented in % AaR/LV is similar between α-TOH treated mice and control mice. Data are presented as means ± SD, **p < 0.01 and ***p < 0.001, n=8–9, Student's t-Test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At week 4, compared to baseline, cardiac output (***p < 0.001) and stroke volume (*p < 0.05) showed significant decreases in the PBS control group as compared to the α-TOH-treated animals (Supplemental Figs. S2B and 1C). There was no significant difference between the two groups for heart rate at baseline or at week 4 post-I/R surgery (Supplemental Fig. S2D). Further central echocardiographic measurements showed a decrease in LV internal diameter at end diastole (p=0.05) and end systole (*p < 0.05) in the α-TOH group compared to the control at week 4 (Supplemental Fig. S3). No difference was observed for both the LV interventricular septal wall and the LV posterior wall.
For a more sensitive and a highly translationally relevant readout, we decided to perform strain and strain-rate analyses [34]. We observed deterioration of the strain pattern in the control mice, while α-TOH treated mice preserved a physiological strain pattern. Radial strain analysis showed a highly significant decrease in control mice as compared to α-TOH-treated mice, both globally (control: 7.5 ± 3.5 vs α-TOH: 30.8 ± 5.9% PK, ***p < 0.001) and in infarct areas (anterior and apex) (control: 3.0 ± 2.5 vs α-TOH: 23.7 ± 5.3% PK, ***p < 0.001). Maximum opposite-wall delay showed significant increases in time for the control group as compared to the α-TOH group (control: 80.5 ± 12.0 vs α-TOH: 32.5 ± 7.5 ms, ***p < 0.001; Fig. 1C and 1D). Longitudinal strain analysis of the global peak (***p < 0.001) and the infarct area (*p < 0.05) obtained similar differences as in the radial strain analysis (Supplemental Fig. S4).
Histological evaluation of infarct size using Evans blue/TTC staining at week 4 post-I/R injury is a valuable parameter for evaluating the efficacy of interventions and cardiac performance. α-TOH-treated mice showed a significant decrease in infarct size (I)/area at risk (AaR) ratio as compared to controls (28.4 ± 7.4 vs 45.0 ± 9.9% I/AaR, respectively; **p < 0.01), while the AaR showed a similar size in all treatment groups, indicating comparable surgical procedures with similar sites of the LAD being ligated (AaR/LV; Fig. 1E).
3.2. Changes in blood cell profile and mRNA expression of inflammatory cytokines and receptors
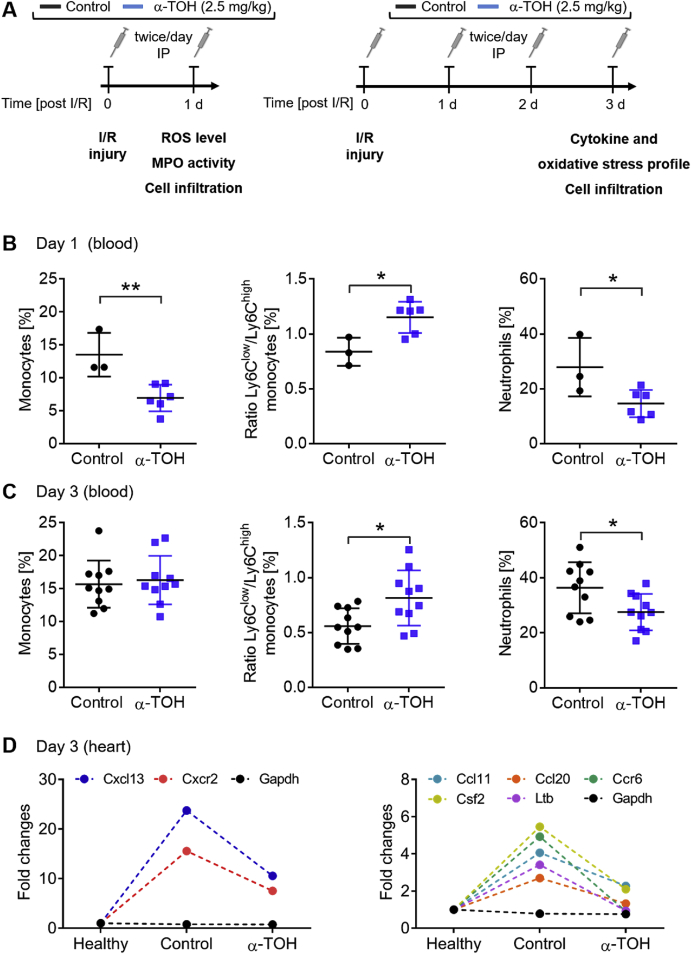
A central part of cardiac I/R injury is a massive pro-inflammatory response in the infarcted myocardium, including inflammatory cell infiltration, cytokine production, and oxidative stress. To study the underlying mechanism systemically and locally, we performed LAD ligation surgeries and investigated the above listed processes in the blood and myocardium at day 1 and 3 post-I/R injury (Fig. 2A).
Fig. 2.
Decrease in systemic and local inflammation in α-TOH-treated animals. A) Design of short-term studies (1 and 3 days post-I/R injury). B) α-TOH treatment reduces systemic inflammation, as assessed in the blood, by reducing total monocyte and neutrophil counts, and shifting the monocyte ratio from pro-inflammatory Ly6Chigh monocytes toward anti-inflammatory Ly6Clow monocytes at day 1 after I/R injury; n=3–6, *p < 0.05 and **p < 0.01, Student's t-Test. C) At day 3 post-I/R, the total monocyte population in the blood is unchanged, although the ratio is still shifted toward anti-inflammatory Ly6Clow monocytes. A significant reduction in neutrophils was also observed; n=10, *p < 0.05, Student's t-Test. D) Local cytokine responses in the myocardium for α-TOH-treated mice, control mice, and healthy mice. Expressions of 7 of the 84 tested genes are strongly upregulated after I/R and diminished in the α-TOH-treated animals compared to the control group; n=10.
Changes in monocyte and neutrophil counts in the blood due to treatment with α-TOH were assessed by flow cytometric analysis after I/R injury (Fig. 2B and C and Supplemental Fig. S5). Compared to control mice, mice treated with α-TOH showed approximately a 50% reduction in blood monocytes at day 1 post-I/R. Importantly, subtype analysis determined a significant shift from pro-inflammatory Ly6Chigh monocytes toward anti-inflammatory Ly6Clow monocytes at day 1 and day 3 post-I/R. Furthermore, neutrophils were significantly decreased in the blood of α-TOH-treated mice at both time points.
At day 3 post-I/R injury, gene expression profiling revealed that α-TOH treatment, compared to the controls, diminished the expression of seven inflammatory cytokines and receptors, which were significantly upregulated after control-treated I/R injury (Fig. 2D). For example, Cxcr2, a receptor expressed on neutrophils that mediates neutrophil migration to sites of injury and inflammation, as well as CCl11, a neutrophil chemoattractant, were strongly downregulated. This observation is in line with the reduction of neutrophils in the blood, as well as the reduction of neutrophils in the infarcted myocardium (see below).
α-TOH reduces oxidation of lipids in the myocardium independent of neutral lipid accumulation and systemic lipid profile.
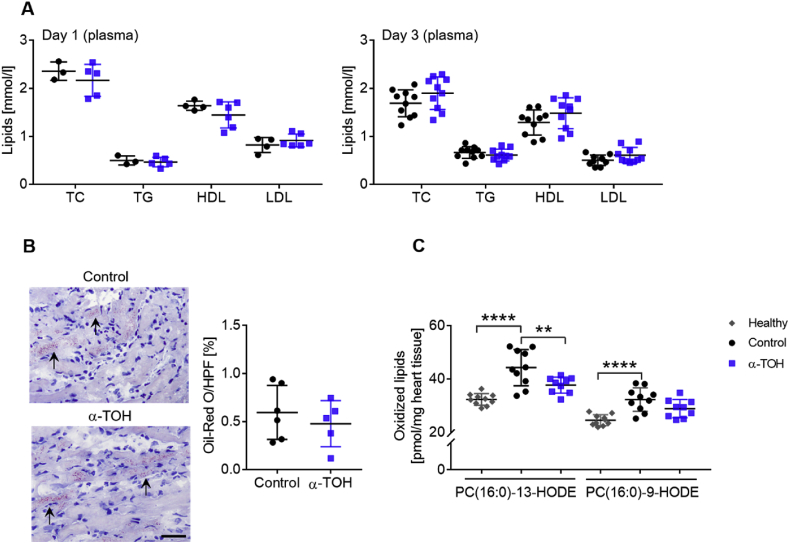
In addition to the inflammatory areas, we investigated the plasma lipid profile and changes in oxidized lipids as markers for oxidative stress in the infarcted myocardium. As well as systemic changes, the heart undergoes structural and functional changes in response to I/R injury, which lead, for example, to changes in the neutral lipid profile as well as the reduction of oxidation of several lipids.
First, we measured plasma lipids at day 1 and day 3 post-I/R and observed no changes in TC, TG, HDL, and LDL between treatment groups (Fig. 3A). Total lipid content in the heart was determined using ORO staining followed by quantitative analysis and showed no difference in total lipid content in the myocardium at three days post-I/R (Fig. 3B). Two major oxidized lipid species, namely PC(16:0–13-HODE) and PC(16:0-9-HODE), were assessed using liquid chromatography electrospray ionization-tandem mass spectrometry. Both oxidized lipids were significantly increased in the infarcted myocardium at day 3 post-I/R in comparison to healthy control samples (13-HODE: 32.3 ± 2.2 vs 44.3 ± 6.8 and 9-HODE 24.5 ± 2.0 vs 32.3 ± 4.4 pmol/mg heart tissue, respectively; ****p < 0.0001). A significant decrease in oxidized PC (16:0–13-HODE) (control: 44.3 ± 6.8 vs α-TOH: 37.7 ± 3.0 pmol/mg heart tissue; **p < 0.01) and a trend toward reduction of oxidized PC (16:0-9-HODE) (32.3 ± 4.4 vs 28.9 ± 3.4 pmol/mg heart tissue) were found in the hearts of α-TOH-treated mice compared to control animals (Fig. 3C).
Fig. 3.
Changes in systemic and local lipids, and oxidized lipids in mice in response to α-TOH treatment. A) Plasma lipids measured 1 and 3 days post-I/R are unchanged in α-TOH-treated mice compared to control mice; n=4–10, one-way Anova with multiple comparison. B) Representative images and quantitative analysis show comparable lipid content in the myocardium as assessed by ORO staining. Scale bar: 30 μm, 200x magnification, n=5–6, Student's t-Test. C) Oxidized lipids in the infarcted area of the myocardium are strongly increased three days after I/R injury and this effect is partly inhibited in mice treated with α-TOH as compared to control mice; n=9–10, **p < 0.01 and ****p < 0.0001, one-way Anova with multiple comparison.
3.3. Decrease in cardiac ROS production in α-TOH-treated mice
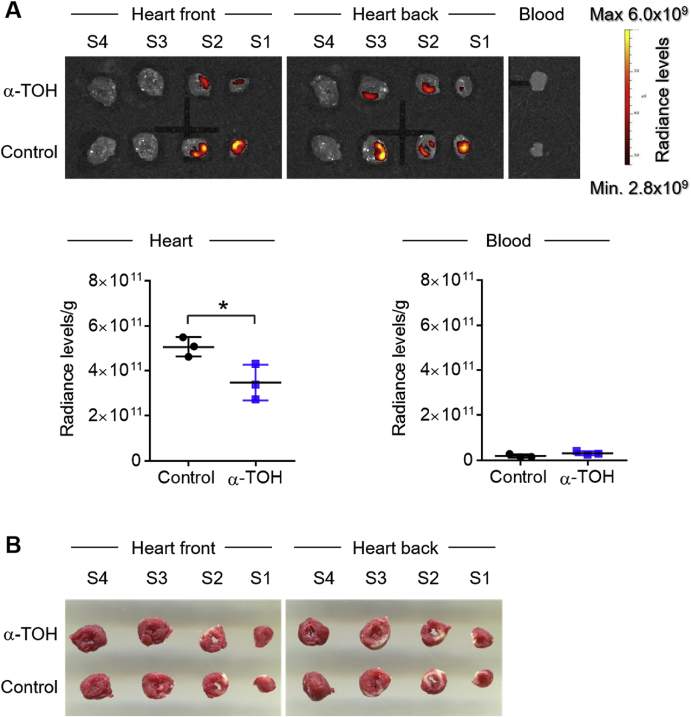
Both ischemic events as well as reperfusion injury lead to excessive ROS production, which ultimately causes tissue damage. With a reduction in oxidized lipids, here we have provided the first evidence that α-TOH treatment leads to an anti-oxidative response in the myocardium after I/R injury. To study this in more detail, we injected a ROS-sensitive nanoprobe, with its fluorescence quenched under normal conditions, while it was able to accumulate in the I/R injury area and ROS-activated fluorescence was then detected using IVIS imaging. Most importantly, IVIS showed a significant decrease of the ROS-dependent fluorescent signal in the α-TOH-treated ischemic/reperfused myocardium compared to control tissue (5.1 × 1011 ± 0.5 × 1011 vs 3.5 × 1011 ± 0.8 × 1011 radiance level/g heart tissue; **p < 0.01). No relevant radiance level was detected for whole blood in all groups (Fig. 4A). The cardiac section was stained with TTC to demonstrate the co-localization of the ROS signal with the infarcted area (Fig. 4B).
Fig. 4.
Treatment of α-TOH reduces production of ROS after I/R injury. A) 2D IVIS scan and quantitative analysis of cardiac sections and blood, 1 day post-I/R. Fluorescence accumulation of a ROS-sensitive fluorescent nanoparticle is significantly reduced in I/R injury area of α-TOH-treated mice compared to control mice (scale bar: 5 mm). Whole blood samples display no accumulation of the dye in all groups; n=3, *p < 0.05, Student's t-Test. B) Representative scans of TTC-stained cardiac sections show infarcted areas within the cardiac sections (scale bar: 5 mm).
Regulation of oxidative stress-related genes, reduction in MPO activity, and decrease in neutrophil infiltration after α-TOH treatment.
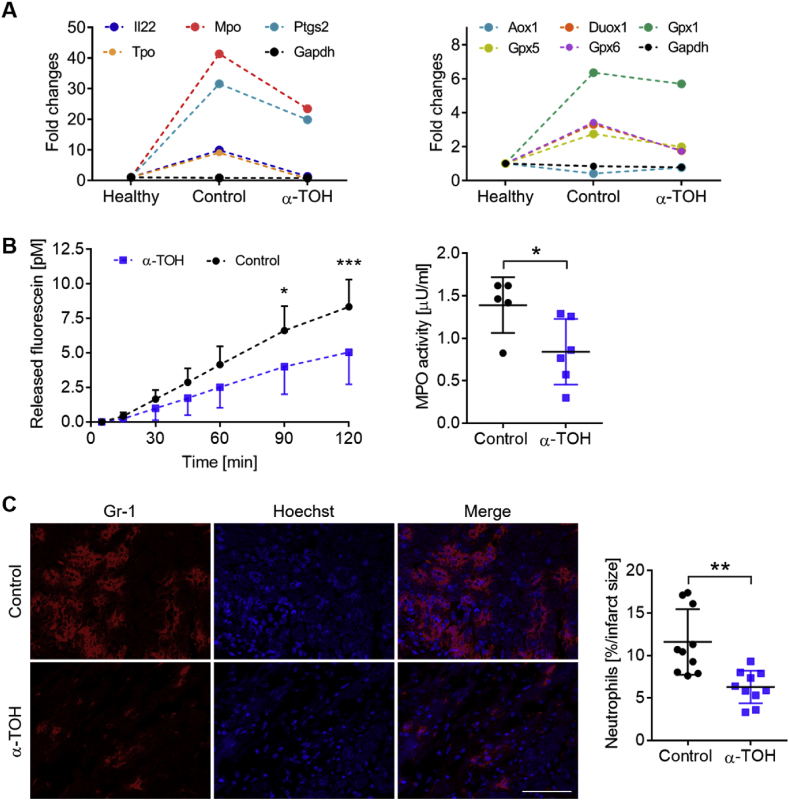
To further confirm the reduction in ROS within the ischemic/reperfused myocardium by α-TOH treatment, the expression of genes involved in oxidative stress were profiled and it was found that the expression of seven oxidative stress-regulating genes were strongly downregulated in α-TOH-treated mice at day 3 post-I/R injury (Fig. 5A). Notably, several glutathione peroxidases (Gpx1, 5, and 6) were downregulated and, most strikingly, MPO was downregulated by more than 43% in mice treated with α-TOH as compared to control mice. Therefore, we continued studying the changes in MPO due to the strong downregulation of its expression, and performed MPO activity measurements in the I/R myocardium at day 1 post-I/R injury. In line with the downregulation of MPO expression, we found a significant decrease in MPO activity of about 40% in the α-TOH-treated group as compared to the control group (0.84 ± 0.3 vs 1.4 ± 0.3 μU/ml, respectively; *p < 0.05; Fig. 5B). As MPO is well-known to be highly correlated with neutrophils, the number of infiltrating neutrophils within the infarcted area was determined and the infiltration of neutrophils was found to be strongly reduced in the infarcted tissue of mice treated with α-TOH (6.28 ± 1.9 vs 11.6 ± 3.9% neutrophils/infarct area, respectively; **p < 0.01; Fig. 5C).
Fig. 5.
Modification of expression of oxidative stress-regulated genes, MPO activity, and neutrophil infiltration. A) Oxidative stress profiler array analysis with cardiac tissue samples for α-TOH-treated mice, control mice, and healthy mice. Seven oxidative stress-related genes show strong upregulation after I/R injury; this upregulation is diminished by α-TOH treatment; n=10. B) Time course of released fluorescein representing MPO activity and quantitative analysis of average MPO activity, which show a decrease in MPO activity in α-TOH-treated mice compared to control mice; n=6, *p < 0.05 and ***p < 0.001, one-way Anova with multiple comparison and Student's t-Test. C) Representative images and quantitative analysis display a reduction of infiltrating neutrophils in the myocardium. Cardiac sections are counterstained with Hoechst dye. Scale bar: 100 μm, 200x magnification, n=10, **p < 0.01, Student's t-Test.
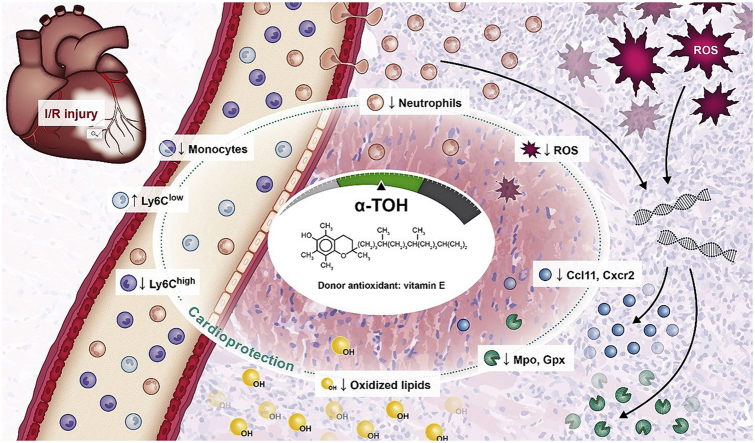
In summary, using a murine I/R injury model we demonstrate the potential of α-TOH in preventing tissue damage and retaining cardiac function after MI. α-TOH protects the heart against (i) oxidative stress-induced tissue damage such as decreased oxidative lipids, reduction in ROS production, and downregulation of the expression of oxidative stress-related genes. Furthermore, it induced (ii) anti-inflammatory changes, such as shifting the ratio of monocyte subpopulations toward anti-inflammatory Ly6Clow monocytes and decreasing the number of infiltrating neutrophils (see graphical abstract).
4. Discussion
The excessive oxidative stress during MI [23,24,26], particularly during reperfusion [27] is associated with a drop in the anti-oxidant defense levels as reported for hydrophilic (vitamin C [35]) and lipophilic vitamins (α-TOH [25,26,36]) circulating in the blood. To compensate for this drop systemically and in the myocardial tissue [37,38], and also to provide the maximal anti-oxidative effect for the cardiac tissue undergoing ischemia and following reperfusion, we applied α-TOH as a treatment during the MI and then for three consecutive days. Our treatment regime reflects clinical conditions, where MI patients could receive their first application of α-TOH either in the ambulance or upon their arrival in the emergency department, before they are transported to the catheter laboratory for reperfusion by percutaneous coronary intervention, and the following days in hospital before discharge.
I/R injury-induced cardiac tissue damage is mainly caused by significant leukocyte infiltration, particularly of monocytes [39] and neutrophils [40], and the subsequent release of pro-inflammatory chemokines and cytokines, as well as ROS. Therefore, inhibiting leukocyte migration is an attractive strategy in finding novel therapeutics for MI. α-TOH has been described as having immunomodulatory ability by affecting monocyte and neutrophil migration into inflamed areas in the lung [17]. In accordance with this, our study shows that α-TOH treatment shifts the monocyte profile in favor of anti-inflammatory Ly6Clow monocytes systemically, and lower neutrophil infiltration locally in the ischemic myocardium, leading to a reduced infarct size and preserved cardiac function. As reported earlier, neutrophil migration and its lipoxygenase-dependent cytokine production enhance the inflammatory burst and thereby increase the expression of pro-inflammatory genes. In parallel, the interaction of neutrophils with ROS during I/R injury [41] has shown the multifactorial importance of neutrophils and neutrophil-derived signaling molecules as further promising targets for MI therapy.
Increased cardiac ROS formation and related signaling-pathway activation during I/R injury are a result of excessive oxidative stress [[42], [43], [44]]. Mitochondria play a pivotal role in ROS formation and mitochondrial membrane potential is an adequate marker for their metabolic activity. As described by Birringer et al., 10 μM α-TOH regenerated mitochondrial membrane potential, followed by decreasing cellular ROS formation in liver HepG2 cells [45]. In addition, formation of superoxide anion has been shown to be inversely correlated with α-TOH content in mitochondria in vitro and in vivo [46]. Protective effects of α-TOH on mitochondrial integrity preserved heart function and improved recovery following I/R [47]. Cardiac tissue is vulnerable to oxidative stress due to the low rates of expression of hydrophilic anti-oxidative detoxification systems [35,48]. However, lipophilic anti-oxidants such as vitamin E are normally abundant in cardiac tissue [47]. Induction of anti-oxidant defense mechanisms after I/R, such as increases in superoxide dismutase, glutathione transferase, and catalase, can be induced by α-TOH [49,50] whereas α-TOH deficiency in heart tissue enhances ROS formation [48,51]. We have studied ROS formation using an innovative ROS-sensitive nanoparticle [33]. Visualization of this ROS-sensitive fluorescent nanoprobe using in vivo imaging shows that I/R-induced ROS formation is decreased by α-TOH, confirming the ROS-scavenging capacity of α-TOH. In support of this hypothesis, as we have shown, α-TOH downregulated the ROS-induced gene expression of MPO in myocardial tissue, specifically its release from neutrophil granules [6]. Furthermore, the partial inhibition of neutrophil migration in the myocardial tissue by α-TOH contributes to the reduced release of MPO.
Excessive ROS production followed by MPO release also causes oxidative modification of cellular macronutrients such as lipids. Malondialdehyde [52], thiobarbituric acid reactive species [49], and hydroperoxides [47,52] are common markers for ROS-induced lipid peroxidation. Enhanced lipid peroxidation after I/R injury and the ability of anti-oxidants to attenuate oxidative stress-induced lipid peroxidation have been reported previously [49,53]. Vitamin E-deficient rats are characterized by significantly higher levels of myocardial lipid peroxidation [54], which is antagonized by α-TOH treatment. One of the major membrane-associated lipid classes prone to be oxidized by ROS is PC, due to its high content of polyunsaturated fatty acyl chains [55]. In addition, oxidized PC-containing phospholipids, generated in cardiomyocytes during I/R, affect the viability of cardiomyocytes and therefore increase infarct size [56]. Treatment with α-TOH has been shown to modulate PC metabolism via phospholipase A [57]. Here, we report and apply a novel technique to measure lipid products directly oxidized during I/R injury using liquid chromatography electrospray ionization-tandem mass spectrometry. Lipid profiles in plasma and the accumulation of lipids, particularly triglycerides, in ischemic myocardial tissue remained unchanged in our experimental setup, however, α-TOH treatment significantly decreased the oxidative modification of lipids. Specifically, α-TOH decreased I/R injury-induced formation of oxidized PC, namely PC (16:0–13-HODE) and PC (16:0-9-HODE). Therefore, we have demonstrated a highly efficient cardioprotective effect of α-TOH through regulating oxidative stress-dependent and stress-independent properties.
Upon uptake in the liver, side chain truncation of all vitamin E forms is initiated by CYP4F2/3A4-dependent ω-hydroxylation, which forms the so-called long-chain metabolite α-13′-OH [58,59]. Subsequently, α-oxidation forms α-13′-COOH via the aldehyde metabolite processed by alcohol and aldehyde dehydrogenase. Following this, β-oxidation-induced side-chain degradation forms the intermediate-chain metabolites and short-chain metabolites, and finally the catabolic end-product of vitamin E, carboxyethyl hydroxychroman (CEHC) [60]. As shown in the work of Farley et al., subcutaneous administration of 100 mg α-TOH/kg BW in rats for 1 week resulted in accumulation of α-TOH (100 nmol/g) and α-CEHC (0.5 nmol/g) in heart tissue, with CEHC concentrations being 200-fold lower compared to α-TOH [61]. As previously described, CEHC-metabolites of vitamin E mediate anti-oxidative [62] and anti-inflammatory [63,64] effects in similar concentrations to α-TOH [63,65]. In human neutrophils, inhibition of PKC translocation and superoxide anion production induced by tocopherols and respective metabolites have been observed [62]. In addition, γ-TOH and γ-CEHC, which increased in humans as a result of supplementation with γ-TOH, have been shown to decrease plasma TNF-α and MPO concentrations [66]. Therefore, the relevance of hepatically formed metabolites have to be considered as contributors to the anti-oxidative and anti-inflammatory effects shown in this study, as described for the vitamin analog Trolox [67]. Therefore, effects of CEHC metabolites in myocardial infarction cannot be excluded completely. Nevertheless, α-CEHC excretion may increase only after exceeding an individual α-TOH threshold (30–50 μM), depending on plasma lipid concentrations [68,69]; and is therefore a marker for (super) optimal α-TOH supply in humans [70]. Since, the aim of our study was to maintain the α-TOH concentration during I/R injury, using α-TOH supplementation of 300 mg/kg/d for 3 days, and not to generate (super) optimal conditions, the formation of CEHC and other metabolites was probably less efficient. Therefore, the contribution of α-CEHC to the observed effects is most likely less relevant for the findings in the study presented here.
The concentration of α-TOH used in our experimental setup is of central importance. The recommended daily oral intake for healthy adults is 15 mg RRR-α-TOH [71,72]. Nevertheless, even higher concentrations have been classified as safe for animals and humans. As observed by the FDA [73], α-TOH has a LD50 > 2000 mg/kg BW in mice, rabbits and rats. Several other species can tolerate oral doses of 200 mg/kg BW [74]. The European Commission Scientific Committee on Food (SCF) approved a daily oral dose of 300 mg α-TOH for humans [75]. In the human trail of Lassnigg et al., 270 mg all-rac-α-TOH which correlates to 180 mg RRR-α-TOH, was intravenously applied after elective cardiac surgery in humans without detecting any side effects [26]. Therefore, 300 mg α-TOH/d (5 mg/kg/d), applied intraperitoneally, has been used in our mouse study. This concentration of α-TOH is slightly higher compared to Lassnigg et al. but is still in the range of the SCF-approved daily dose, in contrast to most other studies which typically use higher doses of α-TOH [29,30]. This demonstrates that even doses of α-TOH within the approved daily intake dose are highly effective and protective in I/R injury when delivered at the crucial time around reperfusion. We hereby provide an experimental design which potentially can be translated to human trials without concern surrounding the safety of α-TOH applications.
The findings presented in our study give hope for a long sought-after therapy for I/R injury, but clearly need to be confirmed in human patients. α-TOH has been extensively tested as a preventive drug with the aim of reducing the rate of cardiovascular events, including MI. However, this approach has been reported to be inefficient, as confirmed by the outcomes of several human intervention and correlation reports published indicating a beneficial effect of vitamin E in cardiac ischemia/reperfusion [29,30,38]. Yau et al. reported that pre-treatment with α-TOH before elective cardiopulmonary bypass showed improvement in myocardial metabolism and ventricular studies [38]. This finding reflects the effects of α-TOH on chronic atherosclerosis, particularly plaque instability and the risk of plaque rupture. However, oxidative stress as the main target for vitamin E therapy will clearly be increased to a much higher level in acute I/R injury compared to chronic atherosclerosis. The potential beneficial effects of α-TOH on infarct size and preservation of cardiac function in MI have been overshadowed and have not been thoroughly investigated. This is based on the negative outcomes of the above-mentioned clinical trials testing of vitamin E for its capability to reduce the cardiovascular event rate. This is surprising as there were a few early function, unfortunately with no clinical significance [38]. Although these reports had limitations such as using ex vivo heart preparations, non-pharmacological doses or limited scope of data and mechanistic work up, these reports indicated the value of a further thorough study. The outcome of our study is noteworthy in the extent of cardio protection shown by vitamin E and the mechanistic insight provided. However, our encouraging preclinical data need to be confirmed in clinical trials with patients presenting with ST-elevation MI, and the use of cardiac enzymes and echocardiography or magnetic resonance imaging to assess α-TOH's potential to preserve cardiac function in patients.
Our study sheds new light on the potential of the acute therapy with α-TOH in patients presenting with MI, and may ultimately offer an effective low-cost treatment for the many patients suffering a MI. α-TOH in a dose of up to 300 mg/d, a concentration equivalent to the one used in our study, is already approved by the SCF and considered safe in humans without adverse effects. This dose would be attractive and highly feasible for the treatment of MI patients and ultimately has the potential to provide a better outcome for patients suffering a MI. As there is currently no drug available in the clinic that can reduce the cardiac damage caused by I/R injury, the potential impact on cardiovascular health would be significant. We postulate that α-TOH therapy/supplementation compensates for the drop seen during I/R injury and would facilitate an adequate anti-oxidative defense within the ischemic and reperfused myocardium. Ultimately, α-TOH holds promise as an inexpensive and readily translatable novel treatment preventing cardiac damage and thereby reducing mortality and morbidity in patients who suffer a MI.
Sources and funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, Wa 3836/1–1, MW), the Australian Research Training Program Scholarship (MLY), the National Heart Foundation of Australia Future Leader Fellowship (XW), the National Health and Medical Research council (NHMRC) of Australia (KHP, GNT1079492 and GNT1163507) and the Victorian Government's Operational Infrastructure Support Program. Other sources of funding include the Forschungskreis der Ernährungsindustrie (FEI) as part of an AiF (Arbeitsgemeinschaft industrieller Forschungsvereinigungen “Otto von Guericke”) project of the Industrielle Gemeinschaftsforschung (IGF), Thüringer Ministerium für Bildung, Wissenschaft und Kultur, Stiftung für Technologie und Forschung, the Deutsche Forschungsgemeinschaft (RTG 1715 “Molecular Signatures of Adaptive Stress Responses” and CRC 1278 “Polymer-based nanoparticle libraries for targeted anti-inflammatory strategies (PolyTarget)”) the German Federal Ministry of Education and Research (nutriCARD, grant agreement number 01EA1411A), and the Free State of Thuringia and the European Social Fund (2016 FGR 0045) to SL.
Declarations of interest
None.
Acknowledgements
We thank Daniela Stallmann and Anke Katharina Müller for their wonderful assistance. We would also like to thank Tobias Ziegler for his assistance with the schematic drawings.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101292.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O'Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. On behalf of the American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee, heart disease and stroke statistics—2019 update: a report from the American heart association. Circulation. 2019;139 doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy D.J., Botker H.E., Engstrom T., Erlinge D., Heusch G., Ibanez B., Kloner R.A., Ovize M., Yellon D.M., Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur. Heart J. 2017;38:935–941. doi: 10.1093/eurheartj/ehw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., Eyassu F., Shirley R., Hu C.-H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A.S.H., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone F., Nencioni A., Mach F., Vuilleumier N., Montecucco F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb. Haemost. 2013;110:501–514. doi: 10.1160/TH13-03-0211. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis N.G., Smith C.W., Entman M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 6.Naegelen I., Beaume N., Plançon S., Schenten V., Tschirhart E.J., Bréchard S. Regulation of neutrophil degranulation and cytokine secretion: a novel model approach based on linear fitting. J. Immunol. Res. 2015;2015:817038. doi: 10.1155/2015/817038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocatta T.J., Pilbrow A.P., Cameron V.A., Senthilmohan R., Frampton C.M., Richards A.M., Winterbourn C.C. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Khan A.A., Alsahli M.A., Rahmani A.H. Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med. Sci. Basel Switz. 2018;6 doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anatoliotakis N., Deftereos S., Bouras G., Giannopoulos G., Tsounis D., Angelidis C., Kaoukis A., Stefanadis C. Myeloperoxidase: expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013;13:115–138. doi: 10.2174/1568026611313020004. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls S.J., Hazen S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza K., Nzirorera C., Kienesberger P.C. Lipid metabolism and signaling in cardiac lipotoxicity. Biochim. Biophys. Acta. 2016;1861:1513–1524. doi: 10.1016/j.bbalip.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Burton G.W., Ingold K.U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981;103:6472–6477. [Google Scholar]
- 13.Nakamura Y.K., Omaye S.T. Alpha-tocopherol modulates human umbilical vein endothelial cell expression of Cu/Zn superoxide dismutase and catalase and lipid peroxidation. Nutr. Res. N. Y. N. 2008;28:671–680. doi: 10.1016/j.nutres.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Uemura M., Manabe H., Yoshida N., Fujita N., Ochiai J., Matsumoto N., Takagi T., Naito Y., Yoshikawa T. Alpha-tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 2002;456:29–37. doi: 10.1016/s0014-2999(02)02639-0. [DOI] [PubMed] [Google Scholar]
- 15.Wallert M., Schmölz L., Galli F., Birringer M., Lorkowski S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014;2:495–503. doi: 10.1016/j.redox.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bou Ghanem E.N., Lee J.N., Joma B.H., Meydani S.N., Leong J.M., Panda A. The alpha-tocopherol form of vitamin E boosts elastase activity of human PMNs and their ability to kill Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 2017;7:161. doi: 10.3389/fcimb.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bou Ghanem E.N., Clark S., Du X., Wu D., Camilli A., Leong J.M., Meydani S.N. The α-tocopherol form of vitamin E reverses age-associated susceptibility to streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment. J. Immunol. Baltim. Md 1950. 2015;194:1090–1099. doi: 10.4049/jimmunol.1402401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaraj S., Hugou I., Jialal I. Alpha-tocopherol decreases CD36 expression in human monocyte-derived macrophages. J. Lipid Res. 2001;42:521–527. [PubMed] [Google Scholar]
- 19.Rode S., Rubic T., Lorenz R.L. alpha-Tocopherol disturbs macrophage LXRalpha regulation of ABCA1/G1 and cholesterol handling. Biochem. Biophys. Res. Commun. 2008;369:868–872. doi: 10.1016/j.bbrc.2008.02.132. [DOI] [PubMed] [Google Scholar]
- 20.Vejux A., Guyot S., Montange T., Riedinger J.-M., Kahn E., Lizard G. Phospholipidosis and down-regulation of the PI3-K/PDK-1/Akt signalling pathway are vitamin E inhibitable events associated with 7-ketocholesterol-induced apoptosis. J. Nutr. Biochem. 2009;20:45–61. doi: 10.1016/j.jnutbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Ng L.-T., Ko H.-J. Comparative effects of tocotrienol-rich fraction, α-tocopherol and α-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem. 2012;134:920–925. doi: 10.1016/j.foodchem.2012.02.206. [DOI] [PubMed] [Google Scholar]
- 22.Huang J., Weinstein S.J., Yu K., Männistö S., Albanes D. Relationship between serum alpha-tocopherol and overall and cause-specific mortality: a 30-year prospective cohort analysis. Circ. Res. 2019;125:29–40. doi: 10.1161/CIRCRESAHA.119.314944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labadarios D., Brink P.A., Weich H.F., Visser L., Louw M.E., Shephard G.S., van Stuijvenberg M.E. Plasma vitamin A, E, C and B6 levels in myocardial infarction. South Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 1987;71:561–563. [PubMed] [Google Scholar]
- 24.Scragg R., Jackson R., Holdaway I., Woollard G., Woollard D. Changes in plasma vitamin levels in the first 48 hours after onset of acute myocardial infarction. Am. J. Cardiol. 1989;64:971–974. doi: 10.1016/0002-9149(89)90792-3. [DOI] [PubMed] [Google Scholar]
- 25.Weisel R.D., Mickle D.A., Finkle C.D., Tumiati L.C., Madonik M.M., Ivanov J., Burton G.W., Ingold K.U. Myocardial free-radical injury after cardioplegia. Circulation. 1989;80:III14–18. [PubMed] [Google Scholar]
- 26.Lassnigg A., Punz A., Barker R., Keznickl P., Manhart N., Roth E., Hiesmayr M. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: a double-blinded, randomized, controlled study. Br. J. Anaesth. 2003;90:148–154. doi: 10.1093/bja/aeg042. [DOI] [PubMed] [Google Scholar]
- 27.Sood R., Narang A.P.S., Abraham R., Arora U., Calton R., Sood N. Changes in vitamin C and vitamin E during oxidative stress in myocardial reperfusion. Indian J. Physiol. Pharmacol. 2007;51:165–169. [PubMed] [Google Scholar]
- 28.Vardi M., Levy N.S., Levy A.P. Vitamin E in the prevention of cardiovascular disease: the importance of proper patient selection. J. Lipid Res. 2013;54:2307–2314. doi: 10.1194/jlr.R026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh N.K., Saleh H.A. Protective effects of vitamin E against myocardial ischemia/reperfusion injury in rats. Saudi Med. J. 2010;31:142–147. [PubMed] [Google Scholar]
- 30.Tripathi Y., Hegde B.M. Effect of alpha-tocopherol pretreatment on infarct size following 90 minutes of ischemia and 4 hours of reperfusion in dogs. Indian J. Physiol. Pharmacol. 1997;41:241–247. [PubMed] [Google Scholar]
- 31.Ziegler M., Alt K., Paterson B.M., Kanellakis P., Bobik A., Donnelly P.S., Hagemeyer C.E., Peter K. Highly sensitive detection of minimal cardiac ischemia using positron emission tomography imaging of activated platelets. Sci. Rep. 2016;6 doi: 10.1038/srep38161. 38161–38161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir J.M., Wong G., Barlow C.K., Greeve M.A., Kowalczyk A., Almasy L., Comuzzie A.G., Mahaney M.C., Jowett J.B.M., Shaw J., Curran J.E., Blangero J., Meikle P.J. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler M., Xu X., Yap M.L., Hu H., Zhang J., Peter K. A self-assembled fluorescent nanoprobe for imaging and therapy of cardiac ischemia/reperfusion injury. Adv. Ther. 2019;2:1800133. [Google Scholar]
- 34.Ziegler M., Hohmann J.D., Searle A.K., Abraham M.-K., Nandurkar H.H., Wang X., Peter K. A single-chain antibody-CD39 fusion protein targeting activated platelets protects from cardiac ischaemia/reperfusion injury. Eur. Heart J. 2018;39:111–116. doi: 10.1093/eurheartj/ehx218. [DOI] [PubMed] [Google Scholar]
- 35.Spoelstra-de Man A.M.E., Elbers P.W.G., Oudemans-van Straaten H.M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care Lond. Engl. 2018;22:70. doi: 10.1186/s13054-018-1996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muzáková V., Kandár R., Vojtísek P., Skalický J., Vanková R., Cegan A., Cervinková Z. Antioxidant vitamin levels and glutathione peroxidase activity during ischemia/reperfusion in myocardial infarction. Physiol. Res. 2001;50:389–396. [PubMed] [Google Scholar]
- 37.Mickle D.A., Weisel R.D., Burton G.W., Ingold K.U. Effect of orally administered alpha-tocopheryl acetate on human myocardial alpha-tocopherol levels. Cardiovasc. Drugs Ther. 1991;5(Suppl 2):309–312. doi: 10.1007/BF00054753. [DOI] [PubMed] [Google Scholar]
- 38.Yau T.M., Weisel R.D., Mickle D.A., Burton G.W., Ingold K.U., Ivanov J., Mohabeer M.K., Tumiati L., Carson S. Vitamin E for coronary bypass operations. A prospective, double-blind, randomized trial. J. Thorac. Cardiovasc. Surg. 1994;108:302–310. [PubMed] [Google Scholar]
- 39.Nahrendorf M., Pittet M.J., Swirski F.K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romson J.L., Hook B.G., Kunkel S.L., Abrams G.D., Schork M.A., Lucchesi B.R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 41.Mullane K.M., Westlin W., Kraemer R. Activated neutrophils release mediators that may contribute to myocardial injury and dysfunction associated with ischemia and reperfusion. Ann. N. Y. Acad. Sci. 1988;524:103–121. doi: 10.1111/j.1749-6632.1988.tb38534.x. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigo R., Hasson D., Prieto J.C., Dussaillant G., Ramos C., León L., Gárate J., Valls N., Gormaz J.G. The effectiveness of antioxidant vitamins C and E in reducing myocardial infarct size in patients subjected to percutaneous coronary angioplasty (PREVEC Trial): study protocol for a pilot randomized double-blind controlled trial. Trials. 2014;15:192. doi: 10.1186/1745-6215-15-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granger D.N., Korthuis R.J. Physiologic mechanisms of postischemic tissue injury. Annu. Rev. Physiol. 1995;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 44.Garlick P.B., Davies M.J., Hearse D.J., Slater T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987;61:757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 45.Birringer M., Lington D., Vertuani S., Manfredini S., Scharlau D., Glei M., Ristow M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic. Biol. Med. 2010;49:1315–1322. doi: 10.1016/j.freeradbiomed.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Lass A., Sohal R.S. Effect of coenzyme Q(10) and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000;14:87–94. doi: 10.1096/fasebj.14.1.87. [DOI] [PubMed] [Google Scholar]
- 47.Venditti P., Napolitano G., Di Stefano L., Agnisola C., Di Meo S. Effect of vitamin E administration on response to ischaemia-reperfusion of hearts from cold-exposed rats. Exp. Physiol. 2011;96:635–646. doi: 10.1113/expphysiol.2011.058289. [DOI] [PubMed] [Google Scholar]
- 48.Janero D.R. Therapeutic potential of vitamin E against myocardial ischemic-reperfusion injury. Free Radic. Biol. Med. 1991;10:315–324. doi: 10.1016/0891-5849(91)90038-5. [DOI] [PubMed] [Google Scholar]
- 49.Dianat M., Esmaeilizadeh M., Badavi M., Samarbaf-Zadeh A.R., Naghizadeh B. Protective effects of crocin on ischemia-reperfusion induced oxidative stress in comparison with vitamin E in isolated rat hearts. Jundishapur J. Nat. Pharm. Prod. 2014;9 doi: 10.17795/jjnpp-17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohanty I.R., Arya D.S., Dinda A., Gupta S.K. Comparative cardioprotective effects and mechanisms of vitamin E and lisinopril against ischemic reperfusion induced cardiac toxicity. Environ. Toxicol. Pharmacol. 2013;35:207–217. doi: 10.1016/j.etap.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Guarnieri C., Flamigni F., Rossoni-Caldarera C., Ferrari R. Myocardial mitochondrial functions in alpha-tocopherol-deficient and -refed rabbits. Adv. Myocardiol. 1982;3:621–627. doi: 10.1007/978-1-4899-5561-6_59. [DOI] [PubMed] [Google Scholar]
- 52.Venditti P., Masullo P., Di Meo S., Agnisola C. Protection against ischemia-reperfusion induced oxidative stress by vitamin E treatment. Arch. Physiol. Biochem. 1999;107:27–34. doi: 10.1076/apab.107.1.27.4355. [DOI] [PubMed] [Google Scholar]
- 53.Andreadou I., Iliodromitis E.K., Farmakis D., Kremastinos D.T. To prevent, protect and save the ischemic heart: antioxidants revisited. Expert Opin. Ther. Targets. 2009;13:945–956. doi: 10.1517/14728220903039698. [DOI] [PubMed] [Google Scholar]
- 54.Coombes J., Powers S., Demirel H., Hamilton K., Jessup J., Vincent H., Shanely A. Vitamin E deficiency fails to affect myocardial performance during in vivo ischemia-reperfusion. Int. J. Vitam. Nutr. Res. 2000;70:293–300. doi: 10.1024/0300-9831.70.6.293. [DOI] [PubMed] [Google Scholar]
- 55.Volinsky R., Kinnunen P.K.J. Oxidized phosphatidylcholines in membrane-level cellular signaling: from biophysics to physiology and molecular pathology. FEBS J. 2013;280:2806–2816. doi: 10.1111/febs.12247. [DOI] [PubMed] [Google Scholar]
- 56.Yeang C., Hasanally D., Que X., Hung M.-Y., Stemankovic A., Chan D., Chaudhary R., Margulets V., Edel A.L., Hoshijima M., Gu Y., Bradford W., Dalton N., Miu P., Cheung D.Y.C., Jassal D.S., Pierce G.N., Peterson K.L., Kirshenbaum L.A., Witztum J.L., Tsimikas S., Ravandi A. Reduction of myocardial ischemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 2018 doi: 10.1093/cvr/cvy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choy P.C., O K., Man R.Y., Chan A.C. Phosphatidylcholine metabolism in isolated rat heart: modulation by ethanol and vitamin E. Biochim. Biophys. Acta. 1989;1005:225–232. doi: 10.1016/0005-2760(89)90041-6. [DOI] [PubMed] [Google Scholar]
- 58.Parker R.S., Sontag T.J., Swanson J.E. Cytochrome P4503a-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 59.Sontag T.J., Parker R.S. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism: NOVEL MECHANISM OF REGULATION OF VITAMIN E STATUS. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 60.Birringer M., Pfluger P., Kluth D., Landes N., Brigelius-Flohé R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002;132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 61.Farley S.M., Leonard S.W., Stevens J.F., Traber M.G. Deuterium-labeled phylloquinone fed to α-tocopherol-injected rats demonstrates sensitivity of low phylloquinone-containing tissues to menaquinone-4 depletion. Mol. Nutr. Food Res. 2014;58:1610–1619. doi: 10.1002/mnfr.201300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varga Z., Kosaras E., Komodi E., Katko M., Karpati I., Balla J., Paragh G., Aisa M.C., Galli F. Effects of tocopherols and 2,2’-carboxyethyl hydroxychromans on phorbol-ester-stimulated neutrophils. J. Nutr. Biochem. 2008;19:320–327. doi: 10.1016/j.jnutbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Grammas P., Hamdheydari L., Benaksas E.J., Mou S., Pye Q.N., Wechter W.J., Floyd R.A., Stewart C., Hensley K. Anti-inflammatory effects of tocopherol metabolites. Biochem. Biophys. Res. Commun. 2004;319:1047–1052. doi: 10.1016/j.bbrc.2004.05.082. [DOI] [PubMed] [Google Scholar]
- 64.Lustgarten M.S., Fielding R.A. Metabolites associated with circulating interleukin-6 in older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2016:glw039. doi: 10.1093/gerona/glw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galli F., Stabile A.M., Betti M., Conte C., Pistilli A., Rende M., Floridi A., Azzi A. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch. Biochem. Biophys. 2004;423:97–102. doi: 10.1016/j.abb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Mah E., Pei R., Guo Y., Ballard K.D., Barker T., Rogers V.E., Parker B.A., Taylor A.W., Traber M.G., Volek J.S., Bruno R.S. γ-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic. Biol. Med. 2013;65:1291–1299. doi: 10.1016/j.freeradbiomed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Mickle D.A., Li R.K., Weisel R.D., Birnbaum P.L., Wu T.W., Jackowski G., Madonik M.M., Burton G.W., Ingold K.U. Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann. Thorac. Surg. 1989;47:553–557. doi: 10.1016/0003-4975(89)90431-1. [DOI] [PubMed] [Google Scholar]
- 68.Horwitt M.K., Harvey C.C., Dahm C.H., Searcy M.T. Relationship between tocopherol and serum lipid levels for determination of nutritional adequacy. Ann. N. Y. Acad. Sci. 1972;203:223–236. doi: 10.1111/j.1749-6632.1972.tb27878.x. [DOI] [PubMed] [Google Scholar]
- 69.Schultz M., Leist M., Petrzika M., Gassmann B., Brigelius-Flohé R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 1995;62:1527S–1534S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]
- 70.Schuelke M., Elsner A., Finckh B., Kohlschütter A., Hübner C., Brigelius-Flohé R. Urinary alpha-tocopherol metabolites in alpha-tocopherol transfer protein-deficient patients. J. Lipid Res. 2000;41:1543–1551. [PubMed] [Google Scholar]
- 71.Deutsche Gesellschaft für Ernährung e. V https://www.dge.de/wissenschaft/referenzwerte (n.d.)
- 72.Food Labeling . U.S. Food and Drug Administration (FDA); 2016. Revision of the Nutrition and Supplement Facts Labels. [PubMed] [Google Scholar]
- 73.FDA . 1978. Tocopherols and Derivatives. [Google Scholar]
- 74.Tomassi G., Silano V. An assessment of the safety of tocopherols as food additives. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1986;24:1051–1061. doi: 10.1016/0278-6915(86)90288-7. [DOI] [PubMed] [Google Scholar]
- 75.European Food Safety Authority, editor. Tolerable Upper Intake Levels for Vitamins and Minerals. European Food Safety Authority; Parma: 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.