Abstract
Background and aims
Pneumothoraces can occur in patients of all ages and genders. We encountered a female adolescent patient who was experiencing recurrent pneumothoraces every several months. She endured an extensive workup to determine an etiology for her pneumothoraces but it was all negative. She was eventually diagnosed with catamenial pneumothorax. This is an established cause of recurrent pneumothorax in adults but is very rare in adolescent patients. Keeping it on the differential for any female of reproductive age with recurrent pneumothoraces may prevent potentially harmful and expensive diagnostic testing and procedures.
Methods
We reported a case of catamenial pneumothorax in an adolescent patient and reviewed the relevant literature.
Results
Our patient was a 14-year-old female patient with recurrent pneumothoraces every several months. She had an extensive procedural and genetic workup performed but no etiology was revealed. Due to a temporal relation of the pneumothoraces to menses, an obstetrics and gynecology consult was obtained. Empirical treatment for catamenial pneumothorax was started with a continuous oral contraceptive with combined estrogen-progestin leading to our patient's complete remission. Three years later our patient has not experienced any relapsing episodes of pneumothorax.
Conclusion
Although rare in younger patients, catamenial pneumothorax should be considered as a cause for recurrent pnueumothoraces in any post-pubertal female.
1. Presentation
A 14-year-old female with a 1- week history of intermittent right-sided chest and back pain and shortness of breath presents to the Emergency Department. She describes the pain as dull in quality, diffuse but non-radiating, and lasts approximately 1 hour before subsiding. The pain is pleuritic in nature and the only alleviating factor identifiable is ibuprofen. The shortness of breath is not related to any exertion or activity and was constant.
There is no past medical history of a specific or relevant medical problem and the patient specifically denies a history of asthma, pneumonia, gastro-esophageal reflux, or broncho-pulmonary dysplasia. The patient was a full term baby born via spontaneous vaginal delivery without any complications. Menarche occurred at 12 years of age. Cycles are regular, occurring every 31 days and lasting for 4 days.
The patient received all of her recommended immunizations for age. She lives with her mother, father, and brother in a house. There are no pets or smokers in the home. There is no history of recent sick contacts, no recent illnesses, and no recent travel. She denies sexual activity and denies alcohol, tobacco, or other illicit substance use.
Family history is significant for asthma in her maternal grandfather but negative specifically for tuberculosis, Connective tissue disease, pneumothorax, or Sarcoidosis.
Vitals signs are within normal limits. She is breathing at a rate of 20 breaths per minute and oxygen saturation is 100% on room air. Height and weight reveal a girl at the 90th and 10th percentiles respectively.
Physical exam reveals an alert, awake female who appears her stated age and is in mild distress. All physical exam findings are within normal limits with the exception of the respiratory exam, which reveals diminished breath sounds in the right upper lung field, and the musculoskeletal exam that reveals a mild scoliosis and a Marfanoid body habitus. A complete blood count and basic metabolic panel are within normal limits. A chest x-ray demonstrates a 2.3cm apical pneumothorax in the right hemithorax.
1.1. Hospital course
This was the patient's first of several presentations. During a 2-year period she developed 9 pneumothoraces both in the right and left hemithorax, all confirmed radiographically. After the first 5 were treated primarily with a non-rebreather mask and symptom management, she had a bilateral video-assisted thoracoscopic surgery (VATS) and a blebectomy of the most highly affected areas. The VATS did not reveal any lesions suggesting a definitive pathology for the pneumothoraces. For 9 months following this procedure, she continued to have recurrent pneumothoraces that occurred approximately every 3 months.
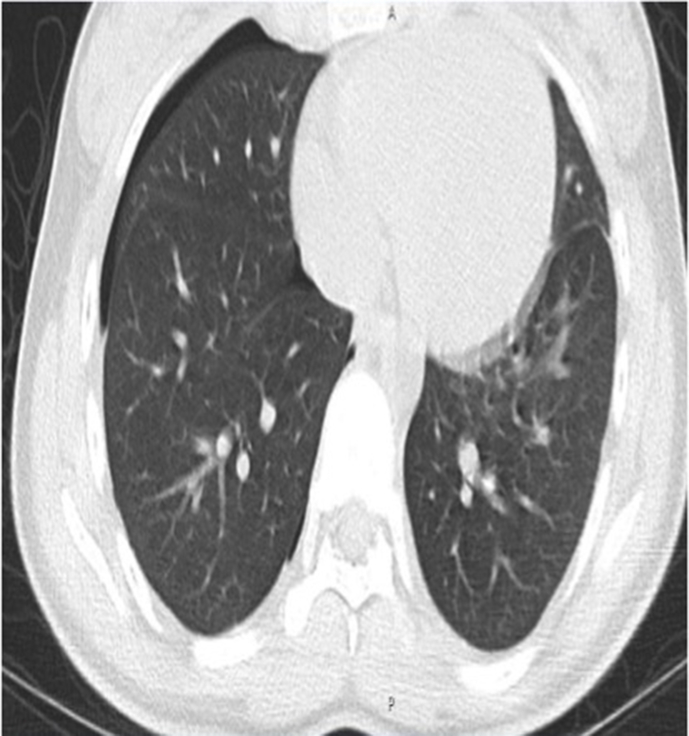
Testing for cystic fibrosis, alpha-1 antitrypsin deficiency, and Fibrillin-1 (to evaluate for Marfan's and adolescent idiopathic scoliosis) was negative. A CT-Chest w/o contrast (see Fig. 1) performed on her 7th presentation displayed a left-sided 2.1cm pneumothorax but did not demonstrate any evidence of blebs or bronchiectasis.
Fig. 1.
Non-contrast CT scan demonstrating one of many right-sided pneumothoraxes in our patient. No bronchiectasis or blebs present on CT.
After her 9th presentation, the obstetrics and gynecology team was consulted. The pneumothoraces always presented within±two days of her menses. She was placed on a trial of continuous combined oral contraceptive pills (COCs), specifically 90 mcg levonorgestrel and 20 mcg ethinyl estradiol tablets (Amethyst™). She achieved complete resolution of her symptoms after this and was thus clinically diagnosed with catamenial pneumothorax. Three years after starting the COC she has remained in total remission and has not experienced another episode of pneumothorax.
2. Discussion
Pneumothoraces come in many shapes and sizes and present with a variety of clinical manifestations. On an upright chest x-ray, a pneumothorax can usually be identified by a white visceral pleural line, edge or interface, which is separated from the parietal pleura by a collection of gas in the apicolateral position. Once identified, the hallmarks of management of pneumothorax include evacuation of the air from the pleural space as well as preventing recurrence of air entering into the pleural space. The American Thoracic Society has established guidelines delineating management based on several features of patient presentation.
A pneumothorax can be primary spontaneous, secondary spontaneous, or traumatic. When traumatic, blunt, crush, or penetrating trauma can usually be identified. When spontaneous, etiologies may be divided into primary or secondary. A Primary pneumothorax occurs without a precipitating event and in the absence of clinical lung disease, thus it is essentially a diagnosis of exclusion. Primary pneumothoraces have an estimated incidence of 4 per 100,000 in males and 1.1 per 100,000 in females with most occurring in patients 16–24 years of age. Many individuals that experience one PSP will experience recurrence. Risk factors identified in individuals with recurrent PSP include tall stature, cigarette smoking, low body weight, Marfan's syndrome, and homocystinuria [1].
Unlike primary pneumothorax, a secondary spontaneous pneumothorax (SSP) happens in the context of a clinically apparent lung disease. They are more common in men than women (6.3 cases per 100,000 versus 2.0 per 100,000) and older patients with a peak incidence of 60–65 years of age [2]. Commonly known conditions predisposing individuals to secondary spontaneous pneumothorax include COPD, CF, asthma, necrotizing pneumonia, interstitial lung disease (including sarcoidosis and idiopathic pulmonary fibrosis), connective-tissue disease (including rheumatoid arthritis, Marfan's syndrome, and ankylosing spondylitis), and cancer (including metastatic disease). All of these conditions can make the lung pleura more susceptible to rupture and subsequent development of pneumothorax. If suspected, consultation with sub-specialists is important in managing and preventing pneumothorax in these patients.
In females of reproductive age like our patient, catamenial pneumothorax (CP) is one explanation for recurrent pneumothorax and was our ultimate diagnosis for this patient. CP is a condition of recurrent pneumothoraces which occur within 48–72 hours from the onset of menses. CP is often associated with thoracic endometriosis syndrome (TES). Endometriosis is a common condition that is characterized by endometrial-like glands present outside of the uterus. Symptoms of endometriosis are extremely variable and dependent largely on the location of the ectopic endometrial tissue. There are several prominent hypotheses that seek to explain the presence of endometrial tissue outside of the reproductive organs and specifically in the thorax as in TES. These include the prostaglandin theory, lymphatic and hematogenous theory, coelomic metaplasia theory, and the retrograde menstruation theory. The mechanism by which blood and air enter the thoracic cavity in CP is not entirely understood. CP secondary to TES is thought to be due to “menstruation” of ectopic endometrial tissue in the thorax secondary to estrogen that allows blood and air to leak into the pleural space leading to pneumothorax and hemothorax. Thoracic endometriosis often occurs after patients have had years of symptomatic pelvic endometriosis suggesting it may represent progression of the disease [8]. Conversely, our patient had no signs of pelvic endometriosis at the time she presented with recurrent pneumothorax, making her case unusual and more diagnostically challenging.
Thoracic endometriosis has been fairly well documented in adults with a median age of 42. However, it has been much less reported or diagnosed in adolescents, though case reports do exist. In one, a 16 year-old female experiencing chest pain 2 days before menstruation eventually had Thoracoscopy which revealed multiple blueberry spots near central tendons of the diaphragm which were biopsied and identified as endometrial glands and stroma [5]. Given that lesions tend to cluster on the diaphragm as described, the incidence of CP increases when surgeons changed their usual VAT procedures to include thorough visualization of the diaphragm in cases highly suspicious for CP [4]. We suspect our patient may have lesions on the diaphragm that were not identified during VATS given low suspicion for CP at the time of the procedure. However, despite the paucity of findings on VATS, we were still able to diagnose our patient with CP using clinical observation given her sudden improvement after starting on oral contraceptives as described in the hospital course. Her case is not only interesting in that she was very young at initial presentation but had no other manifestations of endometriosis or evidence histologically or visually on VATS that would suggest the diagnosis.
As mentioned above, endometrial tissue in the thorax was never identified in our patient. It was therefore more appropriate to diagnose our patient with CP rather than TES. While TES is the most commonly cited explanation for CP other hypotheses do exist to explain the phenomenon. One such theory describes alveolar rupture caused by prostaglandin-induced bronchiolar constriction [7]. Another theory hinges on the concept of air passing from the genital tract to the diaphragm. In one case report demonstrating this theory, a 25 year old female with recurrent pneumothorax was noted on thoracoscopy to have several violet implants with holes along the right hemidiaphragm and bubbles on a pre-operative chest x-ray [3]. These and other theories do not mandate that endometrial tissue be present in the thorax for catamenial pneumothorax occur. Despite differences in pathophysiology, the medical management of catamenial pneumothorax and TES alike both focus on hormonal regulation and production of an anovulatory state.
After our patient was started on an oral continuous oral contraceptive (COC) she had no further recurrence of pneumothorax. Taking a continuous dose of combined estrogen-progestin diminishes ovarian function and suppresses ovulation. Oral contraceptives are preferred over GnRH agonists in the treatment of endometriosis as they have a milder side effect profile and can be used more long term [6]. Procedures such as thoracotomy with pleural abrasion and salpingo-oophorectomy are considered in some patients who continue to experience endometriosis related symptoms despite medical management. Our patient required no surgery or medical treatment as her condition was entirely managed with a COC. She had no residual respiratory complications despite her years long history of recurrent pneumothoraces.
Having a high index of suspicion for catmennial pneumothorax in the appropriate demographic could prevent patients from costly and dangerous workups. Establishing a temporal relationship of pneumothoraces to menstruation as well as improvement in symptoms with hormonal therapy are enough to make the diagnosis. Imaging and VATS are often unable to identify thoracic endometrial mucosa or other anatomic findings to explain symptoms. In addition to hormone therapy, patients will often go on to require pleurodesis of highly affected areas depending on the extent of diaphragmatic involvement and their individualized response to medical management.
3. Conclusion
Catamenial pneumothorax is pneumothorax that occurs within 48–72 hours of menses. It is a rare but important cause of recurrent pneumothorax in women of reproductive age including adolescents. The pathophysiology of catamenial pneumothorax is not completely understood but it is treated with hormonal therapies which produce an anovulatory state. It is often secondary to thoracic endometriosis syndrome in which ectopic endometrial tissue is present in the thorax, usually on the diaphragm. Early recognition of catamenial pneumothorax may help prevent lengthy, costly, and potentially harmful workups as well as recurrent pneumothorax.
Author disclosure
Drs Kramer and Bautista have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.100951.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dotson K., Johnson L.H. Pediatric spontaneous pneumothorax. Pediatr. Emerg. Care. 2012 Jul;28(7):715–720. doi: 10.1097/PEC.0b013e31825d2dd5. ; quiz 721-3, Review. PubMed PMID: 22766594. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y., Xie C., Rodriguez R.M., Light R.W. Factors related to recurrence of spontaneous pneumothorax. Respirology. 2005 Jun;10(3):378–384. doi: 10.1111/j.1440-1843.2005.00715.x. PubMed PMID: 15955153. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa N., Takizawa M., Yachi T., Hiranuma C., Sato H. [Catamenial pneumothorax in a young patient diagnosed by thoracoscopic surgery; report of a case] Kyobu Geka. 2003 Apr;56(4):336–339. PubMed PMID: 12701199. [PubMed] [Google Scholar]
- 4.Shrestha B., Shrestha S., Peters P., Ura M., Windsor M., Naidoo R. Catamenial pneumothorax, a commonly misdiagnosed thoracic condition: multicentre experience and audit of a small case series with review of the literature. Heart Lung Circ. 2019 Jun;28(6):850–857. doi: 10.1016/j.hlc.2019.01.012. Epub 2019 Feb 22. PubMed PMID: 30853525. [DOI] [PubMed] [Google Scholar]
- 5.Sahn S.A., Heffner J.E. Spontaneous pneumothorax. N. Engl. J. Med. 2000 Mar 23;342(12):868–874. doi: 10.1056/NEJM200003233421207. Review. PubMed PMID: 10727592. [DOI] [PubMed] [Google Scholar]
- 6.Olive D.L. Medical therapy of endometriosis. Semin. Reprod. Med. 2003 May;21(2):209–222. doi: 10.1055/s-2003-41327. Review. PubMed PMID: 12917790. [DOI] [PubMed] [Google Scholar]
- 7.Alifano M., Roth T., Broët S.C., Schussler O., Magdeleinat P., Regnard J.F. Catamenial pneumothorax: a prospective study. Chest. 2003 Sep;124(3):1004–1008. doi: 10.1378/chest.124.3.1004. PubMed PMID: 12970030. [DOI] [PubMed] [Google Scholar]
- 8.Carter E.J., Ettensohn D.B. Catamenial pneumothorax. Chest. 1990 Sep;98(3):713–716. doi: 10.1378/chest.98.3.713. Review. PubMed PMID: 2203622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.