Abstract
Gaucher disease (GD) is a lysosomal storage disorder that is associated with bi-allelic pathogenic variants in GBA. Its wide clinical spectrum, ranging from mild organomegaly to significant skeletal and neurological involvement, is partially explained by genotype-phenotype correlations. We present a family, in which all members over two generations presented with at least splenomegaly. Comprehensive clinical, biochemical and genetic workup was required to diagnose GD, which is caused by as many as four distinct GBA genotypes.
Keywords: Gaucher disease, GBA, Genotype-phenotype correlation
1. Introduction
Gaucher disease (GD) is a rare inherited condition. It is caused by bi-allelic variants in the GBA gene, which encodes acid β-glucosidase, also referred to as glucocerebrosidase. Reduced activity of this enzyme in GD patients result in lysosomal accumulation of its substrate glucosylceramide in macrophages. As Gaucher cells, these abnormal macrophages enter the liver, spleen, bone marrow and other organs. The presence of Gaucher cells eventually triggers a wide range of phenotypes including the cardinal symptoms hepato- and/or splenomegaly, bone pain and destruction, and highly abnormal blood profiles; a subset of patients also develops neurological symptoms [1]. Many Gaucher patients are initially monosymptomatic. Due to non-specificity, individual symptoms occurring in isolation are usually not considered to be an indication of a genetic disorder, let alone interpreted as GD. Age at onset and severity of the disease are highly variable and partially depend on the genotype [1]. Together with a lack of awareness due to the condition's rarity, the above factors entail a long diagnostic delay in many patients. Determination of glucocerebrosidase enzymatic activity represents a screening tool that can guide diagnosis [2]. The sphingolipid Lyso-Gb1, an alternative glucocerebroside degradation product, has recently been introduced as an additional diagnostic biomarker [3]. Due to its dynamic nature, it may also provide an objective means for determining disease severity, and for monitoring disease progression as well as therapeutic success [4].
We present a family, in which the presence of hepatomegaly and/or splenomegaly in all family members over two generations initially suggested a non-genetic etiology. However, biochemical and genetic follow up revealed an unusual constellation of three pathogenic GBA haplotypes combined into four distinct genotypes, thereby resulting in variably severe manifestation of GD.
2. Patients and methods
All family members are of Caucasian ethnicity. Following a long odyssey in search for a diagnosis, they were eventually referred to the University Hospital Center ‘Mother Teresa’ in Tirana, which is the only GD center in Albania. All biochemical and genetic tests utilized dried blood spot samples (CentoCard®, CENTOGENE AG, Germany); enzymatic testing of glucocerebrosidase activity was based on standard diagnostic procedures. Lyso-Gb1 was quantified by mass-spectrometry as described previously [3]. The GBA gene was analyzed using a three-step next generation sequencing (NGS) approach: a primary long range PCR first amplified the gene (but not its closely homologous pseudogene). Ordinary PCRs then generated secondary exon-specific products, which were eventually sequenced in an in-house multiplex NGS setting. Description of variants follows the recommendation of the Human Genome Variation Society [5]; historical nomenclature is provided upon first mention.
3. Results
3.1. Clinical presentation
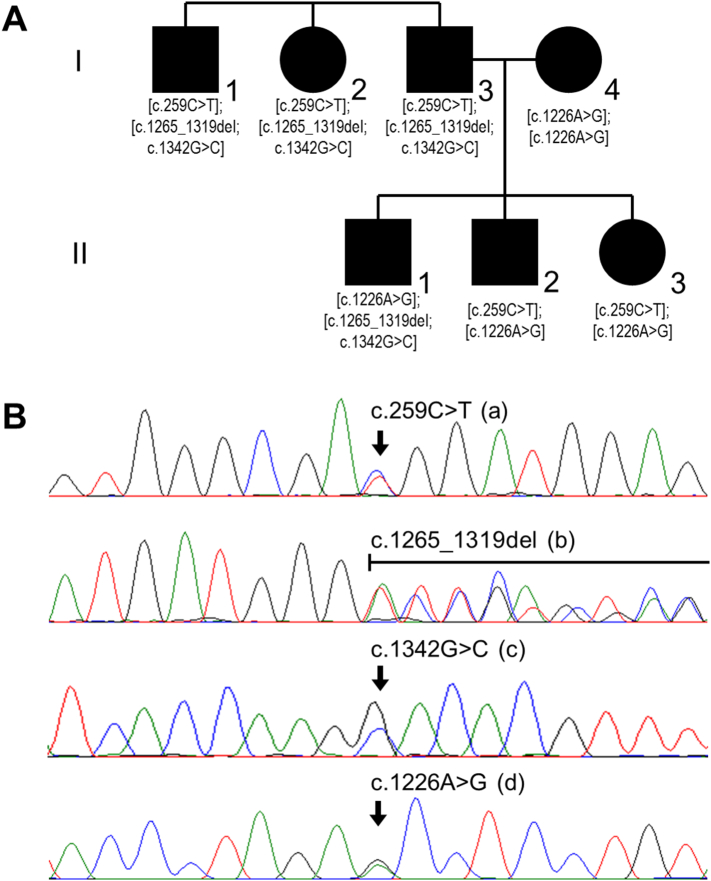
There were a total of seven patients: four in generation I and three in generation II (Fig. 1A, Table 1). Two of the three siblings in generation II presented with only splenomegaly, while their oldest brother additionally showed bone pain and thrombocytopenia. Slight hip dysplasia and a shorter right leg in II-1 are probably also part of the GD phenotype. All three siblings in generation I had presented with severe hepatosplenomegaly that led to splenectomy in their early 20's. In addition, they all reported bone pain and show thrombocytopenia. Bone pain was particularly severe in I-2, who also suffered from epistaxis. I-4 showed only mild hepatosplenomegaly. Following an eventual diagnosis of GD type 1, all patients except for I-4 now receive enzyme replacement therapy (velaglucerase alfa (VPRIV®); Shire, Lexington, MA). Of note, the father of generation I, who is an obligate carrier of either c.259C > T or c.[1265_1319del;1342G > C], reportedly shows signs of Parkinsonism.
Fig. 1.
Family structure and genetic findings. (A) Generation I consists of three affected siblings and one affected spouse, all three offspring in generation II are affected, too. GBA genotypes as eventually detected are indicated below the individuals' symbols (compare Fig. 1B). (B) Exemplary Sanger sequencing image for each variant (in heterozygosity).
Table 1.
Summary of the clinical and genetic findings.
| Patient (compare Fig. 1A) |
I-1 |
I-2 |
I-3 |
I-4 |
II-1 |
II-2 |
II-3 |
|
|---|---|---|---|---|---|---|---|---|
| Gender |
Male |
Female |
Male |
Female |
Male |
Male |
Female |
|
| Age at last examination [yrs] | 44 | 41 | 39 | 43 | 18 | 15 | 8 | |
| Clinical findings | Splenomegaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes (mild) |
| Hepatomegaly | Yes | Yes | Yes | Yes | Yes | – | – | |
| Thrombocytopenia | Yes | Yes | Yes | – | Yes | – | – | |
| Bone pain | Yes | Yes | yes (severe) | – | Yes | – | – | |
| Additional | – | – | epistaxis | – | short right leg | – | – | |
| Overall severity | Severe | Severe | Severe | Mild | Severe | Mild | Mild | |
| GBA genotype | Allele 1 | c.259C > T | c.259C > T | c.259C > T | c.1226A > G | c.1226A > G | c.259C > T | c.259C > T |
| Allele 2 | c.[1265_1319del; c.1342G > C] | c.[1265_1319del; c.1342G > C] | c.[1265_1319del; c.1342G > C] | c.1226A > G | c.[1265_1319del; c.1342G > C] | c.1226A > G | c.1226A > G | |
| Consequences at protein level | From allele 1 | p.Arg87Trp | p.Arg87Trp | p.Arg87Trp | p.Asn409Ser | p.Asn409Ser | p.Arg87Trp | p.Arg87Trp |
| From allele 2 | p.Leu422Profs*4 | p.Leu422Pfros*4 | p.Leu422Profs*4 | p.Asn409Ser | p.Leu422Profs*4 | p.Asn409Ser | p.Asn409Ser | |
| Lyso-Gb1 [ng/μl] | 264 | 1090 | 590 | 64.2 | 115 | 49.6 | 76.3 | |
| Currently under enzyme replacement therapy | Yes | Yes | Yes | No | Yes | Yes | Yes | |
3.2. Biochemical findings
The enzymatic activity of glucocerebrosidase was below the lower limit of quantification in II-2 and II-3, and was thereby pathologically decreased. Subsequent measurement of plasma concentrations of Lyso-Gb1 in all seven family members revealed pathologic values above the cutoff of 10 ng/μl. Values >100 ng/μl were observed in the three siblings of generation I as well as in II-1, while the other three individuals showed more moderate values around 50–80 ng/μl (Table 1).
3.3. Genetic findings
Sequencing of the GBA gene in the three siblings of generation I disclosed the same three heterozygous variants in each: c.259C > T (p.Arg87Trp; “R48W” according to historical nomenclature), c.1265_1319del (p.Leu422Profs*4; “55 bp deletion in exon 9”) and c.1342G > C (p.Asp448His; “D409H”). The spouse of I-3 was found to carry c.1226A > G (p.Asn409Ser; “N370S”) in homozygosity. Accordingly, c.1226A > G was present in heterozygosity in all three offspring. It was associated with c.1265_1319del and c.1342G > C in II-1, but with c.259C > T in II-2 and II-3 (Fig. 1B). Collectively, these observation enabled the definition of three pathogenic haplotypes: c.259C > T, c.[1265_1319del;1342G > C] and c.1226A > G. These are all present in generation I, and were found to be differentially re-assembled in generation II. As a consequence, there are a total of four distinct pathogenic genotypes in the family (compare Fig. 1A).
4. Discussion
The current study reports a family, which is very unusual from a clinical-genetic perspective. When referred, all members over two generations showed clinical symptoms that partially overlapped. As the pedigree was not readily compatible with a classical mode of inheritance (Fig. 1A), a non-genetic infectious etiology had been considered [e.g. [6]], but corresponding tests were negative. The presence of Parkinsonism in a first-degree relative may be considered suggestive of GD [7], but still represents a rather unspecific finding. The diagnosis of GD was ultimately reached at a specialized center, where appropriate biochemical testing and genetic follow-up were eventually initiated in all patients. The family thereby nicely exemplifies that, even in the presence of a positive family history, there are specific challenges for properly diagnosing GD [8].
The unusual clinical transmission pattern in this family may best be described as pseudodominant. This phenomenon refers to the observation of affected individuals in successive generations despite an autosomal recessive mode of inheritance; it is particularly common in populations with high carrier rates [9]. In this family, the presence of bi-allelic pathogenic variants in both parents (rather than in just one) and the distinctiveness of the variants adds further complexity.
All three GBA variants that were detected have been known as pathogenic mutations since the early days of GD genetics [10]. The variants are not only frequent in patients (HGMD), but also observed in heterozygosity in healthy individuals (gnomAD). To the best of our knowledge, however, combined occurrence in a single family is unprecedented. With the exception of c.1226G > A, all variants may be pseudogene-derived [11]. The c.[1265_1319del;1342G > C] allele, harboring two such variants in cis, is particularly suggestive in this respect.
The predicted consequences for the three pathogenic haplotypes vary. While c.[1265_1319del;1342G > C] (p.Leu422Profs*4) is a true loss-of-function allele, c.259C > T (p.Arg87Trp) and c.1226A > G (p.Asn409Ser) result in missense mutated proteins that may retain residual functionality. Moreover, c.1226A > G (p.Asn409Ser) has been shown to represent a particularly mild mutation [12]. The resulting concept of variably pathogenic genotypes [13] is nicely supported by the presented family: individual I-4 carrying p.Asn409Ser in homozygosity has the overall mildest manifestation, while compound heterozygosity for p.Asn409Ser and the ‘ordinary’ missense variant p.Arg87Trp, for p.Asn409Ser and the truncating p.Leu422Profs*4, and for p.Arg87Trp and p.Leu422Profs*4 entail ever more severe phenotypes. This becomes particularly evident in the siblings in generation II: the manifestations in patients II-2 and II-3 represent a mild clinical picture (only spenomegaly) compared with that of patient II-1, who presented far more severely with splenomegaly, hepatomegaly, thrombocytopenia and bone pain (Table 1).
Interestingly, the above genetic and clinical differences seem to also manifest at the metabolic level: Lyso-Gb1 values, covering more than one order of magnitude, nicely correlate with presumed pathogenicity of the genotypes and with clinical manifestation (Table 1). This observation corroborates previous related claims [3]. Together with the accumulating evidence for Lyso-Gb1 to enable therapeutic monitoring in GD [4,14], it also argues for a more widespread utilization of this biomarker, and for an extension of metabolic biomarker development to related genetic disorders [15].
Competing interests
CB, PB and AR are employees of CENTOGENE AG (Rostock, Germany).
Acknowledgments
Acknowledgement
We thank the family for participating.
References
- 1.Pastores G.M., Hughes D.A. Gaucher disease. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., editors. Genereviews((R)) L. J. H. Bean; K. Stephens, and A. Amemiya. Seattle (WA): 1993. [Google Scholar]
- 2.Reuser A.J., Verheijen F.W., Bali D., van Diggelen O.P., Germain D.P., Hwu W.L., Lukacs Z., Muhl A., Olivova P., Piraud M., Wuyts B., Zhang K., Keutzer J. The use of dried blood spot samples in the diagnosis of lysosomal storage disorders – current status and perspectives. Mol. Genet. Metab. Sep-Oct 2011;104(1–2):144–148. doi: 10.1016/j.ymgme.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Rolfs A., Giese A.K., Grittner U., Mascher D., Elstein D., Zimran A., Bottcher T., Lukas J., Hubner R., Golnitz U., Rohle A., Dudesek A., Meyer W., Wittstock M., Mascher H. Glucosylsphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurvitz N., Dinur T., Becker-Cohen M., Cozma C., Hovakimyan M., Oppermann S., Demuth L., Rolfs A., Abramov A., Zimran A., Revel-Vilk S. Glucosylsphingosine (Lyso-Gb1) as a biomarker for monitoring treated and untreated children with Gaucher disease. Int. J. Mol. Sci. Jun 21 2019;20(12) doi: 10.3390/ijms20123033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Dunnen J.T., Dalgleish R., Maglott D.R., Hart R.K., Greenblatt M.S., McGowan-Jordan J., Roux A.F., Smith T., Antonarakis S.E., Taschner P.E. Hgvs recommendations for the description of sequence variants: 2016 update. Hum. Mutat. Jun 2016;37(6):564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 6.Okano M. Recent concise viewpoints of chronic active Epstein-Barr virus infection. Curr. Pediatr. Rev. 2015;11(1):5–9. doi: 10.2174/1573396311666150501002809. [DOI] [PubMed] [Google Scholar]
- 7.O'Regan G., deSoza R., Balestrino R., Schapira A.H. Glucocerebrosidase mutations in Parkinson disease. J. Park. Dis. 2017;7:411–422. doi: 10.3233/JPD-171092. [DOI] [PubMed] [Google Scholar]
- 8.Nalysnyk L., Rotella P., Simeone J.C., Hamed A., Weinreb N. Gaucher disease epidemiology and natural history: a comprehensive review of the literature. Hematology. Mar 2017;22(2):65–73. doi: 10.1080/10245332.2016.1240391. [DOI] [PubMed] [Google Scholar]
- 9.Wilkie Andrew O.M. Els. Wiley; 2018. Dominance and recessivity. [Google Scholar]
- 10.Horowitz M., Zimran A. Mutations causing Gaucher disease. Hum. Mutat. 1994;3(1):1–11. doi: 10.1002/humu.1380030102. [DOI] [PubMed] [Google Scholar]
- 11.Hruska K.S., LaMarca M.E., Scott C.R., Sidransky E. Gaucher disease: mutation and polymorphism Spectrum in the Glucocerebrosidase gene (Gba) Hum. Mutat. May 2008;29(5):567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 12.Fairley C., Zimran A., Phillips M., Cizmarik M., Yee J., Weinreb N., Packman S. Phenotypic heterogeneity of N370s homozygotes with type I Gaucher disease: an analysis of 798 patients from the Icgg Gaucher registry. J. Inherit. Metab. Dis. Dec 2008;31(6):738–744. doi: 10.1007/s10545-008-0868-z. [DOI] [PubMed] [Google Scholar]
- 13.Smith L., Mullin S., Schapira A.H.V. "insights into the structural biology of Gaucher disease." Exp Neurol 298, no. Pt B. Dec 2017:180–190. doi: 10.1016/j.expneurol.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Elstein D., Mellgard B., Dinh Q., Lan L., Qiu Y., Cozma C., Eichler S., Bottcher T., Zimran A. Reductions in Glucosylsphingosine (Lyso-Gb1) in treatment-naive and previously treated patients receiving Velaglucerase alfa for type 1 Gaucher disease: data from phase 3 clinical trials. Mol. Genet. Metab. Sep 2017;122(1–2):113–120. doi: 10.1016/j.ymgme.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Cox T.M. Biomarkers in lysosomal storage diseases: a review. Acta Paediatr. Suppl. Mar 2005;94(447):39–42. doi: 10.1111/j.1651-2227.2005.tb02109.x. [DOI] [PubMed] [Google Scholar]