Highlights
-
•
The study established a subregional-level anatomic alteration profile of subcortical structures in patients with obsessive-compulsive disorder (OCD).
-
•
The OCD patients showed an expansion of the lateral amygdala (right hemisphere) and right pallidum.
-
•
Deformities in pallidum were associated with illness duration and symptom severity of OCD.
-
•
Gender difference in OCD-related morphometric alterations were found in amygdala and caudate.
Key words: Obsessive-compulsive disorder, Shape, Amygdala, Striatum, Gender
Abstract
Background
Subcortical nuclei are important components in the pathology model of obsessive-compulsive disorder (OCD), and subregions of these structures subserve different functions that may distinctively contribute to OCD symptoms. Exploration of the subregional-level profile of structural abnormalities of these nuclei is needed to develop a better understanding of the neural mechanism of OCD.
Methods
A total of 83 medication-free, non-comorbid OCD patients and 93 age- and sex-matched healthy controls were recruited, and high-resolution T1-weighted MR images were obtained for all participants. The volume and shape of the subcortical nuclei (including the nucleus accumbens, amygdala, caudate, pallidum, putamen and thalamus) were quantified and compared with an automated parcellation approach and vertex-wise shape analysis using FSL-FIRST software. Sex differences in these measurements were also explored with an exploratory subgroup analysis.
Results
Volumetric analysis showed no significant differences between patients and healthy control subjects. Relative to healthy control subjects, the OCD patients showed an expansion of the lateral amygdala (right hemisphere) and right pallidum. These deformities were associated with illness duration and symptom severity of OCD. Exploratory subgroup analysis by sex revealed amygdala deformity in male patients and caudate deformity in female patients.
Conclusions
The lateral amygdala and the dorsal pallidum were associated with OCD. Neuroanatomic evidence of sexual dimorphism was also found in OCD. Our study not only provides deeper insight into how these structures contribute to OCD symptoms by revealing these subregional-level deformities but also suggests that gender effects may be important in OCD studies.
1. Introduction
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with obsessions and compulsions as the main symptom profile and has a worldwide lifetime prevalence rate of 1–3% (Adam et al., 2012; Hirschtritt, et al., 2017; Ruscio et al., 2010). Since the early studies, OCD has been associated with the circuitry referred to as the cortico-striato-thalamo-cortical (CSTC) pathway, particularly interconnection across frontal cortical areas (prefrontal and orbitofrontal cortex), the ventral striatum (nucleus accumbens), ventral pallidum and mediodorsal thalamus (Menzies et al., 2008). With the emergence and evolution of modern brain imaging technologies, additional brain regions, especially the amygdala and dorsomedial and dorsolateral striatum (caudate and putamen, respectively), are joining refined neurocircuitry models of OCD (Atmaca et al., 2008; Gottlich et al., 2014; Marsh et al., 2015; Ullrich et al., 2018).
Previous in vivo structural magnetic resonance imaging (MRI) studies of patients with OCD have largely been volume-based or voxel-based, which is not sufficient to detect morphometric alterations at the subregional level of these aforementioned subcortical nuclei. However, a subregional-level analysis may of particular importance for the complex anatomical and functional nature of these structures. Subregions of subcortical nuclei have proven to subserve connections between different and sometimes overlapping regions of the cortex and thalamus, forming organizationally parallel and functionally segregated circuits (Alexander et al., 1986). For instance, the dorsal and ventral subregions of the caudate are involved in the dorsolateral prefrontal (dlPFC) circuit and orbitofrontal cortex (OFC) circuit, respectively (Alexander et al., 1986). External and internal segments of the pallidum (GPe and GPi) are separately recruited in the direct and indirect pathways as part of the intraconnections of the basal ganglia, and are responsible for reinforcement and punishment in the reward system, respectively (Gunaydin and Kreitzer, 2016; Kravitz et al., 2012). Hence, a subregional analysis of these subcortical nuclei in OCD may provide deeper insights into how these abnormalities contribute to OCD symptoms and will enrich the pathology model of OCD. A prior study with a large sample of OCD patients used cross-sectional images to represent basal ganglia anatomy, and they reported deformations in the basal ganglia of OCD patients that may not be accompanied by changes in volume (Pujol et al.,2011). Few articles using surface or vertex-based analyses have found subregional-level abnormalities in the caudate and thalamus; however, these results were inconsistent, possibly due to the small sample size or confounding effects of medication (Choi et al., 2007; Kang et al., 2008; Shaw et al., 2015; Zarei et al., 2011).
Variations in subcortical brain structures and morphology might be affected by clinical and environmental factors, such as age, medication status and comorbidity (Boedhoe et al., 2017; de Wit et al., 2014; Hu et al., 2017), but another considerable proportion of the variation is contributed by sex (Zohar et al., 1999). Sexual dimorphism in patients with OCD has been demonstrated in symptom profile and genetic association studies (Alvarenga et al., 2015; Mathis et al., 2011; Taylor, 2013). In addition, gender differences in subcortical nuclei were also demonstrated in a meta-analysis (Rijpkema et al., 2012; Ruigrok et al., 2014). Therefore, gender as a factor should be taken into consideration in studies of OCD (Mattina and Steiner, 2016; Zohar et al., 1999). However, gender differences have rarely been investigated in patients with OCD using neuroimaging approaches.
In the current study, we recruited a relatively large sample of medication-free, non-comorbid adult patients with OCD that minimized the confounding effects of medications and sex- and age-matched healthy control (HC) subjects, aiming to investigate (1) subregional deformities in subcortical nuclei that are involved in the current CSTC circuit, including the putamen, caudate and nucleus accumbens, pallidum, thalamus and amygdala, using an automatic segmentation and vertex-wise shape analysis protocol; and (2) gender differences in morphometric alterations in these structures using a subgroup analysis stratified by gender. We hypothesize that specific subregions of these subcortical nuclei are involved in the pathology of OCD and that male and female patients show distinct patterns of subcortical abnormalities.
2. Materials and methods
2.1. Participants
This study was approved by the Research Ethics Committee of West China Hospital, Sichuan University, and fully informed written consent was obtained from each participant. All participants were right-handed and native Chinese speakers. A total of 94 OCD patients diagnosed using DSM-IV criteria were recruited from the Mental Health Center in West China Hospital of Sichuan University. Clinical diagnoses were established via the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) by two experienced psychiatrists (B. Li and W. Tang). The Yale-Brown Obsessive Compulsive Scale (YBOCS) was used to assess the severity of OCD symptoms, whereas the 17-item Hamilton Depression Scale (HAMD) and 14-item Hamilton Anxiety Scale (HAMA) were used to rate depressive and anxiety symptoms, respectively. Among these patients, 14 previously received medication for OCD (4 were on clomipramine hydrochloride, 3 on paroxetine hydrochloride, 3 on fluoxetine hydrochloride, 3 on sertraline and 1 on three types of drugs including clomipramine hydrochloride, paroxetine hydrochloride and quetiapine fumarate) but had been medication-free for more than four weeks before the MRI scan, while the remaining patients recruited were medication-naïve. The exclusion criteria were as follows: (1) younger than 18 or older than 60 years of age; (2) psychiatric comorbidity assessed using the SCID; (3) any history of major physical illness, cardiovascular disease or psychiatric or neurological disorder; (4) current or any history of substance abuse or dependence; and (5) pregnancy.
Ninety-five age- and sex-matched HCs were recruited from the local area using poster advertisements and were screened using the SCID (non-patient edition) to confirm the absence of any current or historical psychiatric and neurological illness and the absence of a history of psychiatric illness among first-degree relatives.
2.2. Image acquisition
For all subjects, high-resolution, T1-weighted images were acquired using a volumetric 3-dimensional spoiled gradient recall (SPGR) sequence via a 3.0 T MRI system (EXCITE, General Electric, Milwaukee, WI, USA) with an eight-channel phased array head coil. The acquisition parameters included TR=8.5 ms, TE=3.4 ms, flip angle=12°, and slice thickness=1.0 mm. A field of view (240 × 240 mm2) was used with an acquisition matrix comprising 256 readings of 128 phase encoding steps that produced 156 contiguous coronal slices with a slice thickness of 1.0 mm. The final matrix size of T1-weighted images was automatically interpolated in-plane to 512 × 512, which yielded an in-plane resolution of 0.47 × 0.47 mm2. Foam padding and earplugs were used to reduce head motion and scanner noise.
2.3. Image analysis
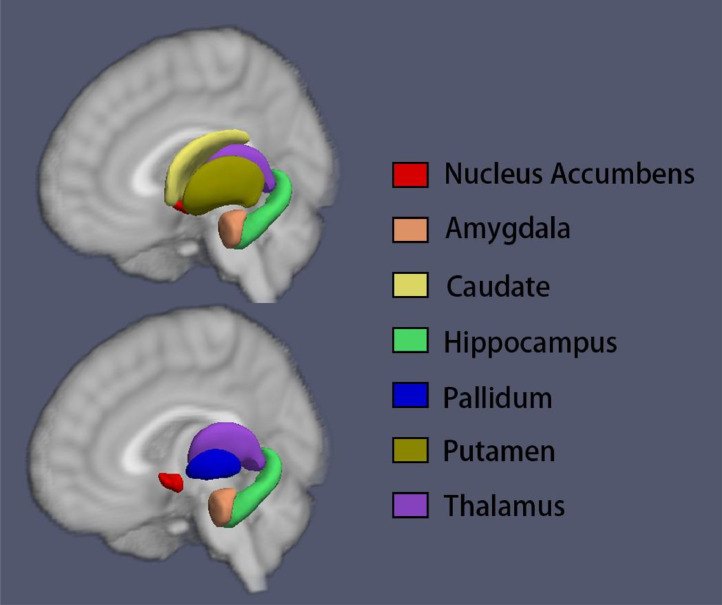
FIRST (Patenaude et al., 2011), a model-based segmentation and registration module implemented in FSL (Jenkinson et al., 2012) software (version 5.0.9, which can be downloaded at the official website: https://fsl.fmrib.ox.ac.uk/) was used to automatically segment the subcortical nuclei, including the nucleus accumbens, caudate, pallidum, putamen, amygdala and thalamus (Fig. 1). Briefly, all subcortical nuclei were segmented using the run_first_all script, which includes initial whole brain image registration, brain tissue extraction, signal intensity normalization, and segmentation of structures with optimal settings for each structure (by default). Whole brain images were registered to a common space based on the nonlinear MNI152 template, while shape variance was reserved after registration. In the second stage, a subcortical mask built from manual segmentation of 128 subjects was used to exclude regions outside the subcortical structures. After alignment, meshes were generated by a deformable model that iteratively updates the vertex locations according to a weighted sum of displacements (Patenaude et al., 2011). After the meshes were generated, localized shape changes were directly compared by analysing vertex locations, which considers the differences in mean vertex position between the groups. To exam the quality of the segmentations, label images were masked on original T1-weighted whole brain images and were visually checked. We followed ENIGMA quality control protocol for subcortical shape (http://enigma.usc.edu/), and consistency was obtained between two independent researchers (Zhang L and Hu X). Subjects (OCD group: 11 out of 94; HC group: 2 out of 95) showing inaccurate segmentation were rolled out in the following analysis. Vertex analysis was then conducted with bvars files (outcome files of run_first_all), and meshes were reconstructed in the MNI space (–useReconMNI). The output files of the vertex analysis were then used in the statistical analysis.
Fig. 1.
An example of a vertex analysis from a healthy subject using FSL. Subcortical nuclei were segmented from the whole brain T1-weighted images using FSL software, and the output files containing mesh parameters for each subcortical structure from each subject were then used for subsequent volumetric and shape analyses.
Volumes of structures were extracted with the run_first_all output files. Furthermore, total intracranial volume (ICV) was obtained by FreeSurfer software (V. 6.0) (http://surfer.nmr.mgh.harvard.edu/), using its standard recon-all pipeline (Fischl et al., 2002)
2.4. Statistical analysis
Statistical analysis for demographic data was conducted using the Statistical Package for the Social Sciences (SPSS, version 17.0) software. Differences between the OCD and HC groups were examined using a two-sample t-test for continuous variables and a chi-square test for categorical variables. Additionally, differences in the YBOCS (obsession, compulsion and overall), HAMA and HAMD scores between male and female patients were further examined using a two-sample t-test.
The differences in nuclei volumes between groups were compared using analysis of covariance (ANCOVA) with age and ICV as covariates. Additional ANCOVA was used to compare volume differences between gender subgroups (male patients vs. male HCs, female patients vs. female HCs) also adjusted for age and ICV. A false discovery rate (FDR) correction was used to correct for multiple testing.
Statistical analysis of nuclei shape was conducted using the implemented module “randomise” in FSL (Winkler et al., 2014). To compare the differences in the shape of the nuclei between OCD patients and HCs, we built a general linear model with diagnosis as the independent variable and the meshes of nuclei as dependent variables (2-sample t-test design), and we then ran a permutation test. Threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009), a method for finding "clusters" in data without defining the clusters in a binary manner, was used for thresholding option, and the family-wise error (FWE) rate was controlled with multiple test corrections (the number of permutation tests was set at 5000). A separate t-test model was built to test shape differences between gender subgroups. This procedure was repeated between medication-naïve patients (n = 69) and 93 HCs to exclude a possible medication effect.
To test correlations between clinical variables with subcortical nuclei vertex shape in the patient group, we built a linear regression model with all clinical variables as independent variables and meshes as dependent variables. For heuristic purposes, the Monte Carlo method was used in “randomise” to correct for multiple testing (the number of permutation tests was set at 500). This correlation test was repeated separately in male patients and female patients.
3. Results
3.1. Demographics
Demographic information regarding the groups of participants is displayed in Table 1. There were no significant differences between all OCD patients and all HC participants in terms of age and gender.
Table 1.
Demographic and clinical variables.
| OCDN = 83 | HCN = 93 | P values | |
|---|---|---|---|
| Age, years, mean (S.D.) | 29.1 (8.8) | 28.1 (10.8) | 0.75 |
| Male | 27.6 (8.1) | 26.9 (11.0) | 0.66 |
| Female | 31.0 (9.3) | 30.0 (10.4) | 0.53 |
| Sex (m/f) | 47/36 | 57/36 | 0.53 |
| Illness duration, years, mean (S.D.) | 7.5 (5.8) | NA | – |
| Male | 7.4 (5.5) | 0.90⁎ | |
| Female | 7.6 (6.2) | ||
| YBOCS score, mean (S.D.) | 21.6 (5.4) | NA | – |
| Male | 22.4 (5.3) | 0.03⁎ | |
| Female | 20.6 (5.5) | ||
| Obsession score, mean (S.D.) | 13.3 (5.0) | NA | – |
| Male | 14.4 (4.7) | 0.60⁎ | |
| Female | 11.9 (5.1) | ||
| Compulsion score, mean (S.D.) | 8.29 (5.2) | NA | – |
| Male | 8.0 (5.2) | 0.14⁎ | |
| Female | 8.6 (5.3) | ||
| HAMA score, mean (S.D.) | 9.17 (4.7) | NA | – |
| Male | 8.9 (4.6) | 0.61⁎ | |
| Female | 9.5 (5.0) | ||
| HAMD score, mean (S.D.) | 9.53 (5.5) | NA | – |
| Male | 9.4 (4.7) | 0.81⁎ | |
| Female | 9.7 (6.5) |
Note: YBOCS, Yale-Brown Obsessive Compulsive Scale; HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale. *Significance for t-test between male and female patients.
There were no significant differences between the male and female OCD patients in terms of demographics and most clinical symptoms. However, YBOCS scores were significantly higher in the male patients than in the female patients (p = 0.03, Table 1).
3.2. Volume
There were no significant volume differences in any nuclei between all OCD patients and all HCs, after FDR correction for multiple hypothesis testing (Supplementary Table 1 & 2). However, the p values for the right amygdala (p = 0.005) and left pallidum (p = 0.037) reached the nominal threshold of significance (p<0.05). The right amygdala (p = 0.007) and left accumbens areas (p = 0.036) showed significance at the nominal threshold between the male patients with OCD and the male HCs (Supplementary Table 2).
3.3. Shape
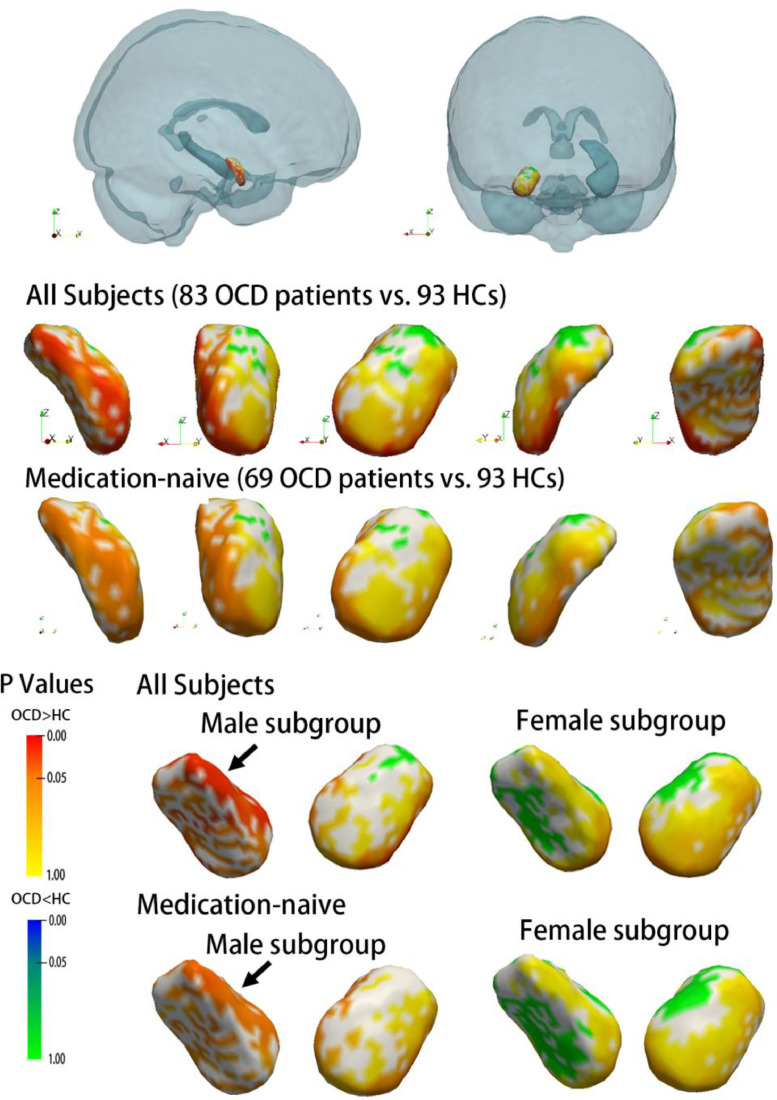
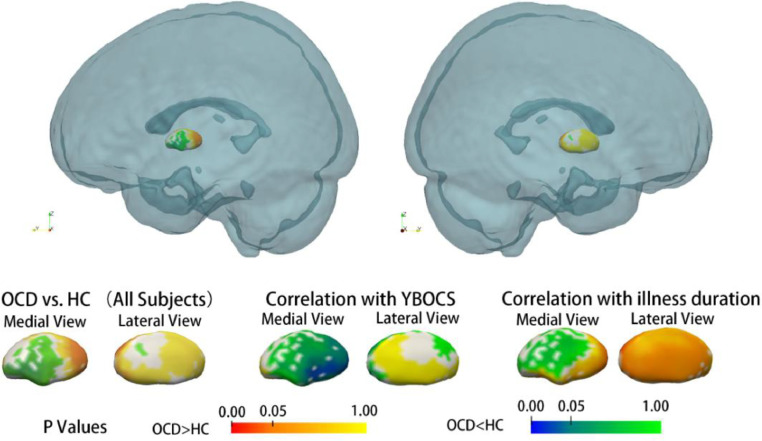
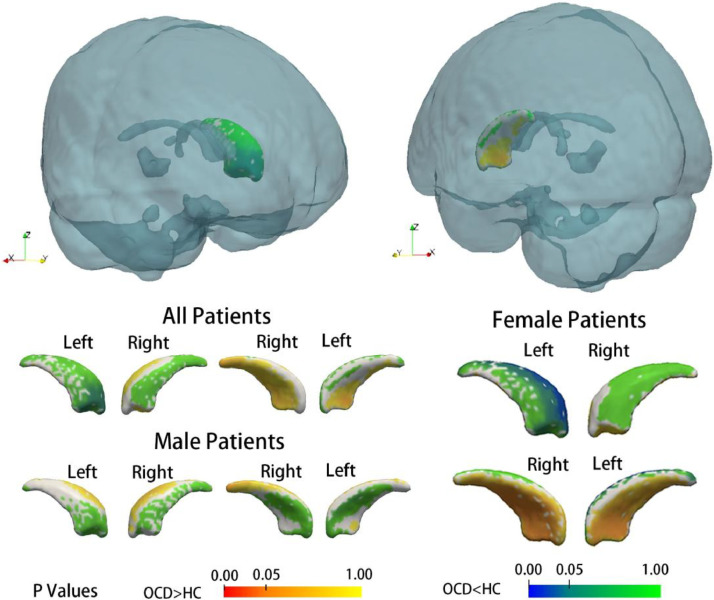
As revealed by vertex-wise shape analysis, all subcortical nuclei showed a trend of lateral shifting in all patients with OCD compared with that in all HCs (Supplementary Figure 1). In particular, the expansion of subregions of the right amygdala and right pallidum survived FWE correction (Fig. 2, Fig. 3). Subsequent subgroup analysis by gender showed gender differences in the nuclei of the amygdala and caudate. The right amygdala showed significant deformation in the male patients with OCD compared with the male HCs, but this effect was not observed in the female patients with OCD (as compared with the female HCs) (Fig. 2). The left caudate showed significant compression on the dorsal medial subregion only in female patients with OCD as compared with female HCs (Fig. 4). To exclude possible age effect, the group comparison was repeated with age as a covariate, and the results remained stable. These deformations remained significant in the comparison between medication-naïve patients and HCs (Supplementary Figure 2)
Fig. 2.
Subregional deformity of the right amygdala in patients with OCD compared with healthy control subjects. The relative position of the right amygdala in the whole brain is shown in the first row, followed by vertex-wise comparison results between the OCD and HC groups. The results of the subgroup analysis by gender are shown in the bottom line. Additional analyses between only medication-naïve subjects and HCs are shown, and the results remained stable. All p values shown were corrected for FWE.
Fig. 3.
Subregional deformity of the pallidum was observed in OCD patients compared with healthy control subjects, and this deformity was associated with longer illness duration. In addition, compression in the medial pallidum was associated with a higher severity of OCD symptoms, as evaluated by the YBOCS score. All p values shown were corrected for multiple testing.
Fig. 4.
Subregional deformity was significant in female patients with OCD compared with female healthy control subjects, but there were no differences in the male patients. The p values shown were corrected for multiple testing using the FWE method.
Significant associations were also found in patients with OCD between clinical variables and subregional deformations of some nuclei. First, a longer illness duration was associated with the bilateral expansion of the lateral pallidum, as shown in all OCD patients (Fig. 3). This association between illness duration and deformation in the pallidum was found only in the female patients in the subsequent analysis by gender subgroup (Supplementary Figure 3). A longer illness duration was also associated with compression on the medial putamen (right hemisphere, all OCD patients), and this effect was stronger in the female patients with OCD than in the male patients with OCD (Supplementary Figure 4).
Second, the severity of the overall clinical symptoms, as evaluated by the YBOCS scores, was associated with measures of the amygdala and pallidum (Supplementary Figure 4). Severe OCD symptoms were associated with compression on the medial side of the left amygdala and compression on the lateral pallidum on the right hemisphere in patients with OCD. These aforementioned associations were found in all OCD patients.
4. Discussion
In the present study, we found shape deformations but not the volume differences in the subcortical nuclei in OCD using a relatively large sample of medication-free patients. The right amygdala and right pallidum were found to be significantly deformed in patients with OCD compared with those in the HCs. Meanwhile, deformation in the pallidum was associated with both illness duration and OCD symptom severity, and deformation in the amygdala was associated with OCD symptom severity. Interestingly, in the male patients with OCD, there was a significant deformity in the amygdala compared with male HCs, while in the female patients with OCD, there was a significant deformity in the caudate compared with female HCs. These aforementioned findings provide neuroanatomic evidence of OCD pathology that has not been detectable by the traditional volumetric approach.
Although the amygdala was not included in the traditional CSTC circuit, which is called the ‘OCD circuit’, it joined this circuit recently for its well-established role in anxiety regulation, fear learning and reinforcement learning that may be implicated in OCD and for its rich interactions with the CSTC circuit (Baxter and Murray, 2002; LeDoux, 2003, 2007; Milad and Rauch, 2012). However, the amygdala consists of several functionally distinct subnuclei that may differentially contribute to OCD symptoms, and this possibility had not been previously clarified in human subjects (LeDoux, 2007). A recent animal study found that increased thalamo-amygdala activity was associated with OCD-like behaviours in mice, and this hyperactivity in the thalamo-amygdala projection was associated with specific morphometric alterations (increased total spine number on dendritic branches) of the lateral amygdala (LA) (Ullrich et al., 2018). Based on the LA function and recent pathology theory of OCD (Huang and Reichardt, 2001; Johansen et al., 2010; Milad and Rauch, 2012), the regional enlargement of the LA found in our study could contribute to OCD symptoms by increasing fear conditioning, although this speculation should be verified in future studies. More importantly, it provided important subregional information that may not be detectable by previous volumetric analyses (Atmaca et al., 2008; Szeszko et al., 2004, 1999).
A larger volume in the pallidum was previously reported in patients with OCD without much information about the location of regional change in this pivotal structure (Boedhoe et al., 2017; Fouche et al., 2016). In the present study, we found an expansion of the dorsal pallidum, also known as the external segment of globus pallidus (GPe), combined with a compressed ventral pallidum, or the internal segment of globus pallidus (GPi). In addition, the expansion of the caudal end of the right pallidum survived correction for multiple testing, and this expansion on the bilateral caudal end of the pallidum was associated with a longer illness duration. Similar results have been found in medicated OCD patients (Shaw et al., 2015). Compression of the ventral pallidum was also associated with higher YBOCS scores and obsession scores in patients with OCD.
In the classical model of intrinsic connectivity of the basal ganglia, the striatum serves as the primary input nucleus, which can be subdivided into a direct pathway for persistent reinforcement and an indirect pathway for transient punishment (Gunaydin and Kreitzer, 2016; Kravitz et al., 2012). The GPe is an important structure in both direct and indirect pathways, but the GPi is only involved in the indirect pathway (Gunaydin and Kreitzer, 2016). Our present findings may indicate a disruption between the direct pathway (reinforcement) and indirect pathway (punishment) in the reward system in patients with OCD. The involvement of both the GPe and GPi in the pathology of OCD is supported by the observation that anti-compulsive effects were only observed when both the entopeduncular nucleus and globus pallidus (the rat equivalent of the primate GPi and GPe) were stimulated in a rat model of OCD (Djodari-Irani et al., 2011; Klavir et al., 2011). However, this speculation remains to be tested in the future studies.
It is also worth noting that most significant deformations in OCD patients as compared with HCs are located in the right hemisphere, suggesting there might be a laterality effect in OCD pathology. Function of the amygdala had been shown to be hemisphere-specific in previous studies. For example, the left amygdala is more frequently reported to exhibit greater activation than the right amygdala in emotion-related tasks, while the right amygdala has been shown to be associated with pain processing (Baas D, et al., 2004; Ji G & Neugebauer V, 2009). Our finding of volume reduction in the right amygdala in OCD is consistent with some previous studies (Szeszko et al., 1999, 2004). Moreover, since right amygdala deformation was found only in male patients, while left caudate deformity was only found in female patients, we postulated this lateral effect might be gender-specific. The underlying mechanism is worth exploring in future studies.
Subgroup analyses stratified by gender found that in the male patients with OCD, compared with the male HCs, the deformity was significant in the amygdala, while in the female patients with OCD, compared with the female HCs, the deformation of the subcortical nuclei was only found in the left caudate, presenting as compression within the dorsolateral head and extending to the tail of the caudate. This particular subregion of the caudate may participate in processes subserving spatial memory, which is impaired in patients with OCD (Alexander et al., 1986; Morein-Zamir et al., 2010). Research has reported significant sex-by-group interactions in the performance of non-verbal memory tasks in OCD patients, and male patients showed more severe impairments than female patients (Segalas et al., 2010). Whether impairment of spatial memory was more profound in female patients is currently unknown, but there are some clinical features we do know that are different in female and male OCD patients. For example, early-onset (before puberty) OCD is more likely to affect males than females, but late-onset (adult) OCD affects more females than males (Taylor, 2011). Female patients are more likely to show symptoms along the dimension of ‘contamination/cleaning’, while male patients have a higher frequency of sexual and religious obsessions (Cherian et al., 2014; Zhang et al., 2013). Our findings might provide a neural basis for the gender differences in the pathology underlying OCD. In male patients, the amygdala abnormality was more profound, while in female patients, implicated differences in the dorsolateral prefrontal circuit was more significant.
The biological meaning of shape alterations in subcortical structures is not clear currently. However, pioneering studies have indicated possible genetic variants that could influence the localized morphometry of subcortical structures. Hibar et al. reported five genetic variants that influence the morphometry of the putamen and caudate nuclei using data collected by the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) consortium (Hibar et al., 2015). These variations in genes could influence the morphometry of subcortical nuclei through various methods, for example, by altering the foetal development process or influencing the synaptic density of related brain regions. In another study comparing subcortical morphometry across OCD patients, their unaffected siblings (which represent a population with a high genetic risk for the disorder) and healthy control subjects also showed similar alterations in brain morphometry that could indicate a genetic basis for brain morphometry (Shaw et al., 2015). While findings in the current study cannot fully reveal the biological mechanism of the pathogenesis of OCD, we expect these findings could provide insights for future studies.
Although we were able to locate subregional impairments in the amygdala and pallidum by vertex-wise analysis, which provides a deeper understanding of how these structures contribute to OCD symptoms, there are limitations in our present study. First, our sample excluded OCD patients with comorbidities. This would help identify OCD-specific features, but the results may not be applicable to the general OCD population. Particularly, the exclusion of patients with comorbid neurodevelopmental disorders such as Tourette's syndrome or autism may induce bias in the sample, especially for male patients. Second, some patients (14 out of 83) had been medicated before recruitment. Although they had been medication-free for over four weeks, and we conducted an additional analysis between medication-naïve patients and HCs finding effects that were similar to those for the comparison between all of the patients and HCs, the long-term effects of the drugs on brain morphometry and shape cannot be excluded. Third, some of the deformities of the structures correlated with clinical measurements but did not survive multiple testing corrections. Hence, the correlations found in the current study should be interpreted with caution. Finally, we admit that the approaches used to generate and compare the meshes might affect the results. However, we believe that the performance of the vertex-based shape analysis in FSL, which was used in this study, is relatively reliable (Hibar et al., 2015).
5. Conclusion
In the current study, with a large sample of medication-free OCD patients, we found a characteristic expansion in the LA and dorsal pallidum. Further, our exploratory analysis provides neuroanatomic evidence of sexual dimorphism in OCD, which had been overlooked in the literature. Taken together, we are able to locate subregional impairments in the amygdala and pallidum by vertex-wise analysis and, by combining known functions of these subregions, we provide insight into how these structures contribute to OCD symptoms.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Ethical statement
The authors declare that they have no conflicts of interest.
This study enrolled human subjects and was approved by the Research Ethics Committee of West China Hospital, Sichuan University; fully informed written consent was obtained from each participant.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Disclosures and Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81671669 and 81171488), the Youth Technology Grant of Sichuan Province (No. 2017JQ0001) and the Post-Doctor Research Project from West China Hospital and Sichuan University (Grant No. 2019HXBH022). This financial support was used for subject enrolment and data collection.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102040.
Appendix. Supplementary materials
References
- Adam Y., Meinlschmidt G., Gloster A.T., Lieb R. Obsessive-compulsive disorder in the community: 12-month prevalence, comorbidity and impairment. Soc. Psychiatry Psychiatr Epidemiol. 2012;47(3):339–349. doi: 10.1007/s00127-010-0337-5. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alvarenga P.G., Cesar R.C., Leckman J.F., Moriyama T.S., Torres A.R., Bloch M.H., do Rosario M.C. Obsessive-compulsive symptom dimensions in a population-based, cross-sectional sample of school-aged children. J. Psychiatr. Res. 2015;62:108–114. doi: 10.1016/j.jpsychires.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Yildirim H., Ozdemir H., Ozler S., Kara B., Ozler Z., Tezcan E. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(5):1283–1286. doi: 10.1016/j.pnpbp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Baxter M.G., Murray E.A. The amygdala and reward. Nat. Rev. Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Baas D., Aleman A., Kahn R.S. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res. Brain Res. Rev. 2004;45(2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. May. [DOI] [PubMed] [Google Scholar]
- Boedhoe P.S., Schmaal L., Abe Y., Ameis S.H., Arnold P.D., Batistuzzo M.C., van den Heuvel O.A. Distinct subcortical volume alterations in pediatric and adult OCD: A worldwide Meta- and Mega-Analysis. Am. J. Psychiatry. 2017;174(1):60–69. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian A.V., Narayanaswamy J.C., Viswanath B., Guru N., George C.M., Bada Math S., Janardhan Reddy Y.C. Gender differences in obsessive-compulsive disorder: findings from a large Indian sample. Asian J. Psychiatr. 2014;9:17–21. doi: 10.1016/j.ajp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Kim S.H., Yoo S.Y., Kang D.H., Kim C.W., Lee J.M., Kwon J.S. Shape deformity of the corpus striatum in obsessive-compulsive disorder. Psychiatry Res. 2007;155(3):257–264. doi: 10.1016/j.pscychresns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- de Wit S.J., Alonso P., Schweren L., Mataix-Cols D., Lochner C., Menchon J.M., van den Heuvel O.A. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatry. 2014;171(3):340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- Djodari-Irani A., Klein J., Banzhaf J., Joel D., Heinz A., Harnack D., Winter C. Activity modulation of the globus pallidus and the nucleus entopeduncularis affects compulsive checking in rats. Behav. Brain Res. 2011;219(1):149–158. doi: 10.1016/j.bbr.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fouche J.P., du Plessis S., Hattingh C., Roos A., Lochner C., Soriano-Mas C., van den Heuvel O.A. Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br. J. Psychiatry. 2016 doi: 10.1192/bjp.bp.115.164020. [DOI] [PubMed] [Google Scholar]
- Gottlich M., Kramer U.M., Kordon A., Hohagen F., Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2014;35(11):5617–5632. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin L.A., Kreitzer A.C. Cortico-Basal ganglia circuit function in psychiatric disease. Annu. Rev. Physiol. 2016;78:327–350. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- Hibar D.P., Stein J.L., Renteria M.E., Arias-Vasquez A., Desrivières S., Jahanshad N., Aribisala B.S. Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546):224. doi: 10.1038/nature14101. Hirschtritt, M. E., Bloch, M. H., & Mathews, C. A. (2017). Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. Jama, 317(13), 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Du M., Chen L., Li L., Zhou M., Zhang L., Gong Q. Meta-analytic investigations of common and distinct grey matter alterations in youths and adults with obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017;78:91–103. doi: 10.1016/j.neubiorev.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Ji G., Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J. Neurophysiol. 2009;102(4):2253–2264. doi: 10.1152/jn.00166.2009. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J.P., Hamanaka H., Monfils M.H., Behnia R., Deisseroth K., Blair H.T., LeDoux J.E. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl. Acad. Sci. U.S.A. 2010;107(28):12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.H., Kim S.H., Kim C.W., Choi J.S., Jang J.H., Jung M.H., Kwon J.S. Thalamus surface shape deformity in obsessive-compulsive disorder and schizophrenia. Neuroreport. 2008;19(6):609–613. doi: 10.1097/WNR.0b013e3282fa6db9. [DOI] [PubMed] [Google Scholar]
- Klavir O., Winter C., Joel D. High but not low frequency stimulation of both the globus pallidus and the entopeduncular nucleus reduces 'compulsive' lever-pressing in rats. Behav. Brain Res. 2011;216(1):84–93. doi: 10.1016/j.bbr.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Kravitz A.V., Tye L.D., Kreitzer A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol. Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Marsh R., Tau G.Z., Wang Z., Huo Y., Liu G., Hao X., Simpson H.B. Reward-based spatial learning in unmedicated adults with obsessive-compulsive disorder. Am. J. Psychiatry. 2015;172(4):383–392. doi: 10.1176/appi.ajp.2014.13121700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis M.A., Alvarenga P., Funaro G., Torresan R.C., Moraes I., Torres A.R., Hounie A.G. Gender differences in obsessive-compulsive disorder: a literature review. Rev. Bras. Psiquiatr. 2011;33(4):390–399. doi: 10.1590/s1516-44462011000400014. [DOI] [PubMed] [Google Scholar]
- Mattina G.F., Steiner M. The need for inclusion of sex and age of onset variables in genetic association studies of obsessive-compulsive disorder: overview. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;67:107–116. doi: 10.1016/j.pnpbp.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends. Cogn. Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein-Zamir S., Craig K.J., Ersche K.D., Abbott S., Muller U., Fineberg N.A., Robbins T.W. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology (Berl) 2010;212(3):357–367. doi: 10.1007/s00213-010-1963-z. [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Soriano-Mas C., Gispert J.D., Bossa M., Reig S., Ortiz H., Olmos S. Variations in the shape of the frontobasal brain region in obsessive-compulsive disorder. Hum. Brain Mapp. 2011;32(7):1100–1108. doi: 10.1002/hbm.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M., Everaerd D., van der Pol C., Franke B., Tendolkar I., Fernandez G. Normal sexual dimorphism in the human basal ganglia. Hum. Brain Mapp. 2012;33(5):1246–1252. doi: 10.1002/hbm.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok A.N., Salimi-Khorshidi G., Lai M.C., Baron-Cohen S., Lombardo M.V., Tait R.J., Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio A.M., Stein D.J., Chiu W.T., Kessler R.C. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol. Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalas C., Alonso P., Labad J., Real E., Pertusa A., Jaurrieta N., Vallejo J. A case-control study of sex differences in strategic processing and episodic memory in obsessive-compulsive disorder. Compr Psychiatry. 2010;51(3):303–311. doi: 10.1016/j.comppsych.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Shaw P., Sharp W., Sudre G., Wharton A., Greenstein D., Raznahan A., Rapoport J. Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol. Psychiatry. 2015;20(2):224–231. doi: 10.1038/mp.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R., MacMillan S., McMeniman M., Lorch E., Madden R., Ivey J., Rosenberg D.R. Amygdala volume reductions in pediatric patients with obsessive-compulsive disorder treated with paroxetine: preliminary findings. Neuropsychopharmacology. 2004;29(4):826–832. doi: 10.1038/sj.npp.1300399. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R., Robinson D., Alvir J.M., Bilder R.M., Lencz T., Ashtari M., Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1999;56(10):913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Taylor S. Early versus late onset obsessive-compulsive disorder: evidence for distinct subtypes. Clin. Psychol. Rev. 2011;31(7):1083–1100. doi: 10.1016/j.cpr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol. Psychiatry. 2013;18(7):799–805. doi: 10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- Ullrich, M., Weber, M., Post, A.M., Popp, S., Grein, J., Zechner, M., Schuh, K. (2018). OCD-like behavior is caused by dysfunction of thalamo-amygdala circuits and upregulated TrkB/ERK-MAPK signaling as a result of SPRED2 deficiency. 23(2), 444–458. doi: 10.1038/mp.2016.232. [DOI] [PMC free article] [PubMed]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Mataix-Cols D., Heyman I., Hough M., Doherty J., Burge L., James A. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol. Psychiatry. 2011;70(11):1083–1090. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu J., Cui J., Liu C. Study of symptom dimensions and clinical characteristics in Chinese patients with OCD. J. Affect. Disord. 2013;151(3):868–874. doi: 10.1016/j.jad.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Zohar J., Gross-Isseroff R., Hermesh H., Weizman A. Is there sexual dimorphism in obsessive-compulsive disorder? Neurosci. Biobehav. Rev. 1999;23(6):845–849. doi: 10.1016/s0149-7634(99)00021-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.