Highlights
-
•
The use of brain-based models of pain were explored in two clinical studies.
-
•
Neurologic pain signature activation decreased following spinal manipulation.
-
•
Spinal manipulation altered the processing of pain-related brain activity.
-
•
We provide evidence for a centrally mediated therapeutic action of spinal manipulation.
-
•
Brain-based models have potential as objective clinical biomarkers of pain.
Keywords: Neck pain, Spinal manipulation, Functional magnetic resonance imaging, Neuroimaging, Pain, Pain measurement, Humans, Randomized controlled trial
Abstract
Background Context
Spinal manipulation (SM) is a common treatment for neck and back pain, theorized to mechanically affect the spine leading to therapeutic mechanical changes. The link between specific mechanical effects and clinical improvement is not well supported. SM's therapeutic action may instead be partially mediated within the central nervous system.
Purpose
To introduce brain-based models of pain for spinal pain and manual therapy research, characterize the distributed central mechanisms of SM, and advance the preliminary validation of brain-based models as potential clinical biomarkers of pain.
Study Design
Secondary analysis of two functional magnetic resonance imaging studies investigating the effect of thoracic SM on pain-related brain activity: A non-controlled, non-blinded study in healthy volunteers (Study 1, n = 10, 5 females, and mean age = 31.2 ± 10.0 years) and a randomized controlled study in participants with acute to subacute neck pain (Study 2, n = 24, 16 females, mean age = 38.0 ± 15.1 years).
Methods
Functional magnetic resonance imaging was performed during noxious mechanical stimulation of the right index finger cuticle pre- and post-intervention. The effect of SM on pain-related activity was studied within brain regions defined by the Neurologic Pain Signature (NPS) that are predictive of physical pain.
Results
In Study 1, evoked mechanical pain (p < 0.001) and NPS activation (p = 0.010) decreased following SM, and the changes in evoked pain and NPS activation were correlated (rRM2 = 0.418, p = 0.016). Activation within the NPS subregions of the dorsal anterior cingulate cortex (dACC, p = 0.012) and right secondary somatosensory cortex/operculum (rS2_Op, p = 0.045) also decreased following SM, and evoked pain was correlated with dACC activity (rRM2 = 0.477, p = 0.019). In Study 2, neck pain (p = 0.046) and NPS (p = 0.033) activation decreased following verum but not sham SM. Associations between evoked pain, neck pain, and NPS activation, were not significant and less clear, possibly due to inadequate power, methodological limitations, or other confounding factors.
Conclusions
The findings provide preliminary evidence that SM may alter the processing of pain-related brain activity within specific pain-related brain regions and support the use of brain-based models as clinical biomarkers of pain.
1. Introduction
Spinal pain is a leading cause of disability, affecting nearly one billion individuals worldwide (Naghavi et al., 2015; Hoy et al., 2010; D Hoy et al., 2014; D Hoy et al., 2014). A challenge in the clinical management of spinal pain is the lack of diagnostic, prognostic, and predictive information to determine which therapy or combination of therapies is most appropriate for an individual patient at a given time. A more nuanced understanding of the neurobiology of spinal pain, the therapeutic action of treatments, and the factors shaping the individual pain experience and treatment response may help realize a new era of patient-centric rehabilitation.
Spinal manipulation (SM) is a common treatment for spinal pain with guideline support when combined with education and exercise (Blanpied et al., 2017; Delitto et al., 2012; Oliveira et al., 2018; Bussieres et al., 2018; Maher et al., 2017; Qaseem et al., 2017). SM is characterized by a mechanical thrust to spinal joints slightly beyond their passive range of motion (Herzog, 2010). SM's clinical rationale has long been guided by the opinion that a mechanical effect on the spine leads to therapeutic mechanical changes (Evans, 2002). However, a purely biomechanical mechanism remains debatable as studies have failed to link specific mechanical effects to meaningful clinical improvement (Lascurain-Aguirrebena et al., 2016; Xia et al., 2017). SM's therapeutic action may instead be partially mediated within the central nervous system (CNS) (Bialosky et al., 2009; Haavik and Murphy, 2012).
Pain is a subjective, psychological phenomenon influenced by multiple physiological and cognitive processes (Gatchel et al., 2007). Clinicians rely mainly on self-reported pain, which is limited by the patient's ability to report his/her pain experience, and may not provide information on the source of pain, its projected natural history, or the proper treatment direction (Williams, 2000; Dansie and Turk, 2013; Schnakers et al., 2010). The development of objective pain biomarkers to complement self-report is of increasing interest (Reddan and Wager, 2018). Functional MRI (fMRI) has had a central role in this effort by mapping pain processing in the CNS, revealing that the perception of pain is not encoded by a single brain area but distributed throughout the brain (Martucci and Mackey, 2018).
The complexity of the pain network makes drawing conclusions on the role of any specific brain region challenging. Higher-level approaches are required to extract meaningful information from the patterns of brain activity. Multivariate pattern analysis and machine-learning techniques are allowing researchers to develop brain-based predictive models of pain that may become valuable biomarkers (Bagarinao et al., 2014; Brown et al., 2011; Ung et al., 2014). In addition to being diagnostic, prognostic, and predictive tools, these approaches can characterize normal and abnormal processes, increasing our mechanistic understanding of pain and identifying new treatment targets (Duff et al., 2015; Kutch et al., 2017).
Here, we perform a secondary analysis of two fMRI studies investigating the effect of thoracic SM on pain-related brain activity. We leverage these datasets and a multivariate brain-based model of physical pain, the Neurologic Pain Signature (NPS), to explore the effect of SM on activity within brain regions predictive of physical pain (Wager et al., 2013). Our purpose is threefold: 1) To introduce brain-based models of pain for spinal pain and manual therapy research, 2) To characterize the distributed central mechanisms of SM, and 3) To advance the preliminary validation of brain-based models as potential clinical biomarkers of pain. We hypothesize that NPS activation and perceived pain will decrease following SM, and we expect the NPS activation to be positively correlated to pain.
2. Materials and methods
2.1. Participants and location
De-identified datasets were obtained from two previously published fMRI studies that investigated changes in pain-related brain activity following thoracic SM using univariate analyses (Sparks et al., 2013; Sparks et al., 2017). The study location, equipment, imaging parameters, and stimulus (location and intensity) were the same across the studies. Study 1 was a non-controlled, non-blinded study in healthy volunteers receiving verum SM only (n = 10). Study 2 utilized a randomized, controlled study design in participants with acute or subacute neck pain (ClinicalTrials.gov Identified: NCT01862705) (Sparks et al., 2017). The participants received either verum (n = 12) or sham (n = 12) SM, and the participants and assessor were blinded to the intervention. The inclusion and exclusion criteria for both studies are summarized in Table 1. The studies were conducted at OSF HealthCare Saint Francis Medical Center (Peoria, IL, USA). The Institutional Review Board at the University of Illinois College of Medicine (Peoria, IL, USA) approved both study protocols. Prior to enrollment, the study procedures and risks were discussed with each participant, and then written informed consent was obtained.
Table 1.
Inclusion and Exclusion Criteria.
| Study 1: Healthy |
| Inclusion |
| • Right handed |
| • No current history of pain, orthopedic, or systemic condition |
| Exclusion |
| • Not fluent in English |
| • Any contraindications to magnetic resonance imaging |
| • Any contraindications to thoracic spinal manipulation |
| • Pregnant or possibly pregnant |
| Study 2: Neck Pain |
| Inclusion |
| • Right handed |
| • Acute to subacute neck pain (< 6 weeks duration) |
| Exclusion |
| • Not fluent in English |
| • Any contraindications to magnetic resonance imaging |
| • Any contraindications to thoracic spinal manipulation |
| • Pregnant or possibly pregnant |
| • History of traumatic neck pain or cervical surgery |
| • Diagnosis of cervical radiculopathy, myelopathy, fibromyalgia, vascular disease, or Raynaud's phenomenon |
| • Red flags suggestive of non-musculoskeletal origin of pain including metabolic disorders, osteoporosis, tumor, or rheumatoid arthritis |
2.2. Noxious mechanical stimulation
To study the effect of SM on pain-related brain activity, noxious mechanical stimuli were applied manually to the cuticle of the right index finger with von Frey filaments. Several studies have used a similar combination of von Frey filaments and fMRI to study central mechanisms of pain processing (Taylor and Davis, 2009; Ghazni et al., 2010; Maihofner et al., 2005). In Study 1, participants underwent a pain thresholding procedure on the day prior to imaging in which a graduated succession of filaments (starting filament 2.83 size (0.07 g)) were applied in 5 s durations with a 20 s interstimulus interval. The pain threshold was defined as the least intensity stimulus at which the stimulus changed from pressure to pain. The 6.65 size (300 g) von Frey filament elicited pain in every participant and was therefore used as the stimulus intensity for functional imaging in Study 1. The same stimulus intensity was also used in Study 2. Functional imaging was performed in 5 min runs during alternating 15 s blocks of noxious mechanical stimulation of the right index finger cuticle and no stimulation pre- and post-intervention. During the stimulation blocks, the stimuli were applied manually at 1 Hz. The stimulation protocol is designed to elicit temporal summation of second pain (TSSP). TSSP is the human analog to the animal “wind-up” phenomenon and is hypothesized to be centrally mediated (Staud et al., 2001). Following each run, participants rated the intensity of the index finger stimulus using the 11-point numerical pain rating scale (NPRS) with anchors of no pain (0) and worst imaginable pain (10). In Study 2, participants also used the NPRS to rate their neck pain at baseline and pre- and post-intervention, and the investigator administering the stimuli and assessing the evoked pain and neck pain intensity was blinded to treatment assignment. Functional imaging was also performed during noxious mechanical stimulation of the right great toe pre- and post-intervention in Study 2; however, since the right great toe stimulation protocol was not performed in Study 1, these findings were not included in the present analysis but have been reported previously in Sparks et al. (2017) (Sparks et al., 2017). The great toe stimulation was performed in separate functional imaging runs from the index finger stimulation, and the index finger stimulation runs always preceded the great toe stimulation.
2.3. Image acquisition
Imaging was performed on a 3T Signa HDx General Electric magnetic resonance scanner equipped with an 8-channel head coil (General Electric, Milwaukee, Wisconsin, USA). Participants were placed supine on the scanner bed in a hook-lying position with a foam bolster under the knees. Ear plugs were provided to attenuate the scanner noise, and foam pads were used to secure the head and minimize participant motion during scanning. For spatial normalization of the functional images to template space, a high-resolution T1-weighted structural image of the whole brain was initially obtained using a three-dimensional fast spoiled gradient-echo sequence without fat suppression (flip angle = 12°, repetition time = 8 ms, echo time = 3 ms, bandwidth = 62.4 kHz, field-of-view = 250 mm × 250 mm, matrix size = 256 × 256, and resolution = 0.98 mm × 0.98 mm × 1.00 mm). Whole-brain functional imaging was then performed during noxious mechanical stimulation of the right index finger cuticle pre- and post-intervention using a T2*-weighted two-dimensional gradient-echo echo-planar-imaging sequence (flip angle = 90°, repetition time = 3000 ms, echo time = 30 ms, phase-encoding = anterior-posterior, ASSET acceleration factor = 2, field-of-view = 240 mm × 240 mm, matrix size = 64 × 64, resolution = 3.75 mm × 3.75 mm, slice thickness = 3.00 mm, number of slices = 50, slice-timing = interleaved, dummy volumes = 4, and volumes per run = 100).
2.4. Spinal manipulation
Participants received a single session of either verum (Studies 1 and 2) or sham (Study 2) thoracic SM immediately following the pre-intervention functional imaging. Thoracic SM is commonly used in the treatment of non-specific neck pain with effectiveness similar to cervical SM (Cross et al., 2011; Huisman et al., 2013; Masaracchio et al., 2019). In Study 2, participants were randomly assigned to the verum or sham intervention using a computer-generated sequence. The SM procedure was performed without repositioning the participant on the scanner table or removing the participant from the head coil. The verum SM intervention consisted of a high-velocity low-amplitude end-range force applied manually along an anterior-to-posterior vector through the elbows and directed to the mid-thoracic spine (Cleland et al., 2007; Puentedura et al., 2011). To accomplish this, the experimenter's manipulative hand was used to stabilize the inferior vertebra of the targeted motion segment (approximately the T4-5 motion segment), and the experimenter used her body weight to push down through the participant's arms. For the sham intervention, the experimenter's hands were placed in the same position as the verum intervention; however, the investigator's hands slid across the skin with minimal pressure to mimic the contact in the verum intervention, but no counterforce or thrust was directed toward the motion segment. The sham SM procedure has been demonstrated to be a valid sham compared to verum SM targeting the thoracic region (Michener et al., 2015). The time from the end of the pre-intervention functional imaging run to the start of the post-intervention run was less than 5 min.
2.5. First-Level analysis
Image processing and statistical analysis of the functional images were performed using the Oxford Center for fMRI of the Brain's (FMRIB) Software Library (FSL, Version 5.0.9, University of Oxford, Oxford, UK) (Jenkinson et al., 2012). The functional time series was motion corrected (MCFLIRT), slice-timing corrected, brain extracted (BET), spatially smoothed (5 mm3 FWHM), and band-pass temporal filtered (sigma = 15.0 s). The mechanical stimuli were modeled as a single box-car function with alternating 15 s blocks of stimulation and no stimulation. First-level activation maps of the preprocessed time series were generated using FMRIB's Improved Linear Model (FILM) with pre-whitening (Woolrich et al., 2001; Worsley, 2001). The design matrix included the hemodynamic response function (gamma, phase 0 s, standard deviation 3 s, mean lag 6 s) convolved stimulation vector as an explanatory variable and the temporal derivatives of the stimulation vector and the six motion parameters from motion correction (3 translations and 3 rotations) as covariates of no interest. The first-level activation maps were then spatially normalized to standard space (2 mm3 MNI-152 standard template) using boundary-based registration (BBR) for the functional to structural transformation and non-linear registration (FNIRT) for the structural to template transformation (Greve and Fischl, 2009; Andersson et al., 2010).
2.6. Neurologic pain signature
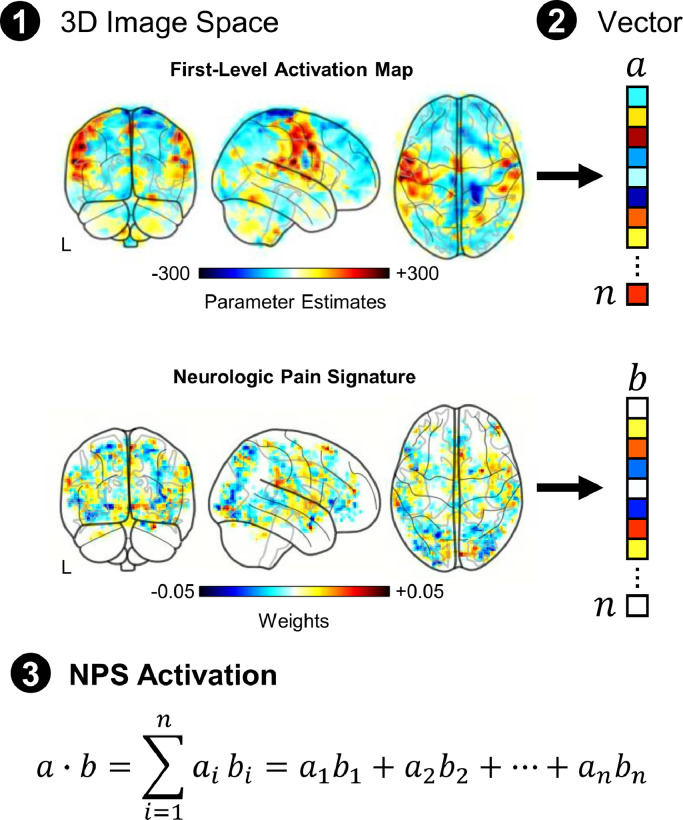
The Cognitive and Affective Neuroscience Lab (CANlab, University of Colorado, Boulder, CO, USA) neuroimaging analysis tools, an open-source collection of tools for interactive analysis of neuroimaging data (https://canlab.github.io/), were used to assess the NPS activation pre- and post-intervention. The CANlab tools are based in MATLAB (Version R2017b, Mathworks, Natick, MA, USA) and utilize several functions from SPM (Version 8, Wellcome Centre for Human Neuroimaging, University College London, London, UK) (Ashburner, 2012). The NPS activation was calculated by taking the dot product of the NPS pattern weights and the stimulus parameter estimate images from each participant's first-level analysis (Fig. 1). The dot product integrates the multivariate pattern of activity to a single scalar value, providing a quantitative metric of NPS activation (arbitrary units). Activation was then further explored in the NPS subregions with positive (higher predicted pain with higher activity) and negative (lower predicted pain with higher activity) predictive weights. The NPS positive subregions include the vermis, right mid-insula (rIns), right primary visual cortex (rV1), right thalamus (rThal), left mid-insula (lIns), right dorsal posterior insula (rdplns), right secondary somatosensory cortex/operculum (rS2_Op), and dorsal anterior cingulate cortex (dACC). The NPS negative subregions include the right lateral occipital complex (rLOC), left lateral occipital complex (lLOC), right posterior lateral occipital complex (rpLOC), pregenual anterior cingulate cortex (pgACC), left superior temporal sulcus (lSTS), right inferior parietal lobule (rIPL), and posterior cingulate cortex (PCC). To account for differences in subregion volumes and facilitate visualization of the activation across the subregions, the activation within each subregion was normalized by the ℓ1-norm of the corresponding NPS subregion pattern weights.
Fig. 1.
To investigate the effect of SM on evoked mechanical pain-related brain activity, we focused on activation within brain regions predictive of physical pain using the Neurologic Pain Signature (NPS). The NPS is a multivariate brain activation pattern that has been shown to be sensitive and specific to experimentally-evoked pain at the individual level (Wager et al., 2013). The NPS activation was calculated by taking the dot product of the NPS pattern weights and the stimulus parameter estimate images from each participant's first-level analysis. The dot product provides a single scalar value to quantify the NPS activation (arbitrary units).
2.7. Statistical analysis
As our a priori hypotheses were directional, one-tailed statistical tests were performed to investigate the effect of time (pre- and post-intervention) on evoked pain, NPS activation, and, in Study 2 only, neck pain. All other analyses were performed using two-tailed tests, which included the exploratory analysis of activation within the different NPS subregions. Repeated measures correlations (RMCORR) were performed to assess associations between the clinical measures and NPS activation pre- and post-intervention. RMCORR estimates the common regression slope (i.e., fixed slopes and varying intercepts) to quantify the association shared among the participants without aggregation of the repeated measures or violation of the assumption of independence of observations and has been reported to have increased statistical power (Bakdash and Marusich, 2017). Statistical analyses were performed in RStudio (Version 1.1.442, Boston, Maryland, USA). For all statistical tests, an α < 0.05 was considered statistically significant. Study 2 was not powered to examine between-group changes in NPS activation following SM, so only within-group changes were investigated. A power analysis using G*Power (Universität Düsseldorf, Düsseldorf, Germany) demonstrated that for a repeated measures ANOVA (within-between interaction, partial η2 = 0.093, effect size = 0.320, α = 0.05, number of groups = 2, number of measures = 2, correlation among repeated measures = 0.30) a sample size of 15 or 19 participants per group was necessary for 80% or 90% power, respectively (Faul et al., 2009; Faul et al., 2007).
3. Results
All participants completed the entire study protocol with no reported adverse events, and no data were excluded from the analyses. Participant motion during functional imaging was very low. The mean absolute and mean relative displacements were < 0.5 mm for all participants and runs. Age and gender did not statistically differ between the groups. Baseline evoked pain was significantly lower for the Study 2 Verum SM group compared to the Study 1 Verum SM group (two-tailed independent samples t-test, t = 3.468, p = 0.002) and Study 2 Sham SM group (t = 2.845, p = 0.010), indicating that while the same stimulus intensity was used for each participant, baseline evoked pain was not matched across the groups (Table 2).
Table 2.
Sample Characteristics.
| Study 1: Healthy | Study 2: Neck Pain | ||
|---|---|---|---|
| Verum SM (n = 10) | Verum SM (n = 12) | Sham SM (n = 1 2) | |
| Age, years | 31.2 ± 10.0 | 36.2 ± 15.1 | 39.8 ± 15.0 |
| Female sex, n | 5 | 8 | 8 |
| Pre-SM Evoked Pain, NPRS | 4.5 ± 1.1 | 2.6 ± 1.5*,† | 4.5 ± 1.8 |
| Baseline Neck Pain, NPRS | N/A | 3.0 ± 1.5 | 3.3 ± 1.8 |
| Pre-SM Neck Pain, NPRS | N/A | 2.1 ± 2.2 | 2.7 ± 2.4 |
SM=spinal manipulation.
NPRS, numerical pain rating scale (0–10).
N/A=not applicable.
Values are mean ± SD unless otherwise noted.
Statistically significant difference from Study 1, p < 0.01.
Statistically significant difference from Study 2 Sham SM, p < 0.05.
3.1. Study 1: healthy
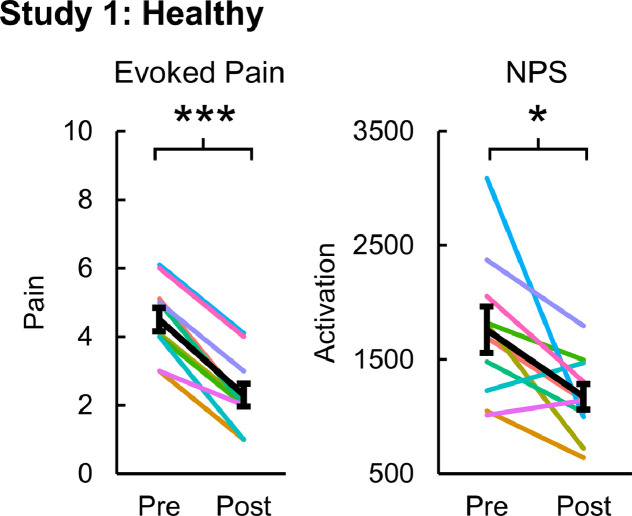
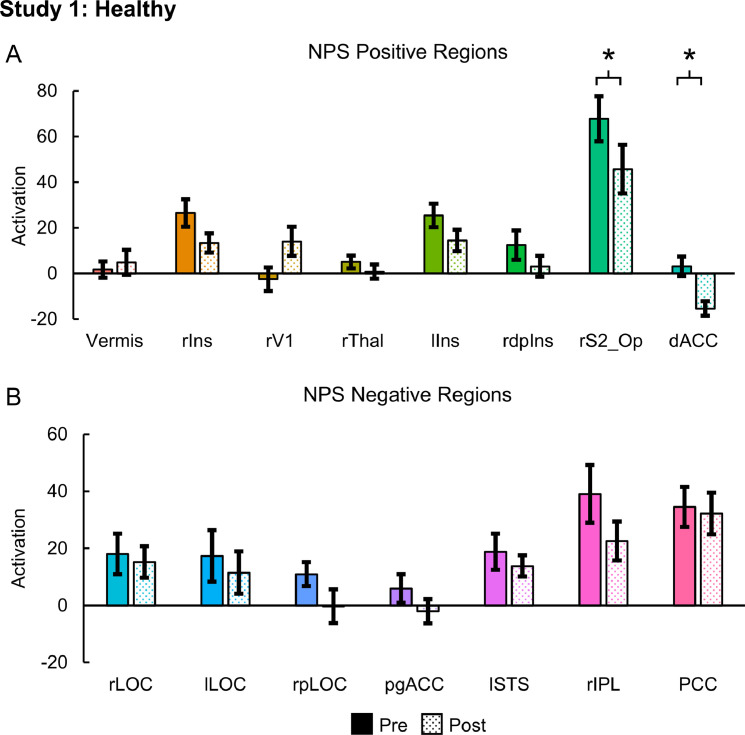
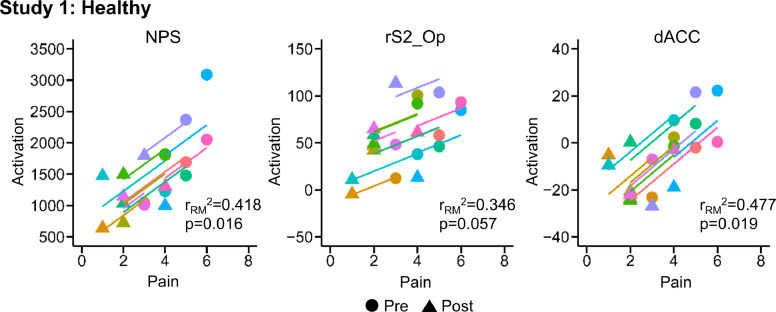
Evoked pain (mean ± standard error (SE)) significantly decreased from 4.50 ± 0.34 to 2.30 ± 0.34 post-intervention (one-tailed paired samples t-test, t = 11.000, p < 0.001). NPS activation (mean ± SE) also significantly decreased from 1760.17 ± 201.79 to 1174.60 ± 112.42 post-intervention (one-tailed paired samples t-test, t = 2.826, p = 0.010) (Fig. 2). Within the NPS positive subregions, the rS2_Op (two-tailed paired samples t-test, t = 2.320 p = 0.045) and dACC (t = 3.130 p = 0.012) activation significantly decreased post-intervention. The rS2_Op and dACC activation decreased from 67.78 ± 9.88 and 3.14 ± 4.25, respectively, to 45.66 ± 10.65 and −15.39 ± 3.18, respectively. No significant changes in activation within the NPS negative subregions were present (Fig. 3). NPS activation was strongly correlated to evoked pain (one-tailed RMCORR, rRM = 0.647, p = 0.016). Within the NPS subregions showing significant changes, dACC activation was also strongly correlated to evoked pain (two-tailed RMCORR, rRM = 0.691, p = 0.019) while rS2_Op activation tended to be moderately correlated to evoked pain (rRM = 0.588, p = 0.057) (Fig. 4).
Fig. 2.
Evoked pain and Neurologic Pain Signature (NPS) activation pre- and post-intervention for the healthy volunteers receiving verum spinal manipulation (SM). Both evoked pain and NPS activation decreased following SM. NPS activation is in arbitrary units. One-tailed paired samples t-tests, *p<0.05, and ***p<0.001. Error bars = ± standard error.
Fig. 3.
Neurologic Pain Signature (NPS) subregion activation pre- and post-intervention for the healthy volunteers receiving verum spinal manipulation (SM). A) Activation within the NPS positive regions of the right secondary somatosensory cortex/operculum (rS2_Op) and the dorsal anterior cingulate cortex (dACC) decreased following SM. B) No significant changes were identified within any of the NPS negative regions. NPS activation is in arbitrary units. Two-tailed paired samples t-tests, *p < 0.05. Error bars = ± standard error. rIns = right mid-insula, rV1 = right primary visual cortex, rThal = right thalamaus, lIns = left mid-insula, rdplns = right dorsal posterior insula, rLOC = right lateral occipital complex, lLOC = left lateral occipital complex, rpLOC = right posterior lateral occipital complex, pgACC = pregenual anterior cingulate cortex, lSTS = left superior temporal sulcus, rIPL = right inferior parietal lobule, and PCC = posterior cingulate cortex.
Fig. 4.
Scatter plots for the repeated measures correlations (RMCORR) between evoked pain and Neurologic Pain Signature (NPS) activation and NPS subregion activation for the healthy volunteers receiving verum spinal manipulation (SM). The observations from the same participant are shown in the same color, and the colored lines show the RMCORR fit for each participant (i.e., fixed slopes and varying intercepts). Evoked pain was positively correlated to NPS activation. Only correlations within subregions showing significant differences in Study 1 were explored. Within the subregions, evoked pain was positively correlated to the dorsal anterior cingulate cortex (dACC) activation and tended to be positively correlated to right somatosensory cortex (rS2_Op) activation. NPS activation was hypothesized to be positively correlated with evoked pain, so one-tailed tests were performed. Two-tailed tests were performed for the exploratory analysis within the NPS subregions. NPS activation is in arbitrary units.
3.2. Study 2: neck pain
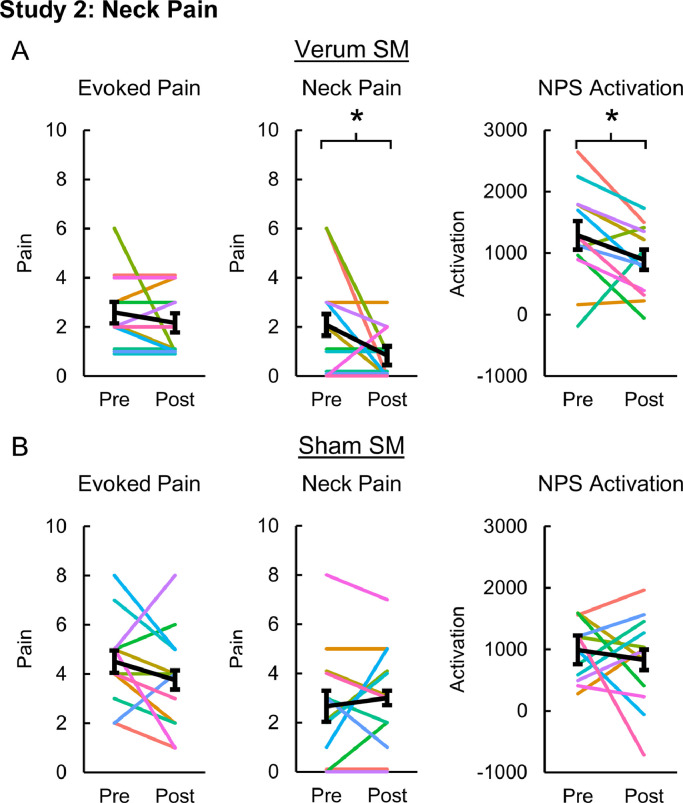
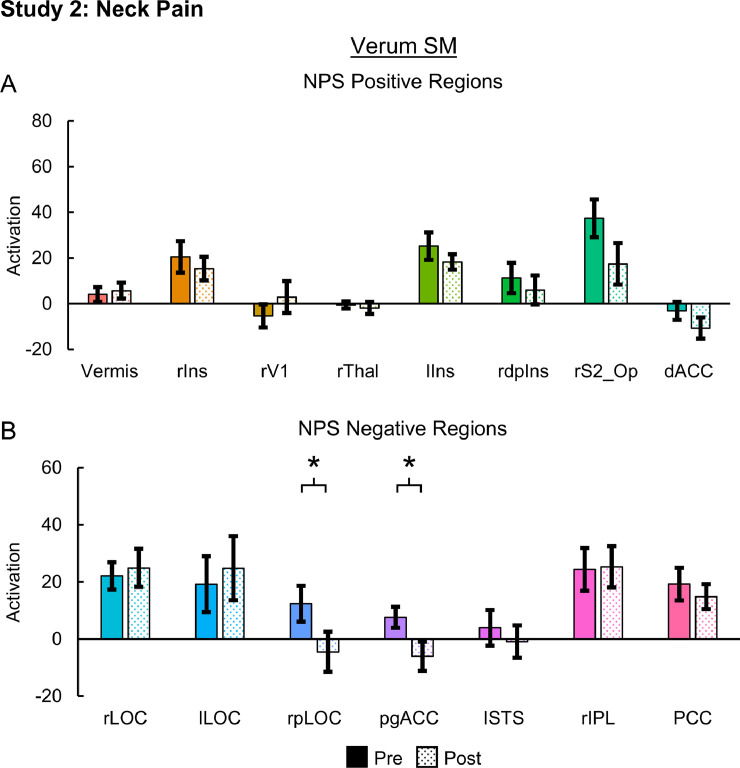
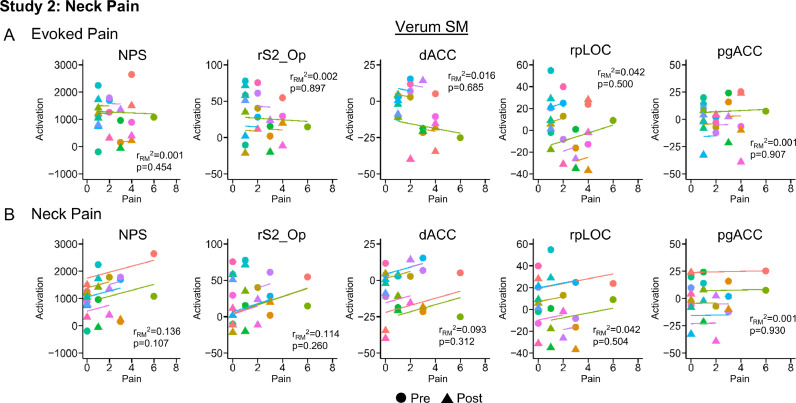
Evoked pain was 2.58 ± 0.43 and 4.50 ± 0.51 pre-intervention and 2.17 ± 0.39 and 3.75 ± 0.60 post-intervention for the verum and sham SM groups, respectively. Evoked pain did not significantly change in either the verum (one-tailed paired samples t-test, t = 0.923, p = 0.188) or sham SM groups (t = 1.295, p = 0.111). Neck pain (mean ± SE) significantly decreased from 2.08 ± 0.63 to 0.83 ± 0.30 post-intervention in the verum SM group (one-tailed paired samples t-test, t = 1.850, p = 0.046), but no significant change in neck pain was identified following sham SM (one-tailed paired t-test, t = −0.650, p = 0.264). Neck pain for the sham SM group was 2.67 ± 0.69 and 3.00 ± 0.62 pre- and post-intervention, respectively. NPS activation significantly decreased from 1288.95 ± 233.84 to 890.47 ± 166.04 post-intervention in the verum SM group (one-tailed paired samples t-test, t = 2.039, p = 0.033) while no significant changes in NPS activation were identified in the sham SM group (t = 0.628, p = 0.272). NPS activation for the sham SM group was 989.79 ± 137.78 and 829.23 ± 216.92 pre- and post-intervention, respectively (Fig. 5). Next, we explored activation within the NPS subregions in the verum SM group. Within the NPS positive subregions, no significant changes in activation were present. Within the NPS negative subregions, the rpLOC (two-tailed paired samples t-test, t = 2.548, p = 0.027) and pgACC (t = 2.412, p = 0.035) activation significantly decreased post-intervention. The rpLOC and pgACC activation decreased from 12.33 ± 6.24 and 7.58 ± 3.70, respectively, to −4.52 ± 7.02 and −6.04 ± 5.12, respectively (Fig. 6). No significant changes in NPS activation within the positive or negative subregions were identified in the sham SM group. Next correlations between NPS and NPS subregion (rS2_Op, dACC, rpLOC, and pgACC) activation, evoked pain, and neck pain were explored in the verum SM group. While no significant correlations were present, the NPS activation tended to be weakly correlated to neck pain (one-tailed RMCORR, rRM = 0.369, p = 0.107) (Fig. 7).
Fig. 5.
Evoked pain, neck pain, and Neurologic Pain Signature (NPS) activation pre- and post-intervention for the neck pain participants receiving verum (A) and sham (B) spinal manipulation (SM). Neck pain and NPS activation decreased following verum SM but not evoked pain. No significant changes in evoked pain, neck pain, or NPS activation were seen following sham SM. NPS activation is in arbitrary units. One-tailed paired samples t-tests, *p < 0.05. Error bars = ± standard error.
Fig. 6.
Neurologic Pain Signature (NPS) subregion activation pre- and post-intervention for the neck pain participants receiving verum spinal manipulation (SM). A) No significant changes were identified within any of the NPS positive regions. B) Activation within the NPS negative regions of the right posterior lateral occipital complex (rpLOC) and pregenual anterior cingulate cortex (pgACC) decreased following SM. NPS activation is in arbitrary units. Two-tailed paired samples t-tests, *p < 0.05. Error bars = ± standard error. rIns = right mid-insula, rV1 = right primary visual cortex, rThal = right thalamaus, lIns = left mid-insula, rdplns = right dorsal posterior insula, rS2_Op = right secondary somatosensory cortex/operculum, dACC = dorsal anterior cingulate cortex, rLOC = right lateral occipital complex, lLOC = left lateral occipital complex, lSTS = left superior temporal sulcus, rIPL = right inferior parietal lobule, and PCC = posterior cingulate cortex.
Fig. 7.
Scatter plots for the repeated measures correlations (RMCORR) between evoked pain (A) and neck pain (B) and Neurologic Pain Signature (NPS) activation and NPS subregion activation for the neck pain participants receiving verum spinal manipulation (SM). The observations from the same participant are shown in the same color, and the colored lines show the RMCORR fit for each participant (i.e., fixed slopes and varying intercepts). Only correlations within subregions showing significant differences in Studies 1 and 2 were explored. While no significant correlations were identified for evoked pain or neck pain in the neck pain participants receiving verum SM, a non-significant positive weak correlation between neck pain and NPS activation (p = 0.107) was present. NPS activation is in arbitrary units. NPS activation was hypothesized to be positively correlated with evoked pain and neck pain, so one-tailed tests were performed. Two-tailed tests were performed for the exploratory analysis within the NPS subregions. rS2_Op=right secondary somatosensory cortex/operculum, dACC=dorsal anterior cingulate cortex, rpLOC=right posterior lateral occipital complex, and pgACC=pregenual anterior cingulate cortex.
4. Discussion
We provide evidence for decreased NPS activation following thoracic SM. First in a non-controlled, non-blinded study of healthy volunteers (Study 1), we showed that evoked pain intensity, NPS activation, and activation within the NPS positive subregions of the dACC and rS2_Op decreased following SM. Additionally, evoked pain was strongly positively correlated to overall NPS activation and activation within the dACC, further supporting the association between SM-induced changes in evoked pain and NPS activation. The study design, however, prevents drawing any causal conclusions, as experimenter and observer bias, placebo, habituation, and demand characteristics may have confounded the findings. Moreover, the use of healthy volunteers reduces the ecological validity of the findings, as SM is clinically used for spinal pain. In a randomized controlled trial of neck pain participants (Study 2), we addressed some of these limitations and provide further evidence of decreased NPS activation following verum SM but not sham SM. The associations between changes in NPS activation, evoked pain, and neck pain in this clinical population, however, were not significant and less clear. Despite the limitations discussed below, when taken together, the findings provide evidence that SM may alter pain-related brain activity within brain regions specific to the processing of physical pain, supporting a possible central mechanism of SM and providing further validation of the NPS as a clinical biomarker of pain.
SM-induced changes in evoked pain-related brain activity in Studies 1 and 2 were previously explored using conventional brain mapping approaches (Sparks et al., 2013; Sparks et al., 2017). For example in Study 1 using a univariate voxelwise analysis with standard statistical thresholds, evoked-pain activity was mapped to brain areas commonly reported in experimental pain studies including the cerebellum, amygdala, thalamus, periaqueductal gray, insular cortices, anterior cingulate cortex, somatosensory cortices, and supplemental motor and premotor areas. Post-SM activity decreased in the left postcentral and precentral gyri, right supramarginal gyrus, anterior cingulate cortex, right superior parietal lobule, right cerebellum, and right insular cortex (Sparks et al., 2013). Conventional brain mapping can reveal differences in brain activity due to physiological processes, following treatment, or between groups, aiding in our understanding of brain mechanisms and hypothesis generation. However, the spatial location and extent of activity are dependent on the statistical thresholding employed, and the maps themselves provide no information on the interactions between brain regions or any predictive information, precluding their use as a biomarker. In contrast, brain-based models such as the NPS provide a quantitative measure that can make predictions across individuals and, in the case of the NPS, predict pain.
The NPS was developed by training a multivariate regression model to predict pain intensity from fMRI maps of varying intensity experimental thermal stimuli in healthy volunteers. In independent datasets, the NPS model was shown to track perceived pain intensity, classify between innocuous and painful stimuli, be specific to physical pain versus social pain, and decrease with opioid analgesics (Wager et al., 2013). The sensitivity and specificity of the NPS has been further demonstrated showing that experimental electrical pain activates the NPS but not vicarious pain, pain anticipation, or pain recall (Brascher et al., 2016; Krishnan et al., 2016; Ma et al., 2016; Woo et al., 2015). Recently, the NPS was expanded to fibromyalgia using mechanical pressure pain; mechanical sensitivity is a clinical hallmark of fibromyalgia. Fibromyalgia participants displayed higher NPS positive subregion responses to painful stimuli than healthy controls (stimulus pressure matched across participants), and the NPS positive subregion activity mediated the relationship between the classification of fibromyalgia and healthy controls and pain intensity and unpleasantness (Lopez-Sola et al., 2017). These findings provide evidence of increased activity within specific pain-related brain regions in fibromyalgia, consistent with peripheral and central sensitization which are two mechanisms thought to at least partially underlie the persistence of pain in fibromyalgia, and further support the use of the NPS as a potential index of central sensitization. In the same study, combining several fMRI-based models (NPS, fibromyalgia-pain signature, and multisensory signature models) into a single model, resulted in 92% and 94% cross-validated sensitivity and specificity, respectively, for out-of-sample participants, demonstrating that brain-based models may have potential as clinical biomarkers in fibromyalgia.
A challenge that exists in pain neuroimaging is how to image the clinical pain experience. Many studies have used evoked experimental pain to investigate pain processing in clinical pain conditions, such as mechanical pain in this study. While evoked mechanical pain appears useful in fibromyalgia, it may not be an ideal surrogate of pain in other clinical pain conditions, such as neck pain. This may explain some of the discrepancies in the findings reported. In Study 1, evoked pain decreased post-SM and strongly correlated to NPS activation; however, in Study 2, while NPS activation decreased following verum SM, evoked mechanical pain did not decrease, and no significant associations between NPS activation, evoked pain, and neck pain were present. Evoked mechanical pain and the experience of neck pain are not equivalent, and the presence of one may have influenced the perception of the other, possibly through mechanisms related to conditioned pain modulation such as diffuse noxious inhibitory control (Damien et al., 2018). Improved, more ecologically valid fMRI measures of the clinical pain experience should lead to more accurate brain-based models of clinical pain, but how can we better image clinical pain?
One strategy includes continuously tracking self-reported spontaneous clinical pain during imaging to encode the brain activity underlying self-reported clinical pain (Apkarian et al., 2001; Baliki et al., 2006). Other approaches have used mechanical maneuvers to evoke pain that is more representative of the clinical condition, such as rectal distention in irritable bowel syndrome, phasic hip movements for hip osteoarthritis, and exercise to induce back pain (Gram et al., 2017; Kong et al., 2013; Mertz et al., 2000). Similar methods could be used to map clinical neck pain and study changes in neck pain-related NPS activation. Another approach is the use of resting state fMRI in which brain signals are collected over time without the participant engaged in a task. The fluctuations in spontaneous clinical pain should, in theory, cause detectable fluctuations in the brain signals related to the clinical pain state. Resting state networks are well understood to be altered in chronic pain (Martucci et al., 2015; Kutch et al., 2015; Kilpatrick et al., 2014; Jiang et al., 2016; Baliki et al., 2014). Recently, Mano et al. (2018) used a multi-site resting state fMRI dataset of chronic back pain participants and their healthy counter-parts from the United Kingdom and Japan to train a classification model and reported 63% accuracy in an independent test set from the United States, demonstrating the potential for generalizable brain-based resting state models of clinical pain (Mano et al., 2018). Resting state connectivity has also been used to predict recovery from subacute back pain and may change post-SM in exercise-induced back pain (Vachon-Presseau et al., 2016; Gay et al., 2014).
Employing methods similar to the NPS, brain-based models could be developed to predict treatment response using experimental evoked pain maps, clinical pain maps, resting state fMRI measures, or any combination of these features. The predictive brain regions could provide valuable information on the neurobiological mechanisms of treatment and neurobiological state of treatment responders. When building these models, patient expectations should be considered. Expectations appear to modulate the patterns of neural activation and the pain experience in individuals with knee osteoarthritis, and expectations may shape the patterns of neural activation as well as the clinical outcomes and experiences of individuals seeking neck pain treatment (Gollub et al., 2018). In contrast, two recent studies demonstrated that while self-regulation and placebo reduce pain responses, they had little effect on NPS activation, indicating that the NPS may be responsive to the primary processing of nociceptive information versus higher level cognitive aspects of the pain experience (Woo et al., 2015; Zunhammer et al., 2018). These studies, however, used experimental evoked pain in healthy participants, and the findings may not translate to clinical pain conditions. Monitoring treatment expectation should be performed in future studies to explore its influence.
The clinical rationale regarding SM has long been guided by a biomechanical perspective, in which SM is applied to correct aberrant joint mechanics leading to therapeutic mechanical changes in the spine. Links between biomechanical changes and clinical improvements, however, have only been weakly supported in the literature (Lascurain-Aguirrebena et al., 2016; Xia et al., 2017). A growing body of evidence is pointing towards neurophysiologic mechanisms of action underlying the pain modulating effects of SM including both spinal and supraspinal mechanisms (Bialosky et al., 2009; Haavik and Murphy, 2012). The reduction in NPS activation suggests a decrease in the nociceptive information reaching supraspinal areas. SM is known to activate large diameter mechanoreceptors that in turn can inhibit the transmission of nociceptive signals at the spinal cord (Pickar, 2002). Additionally, the activation of descending pain inhibitory pathways could also reduce nociceptive signaling, and the activation of these inhibitory systems may explain how thoracic SM could affect nociceptive processing at more superior cervical spinal cord segments (i.e., right index finger stimulation corresponds to the C7 dermatome) as reported in this study. Distraction and/or patient expectation for pain relief may further explain the non-segmental effects of SM on pain reduction (Bishop et al., 2011; Cleland et al., 2010; Bialosky et al., 2008). Segmental inhibition, descending inhibition, and other supraspinal processes not yet fully understood, may contribute to the overall therapeutic action of SM (Pickar, 2002; Skyba et al., 2003; Wright, 1995). Technical advancements in imaging have made spinal cord fMRI, and, more recently, simultaneous spinal cord-brain fMRI, possible (Weber II et al., 2018; Weber et al., 2016; Weber et al., 2016; Islam et al., 2019; Martucci et al., 2019). Such advances may allow us to better characterize spinal and supraspinal patterns of activity, providing a more complete assessment of nociceptive and pain processes and further refinement of the NPS and other neuroimaging-based models of pain (Kornelsen and Mackey, 2007). Future work using a better surrogate for imaging the neck pain experience and simultaneous spinal cord-brain fMRI may allow us to better identify the spinal and supraspinal mechanisms of SM and develop a more comprehensive understanding of the neurophysiological mechanisms of SM.
We acknowledge the limitation of testing and reporting on a small sample size, especially in Study 2, which was not powered to identify between-group differences in NPS activation, limiting the analyses to within-group differences. Secondly, Study 2 consisted of a single session of SM, and in clinical practice, patients likely receive a multimodal approach, more than one intervention targeting the spine, and the inclusion of education, exercise, and lifestyle recommendations. Future studies could also include multiple treatment sessions, more typical of a standard clinical protocol to increase the external validity of the findings. Longitudinally tracking the clinical and brain-based measures over the course of treatment would further strengthen our understanding of the central mechanisms of SM and their causal connections to treatment response. Another limitation in Study 2 is that the adequacy of the blinding intervention was not assessed, and a placebo response cannot be ruled out. Validated measures of treatment expectation and blinding should be included in future work to study their influence on treatment response. Finally, as we only investigated effects in healthy volunteers and participants with acute to subacute neck pain, care should be taken when extrapolating any findings to chronic pain.
5. Conclusions
We provide preliminary evidence that SM may alter the processing of pain-related brain activity within specific pain-related brain regions, supporting the use of brain-based models such, as the NPS, as clinical biomarkers of pain. Future work should aim to improve brain-based biomarker models of neck pain for use in larger randomized controlled trials. These biomarkers have potential to allow us to better understand the central mechanisms of SM, predict treatment response, and optimize the delivery of treatment (technique, frequency, and duration).
Declaration of Competing Interest
Drs. Weber, Wager, Mackey and Elliott report grants from the National Institutes of Health during the conduct of this study. Dr. Wager reports a Small Business Innovation Research grant with WaviMed. Dr. Wager is also on the scientific advisory board of Curable Health, Inc., has consulted for GSK and Cognifisense, has performed contract work for PainQX, and has been issued two patents: US 2016/0,054,409 fMRI-based Neurologic Signature of Physical Pain (PCT/US14/3353) and US 2018/0,055,407 Neurophysiological Signatures for Fibromyalgia (CU4199B-PPA1). Dr. Elliott is an advisory member for the board of directors of the Journal of Orthopaedic and Sports Physical Therapy, an editorial board member for the Journal of Orthopaedic and Sports Physical Therapy, an editorial board member for Musculoskeletal Science and Practice, and an advisory board member for Spine. Drs. Liu and Sparks declare no competing interests.
Acknowledgements
The original studies were supported by grants from the Orthopedic Section of the American Physical Therapy Association and OSF HealthCare Saint Francis Medical Center. The secondary analyses were supported by grants from the National Institute on Drug Abuse under award numbers T32DA035165 and K24DA029262 and the National Institute of Neurological Disorders and Stroke under award number K23NS104211. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Andersson J.L.R., Jenkinson M., Smith S. 2010. Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2. [Google Scholar]
- Apkarian A.V., Krauss B.R., Fredrickson B.E., Szeverenyi N.M. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci. Lett. 2001;299(1–2):57–60. doi: 10.1016/s0304-3940(01)01504-x. [DOI] [PubMed] [Google Scholar]
- Ashburner J. SPM: a history. Neuroimage. 2012;62(2):791–800. doi: 10.1016/j.neuroimage.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagarinao E., Johnson K.A., Martucci K.T. Preliminary structural mri based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014;155(12):2502–2509. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakdash J.Z., Marusich L.R. Repeated measures correlation. Front Psychol. 2017;8:456. doi: 10.3389/fpsyg.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Chialvo D.R., Geha P.Y. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(47):12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Mansour A.R., Baria A.T., Apkarian A.V. Functional reorganization of the default mode network across chronic pain conditions. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialosky J.E., Bishop M.D., Price D.D., Robinson M.E., George S.Z. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man. Ther. 2009;14(5):531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialosky J.E., Bishop M.D., Robinson M.E., Barabas J.A., George S.Z. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop M.D., Bialosky J.E., Cleland J.A. Patient expectations of benefit from common interventions for low back pain and effects on outcome: secondary analysis of a clinical trial of manual therapy interventions. J. Man. Manip. Ther. 2011;19(1):20–25. doi: 10.1179/106698110X12804993426929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied P.R., Gross A.R., Elliott J.M. Neck pain: Revision 2017. J. Orthop. Sports. Phys. Ther. 2017;47(7):A1–a83. doi: 10.2519/jospt.2017.0302. [DOI] [PubMed] [Google Scholar]
- Brascher A.K., Becker S., Hoeppli M.E., Schweinhardt P. Different brain circuitries mediating controllable and uncontrollable pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36(18):5013–5025. doi: 10.1523/JNEUROSCI.1954-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.E., Chatterjee N., Younger J., Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS ONE. 2011;6(9):e24124. doi: 10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussieres A.E., Stewart G., Al-Zoubi F. Spinal manipulative therapy and other conservative treatments for low back pain: a guideline from the canadian chiropractic guideline initiative. J. Manipulative Physiol. Ther. 2018;41(4):265–293. doi: 10.1016/j.jmpt.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Cleland J.A., Childs J.D., Fritz J.M., Whitman J.M., Eberhart S.L. Development of a clinical prediction rule for guiding treatment of a subgroup of patients with neck pain: use of thoracic spine manipulation, exercise, and patient education. Phys. Ther. 2007;87(1):9–23. doi: 10.2522/ptj.20060155. [DOI] [PubMed] [Google Scholar]
- Cleland J.A., Mintken P.E., Carpenter K. Examination of a clinical prediction rule to identify patients with neck pain likely to benefit from thoracic spine thrust manipulation and a general cervical range of motion exercise: multi-center randomized clinical trial. Phys. Ther. 2010;90(9):1239–1250. doi: 10.2522/ptj.20100123. [DOI] [PubMed] [Google Scholar]
- Cross K.M., Kuenze C., Grindstaff T.L., Hertel J. Thoracic spine thrust manipulation improves pain, range of motion, and self-reported function in patients with mechanical neck pain: a systematic review. J. Orthop. Sports. Phys. Ther. 2011;41(9):633–642. doi: 10.2519/jospt.2011.3670. [DOI] [PubMed] [Google Scholar]
- Damien J., Colloca L., Bellei-Rodriguez C.E., Marchand S. Pain modulation: From conditioned pain modulation to placebo and nocebo effects in experimental and clinical pain. Int. Rev. Neurobiol. 2018;139:255–296. doi: 10.1016/bs.irn.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansie E.J., Turk D.C. Assessment of patients with chronic pain. Br. J. Anaesth. 2013;111(1):19–25. doi: 10.1093/bja/aet124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delitto A., George S.Z., Van Dillen L.R. Low back pain. J. Orthop. Sports. Phys. Ther. 2012;42(4):A1–57. doi: 10.2519/jospt.2012.42.4.A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff E.P., Vennart W., Wise R.G. Learning to identify cns drug action and efficacy using multistudy fMRI data. Sci. Transl. Med. 2015;7(274) doi: 10.1126/scitranslmed.3008438. 274ra16. [DOI] [PubMed] [Google Scholar]
- Evans D.W. Mechanisms and effects of spinal high-velocity, low-amplitude thrust manipulation: previous theories. J. Manipulative. Physiol. Ther. 2002;25(4):251–262. doi: 10.1067/mmt.2002.123166. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Gatchel R.J., Peng Y.B., Peters M.L., Fuchs P.N., Turk D.C. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol. Bull. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Gay C.W., Robinson M.E., George S.Z., Perlstein W.M., Bishop M.D. Immediate changes after manual therapy in resting-state functional connectivity as measured by functional magnetic resonance imaging in participants with induced low back pain. J. Manipulative. Physiol. Ther. 2014;37(9):614–627. doi: 10.1016/j.jmpt.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazni N.F., Cahill C.M., Stroman P.W. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR American journal of neuroradiology. 2010;31(4):661–667. doi: 10.3174/ajnr.A1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub R.L., Kirsch I., Maleki N. A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. The journal of pain: official journal of the American Pain Society. 2018;19(5):515–527. doi: 10.1016/j.jpain.2017.12.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram M., Erlenwein J., Petzke F. The cortical responses to evoked clinical pain in patients with hip osteoarthritis. PLoS ONE. 2017;12(10) doi: 10.1371/journal.pone.0186400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik H., Murphy B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2012;22(5):768–776. doi: 10.1016/j.jelekin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Herzog W. The biomechanics of spinal manipulation. J. Bodyw. Mov. Ther. 2010;14(3):280–286. doi: 10.1016/j.jbmt.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Blyth F., Buchbinder R. The epidemiology of low back pain. Best practice & research Clinical rheumatology. 2010;24(6):769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Hoy D., March L., Brooks P. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- Hoy D., March L., Woolf A. The global burden of neck pain: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73(7):1309–1315. doi: 10.1136/annrheumdis-2013-204431. [DOI] [PubMed] [Google Scholar]
- Huisman P.A., Speksnijder C.M., de Wijer A. The effect of thoracic spine manipulation on pain and disability in patients with non-specific neck pain: a systematic review. Disabil. Rehabil. 2013;35(20):1677–1685. doi: 10.3109/09638288.2012.750689. [DOI] [PubMed] [Google Scholar]
- Islam H., Law C.S.W., Weber K.A., Mackey S.C., Glover G.H. Dynamic per slice shimming for simultaneous brain and spinal cord fMRI. Magn. Reson. Med. 2019;81(2):825–838. doi: 10.1002/mrm.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Oathes D., Hush J. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain. 2016;157(9):1970–1978. doi: 10.1097/j.pain.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L.A., Kutch J.J., Tillisch K. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J. Urol. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Spaeth R.B., Wey H.Y. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol. Pain. 2013;9:43. doi: 10.1186/1744-8069-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornelsen J., Mackey S. Potential clinical applications for spinal functional MRI. Curr. Pain Headache. Rep. 2007;11(3):165–170. doi: 10.1007/s11916-007-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Woo C.W., Chang L.J. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife. 2016;5 doi: 10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch J.J., Labus J.S., Harris R.E. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017;158(6):1069–1082. doi: 10.1097/j.pain.0000000000000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch J.J., Yani M.S., Asavasopon S. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research network neuroimaging study. NeuroImage Clinical. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascurain-Aguirrebena I., Newham D., Critchley D.J. Mechanism of action of spinal mobilizations: A systematic review. Spine (Phila Pa 1976) 2016;41(2):159–172. doi: 10.1097/BRS.0000000000001151. [DOI] [PubMed] [Google Scholar]
- Lopez-Sola M., Woo C.W., Pujol J. Towards a neurophysiological signature for fibromyalgia. Pain. 2017;158(1):34–47. doi: 10.1097/j.pain.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang C., Luo S. Serotonin transporter polymorphism alters citalopram effects on human pain responses to physical pain. Neuroimage. 2016;135:186–196. doi: 10.1016/j.neuroimage.2016.04.064. [DOI] [PubMed] [Google Scholar]
- Maher C., Underwood M., Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736–747. doi: 10.1016/S0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- Maihofner C., Forster C., Birklein F., Neundorfer B., Handwerker H.O. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain. 2005;114(1–2):93–103. doi: 10.1016/j.pain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Mano H., Kotecha G., Leibnitz K. Classification and characterisation of brain network changes in chronic back pain: A multicenter study. Wellcome open research. 2018;3:19. doi: 10.12688/wellcomeopenres.14069.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci K.T., Mackey S.C. Neuroimaging of pain: Human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology. 2018;128(6):1241–1254. doi: 10.1097/ALN.0000000000002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci K.T., Shirer W.R., Bagarinao E. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP research network. Pain. 2015;156(9):1755–1764. doi: 10.1097/j.pain.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci K.T., Weber K.A., 2nd, Mackey S.C. Altered cervical spinal cord resting-state activity in fibromyalgia. Arthritis & rheumatology (Hoboken, NJ) 2019;71(3):441–450. doi: 10.1002/art.40746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaracchio M., Kirker K., States R., Hanney W.J., Liu X., Kolber M. Thoracic spine manipulation for the management of mechanical neck pain: a systematic review and meta-analysis. PLoS ONE. 2019;14(2) doi: 10.1371/journal.pone.0211877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz H., Morgan V., Tanner G. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118(5):842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- Michener L.A., Kardouni J.R., Sousa C.O., Ely J.M. Validation of a sham comparator for thoracic spinal manipulation in patients with shoulder pain. Man. Ther. 2015;20(1):171–175. doi: 10.1016/j.math.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M., Wang H., Lozano R. Global, regional and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C.B., Maher C.G., Pinto R.Z. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2018;27(11):2791–2803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- Pickar J.G. Neurophysiological effects of spinal manipulation. The spine journal: official journal of the North American Spine Society. 2002;2(5):357–371. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- Puentedura E.J., Landers M.R., Cleland J.A., Mintken P.E., Huijbregts P., Fernandez-de-Las-Penas C. Thoracic spine thrust manipulation versus cervical spine thrust manipulation in patients with acute neck pain: a randomized clinical trial. J. Orthop. Sports. Phys. Ther. 2011;41(4):208–220. doi: 10.2519/jospt.2011.3640. [DOI] [PubMed] [Google Scholar]
- Qaseem A., Wilt T.J., McLean R.M., Forciea M.A. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann. Intern. Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- Reddan M.C., Wager T.D. Modeling pain using fMRI: From regions to biomarkers. Neurosci. Bull. 2018;34(1):208–215. doi: 10.1007/s12264-017-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakers C., Chatelle C., Vanhaudenhuyse A. The nociception coma scale: a new tool to assess nociception in disorders of consciousness. Pain. 2010;148(2):215–219. doi: 10.1016/j.pain.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Skyba D.A., Radhakrishnan R., Rohlwing J.J., Wright A., Sluka K.A. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106(1–2):159–168. doi: 10.1016/s0304-3959(03)00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks C., Cleland J.A., Elliott J.M., Zagardo M., Liu W.C. Using functional magnetic resonance imaging to determine if cerebral hemodynamic responses to pain change following thoracic spine thrust manipulation in healthy individuals. J. Orthop. Sports. Phys. Ther. 2013;43(5):340–348. doi: 10.2519/jospt.2013.4631. [DOI] [PubMed] [Google Scholar]
- Sparks C.L., Liu W.C., Cleland J.A. Functional magnetic resonance imaging of cerebral hemodynamic responses to pain following thoracic thrust manipulation in individuals with neck pain: a randomized trial. J. Manipulative. Physiol. Ther. 2017;40(9):625–634. doi: 10.1016/j.jmpt.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Staud R., Vierck C.J., Cannon R.L., Mauderli A.P., Price D.D. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1–2):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Taylor K.S., Davis K.D. Stability of tactile- and pain-related fMRI brain activations: an examination of threshold-dependent and threshold-independent methods. Hum. Brain. Mapp. 2009;30(7):1947–1962. doi: 10.1002/hbm.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H., Brown J.E., Johnson K.A., Younger J., Hush J., Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cerebral cortex (New York, NY: 1991) 2014;24(4):1037–1044. doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon-Presseau E., Tetreault P., Petre B. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain: a journal of neurology. 2016;139(Pt 7):1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. An fMRI-based neurologic signature of physical pain. The New England journal of medicine. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber II K.A., Sentis A.I., Bernadel-Huey O.N. Thermal stimulation alters cervical spinal cord functional connectivity in humans. Neuroscience. 2018;369:40–50. doi: 10.1016/j.neuroscience.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K.A., 2nd, Chen Y., Wang X., Kahnt T., Parrish T.B. Functional magnetic resonance imaging of the cervical spinal cord during thermal stimulation across consecutive runs. Neuroimage. 2016;143:267–279. doi: 10.1016/j.neuroimage.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K.A., 2nd, Chen Y., Wang X., Kahnt T., Parrish T.B. Lateralization of cervical spinal cord activity during an isometric upper extremity motor task with functional magnetic resonance imaging. Neuroimage. 2016;125:233–243. doi: 10.1016/j.neuroimage.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.C.d.C., Davies H.T., Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain. 2000;85(3):457–463. doi: 10.1016/S0304-3959(99)00299-7. [DOI] [PubMed] [Google Scholar]
- Woo C.W., Roy M., Buhle J.T., Wager T.D. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 2015;13(1) doi: 10.1371/journal.pbio.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation images. In: Jezzard P., Matthews P.M., Smith S.M., editors. Functional MRI: An Introduction to Methods. Oxford University Press; USA: 2001. eds. [Google Scholar]
- Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther. 1995;1(1):11–16. doi: 10.1054/math.1995.0244. [DOI] [PubMed] [Google Scholar]
- Xia T., Long C.R., Vining R.D. Association of lumbar spine stiffness and flexion-relaxation phenomenon with patient-reported outcomes in adults with chronic low back pain - a single-arm clinical trial investigating the effects of thrust spinal manipulation. BMC Complement Altern. Med. 2017;17(1):303. doi: 10.1186/s12906-017-1821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunhammer M., Bingel U., Wager T.D. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol. 2018;75(11):1321–1330. doi: 10.1001/jamaneurol.2018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]