Abstract
Background
Pollinex Quattro Grass (PQ Grass) is an effective, well-tolerated, short pre-seasonal subcutaneous immunotherapy to treat seasonal allergic rhinoconjunctivitis (SAR) due to grass pollen. In this Phase II study, 4 cumulative doses of PQ Grass and placebo were evaluated to determine its optimal cumulative dose.
Methods
Patients with grass pollen-induced SAR were randomised to either a cumulative dose of PQ Grass (5100, 14400, 27600 and 35600 SU) or placebo, administered as 6 weekly subcutaneous injections over 31–41 days (EudraCT number 2017-000333-31). Standardized conjunctival provocation tests (CPT) using grass pollen allergen extract were performed at screening, baseline and post-treatment to determine the total symptom score (TSS) assessed approximately 4 weeks after dosing. Three models were pre-defined (Emax, logistic, and linear in log-dose model) to evaluate a dose response relationship.
Results
In total, 95.5% of the 447 randomized patients received all 6 injections. A highly statistically significant (p < 0.0001), monotonic dose response was observed for all three pre-specified models. All treatment groups showed a statistically significant decrease from baseline in TSS compared to placebo, with the largest decrease observed after 27600 SU (p < 0.0001). The full course of 6 injections was completed by 95.5% of patients. Treatment-emergent adverse events were similar across PQ Grass groups, and mostly mild and transient in nature.
Conclusions
PQ Grass demonstrated a strong curvilinear dose response in TSS following CPT without compromising its safety profile.
Keywords: Allergen immunotherapy, Allergoid, Cumulative dose, Curvilinear dose response, Grass pollen
Abbreviations: ARC, adverse reaction complexes; ADRs, adverse drug reactions; AE, adverse events; ANCOVA, analysis of covariance; AIT, allergen immunotherapy; CIA-CPT, Culture – Independent Assessment of the Conjunctival Provocation Test; CPT, conjunctival provocation test; EAACI, European Academy of Allergy and Clinical Immunology; EMA, European Medicine Agency; FAS, Full Analysis Set; FEV, forced expiratory volume; FVC, forced vital capacity; HEP, Histamine Equivalent Potency; LPS, lipopolysaccharide; MedDRA, Medical Dictionary for Regulatory Activities; MCP-Mod, Multiple Comparison Procedure and Modelling; MCT, microcrystalline tyrosine; mFAS, Modified Full Analysis Set; MPL, Monophosphoryl Lipid A; PPS, Per Protocol Set; SAEs, serious adverse events; SAF, safety set; SAR, seasonal allergic rhinoconjunctivitis; SD, standard deviation; SU, standardized units; TEAEs, treatment-emergent adverse events; TLR, Toll-like receptor; TSS, Total Symptom Score
Introduction
Grass pollen represents 25–35% of the earth's vegetation, and depending on climate and geography, represents a major contributor of airborne allergens during spring and summer. Depending on climate and region, global sensitization rates to grass pollen vary between 1% and 30% of the general population.1, 2, 3, 4, 5 Seasonal allergic rhinoconjunctivitis (SAR) is a type I allergic disease to common aeroallergens, such as pollen which is estimated to affect 400 million people worldwide.6, 7, 8, 9, 10
The only treatment that addresses the underlying cause of SAR includes allergen immunotherapy (AIT), which aims to provide long-term relief via immunomodulation and has been practiced since 1911.11 A systematic review of the Cochrane database established the efficacy of AIT in both improving symptoms and reducing anti-allergic medication in SAR.12 Efficacy of AIT is related to the cumulative dose of the allergen.13 The use of native extracts in finding the optimal cumulative dose is often limited by systemic reactions such as anaphylaxis.14 The introduction of depot adjuvants to prolong immune exposure provided scope in dose adaptations and improved tolerability profiles.15 However, such conventional subcutaneous approaches to AIT still require up to 72 injections over 3–5 years resulting in high and wide-ranging withdrawal and non-compliance rates.16, 17, 18 The introduction of hypoallergenic formulations such as allergoids have enabled updosing that achieve optimal efficacious cumulative doses faster with high tolerability.19
The amino acid L-tyrosine was developed as an alternative depot platform to aluminium hydroxide (alum) to deliver AIT as well as other vaccines. It is referred to as microcrystalline tyrosine (MCT) which reflects its physicochemical properties. MCT shows high adsorption of proteins at neutral pH and enhances the induction of immunoglobulin (Ig) G antibodies without IgE stimulation. In addition, its half-life is 48 hours at the site of injection during which time it delivers a sustained release of antigens for prolonged immune exposure, but unlike alum, is fully metabolized within the body.14,20,21 MCT has been shown to facilitate allergen-specific IgG4 antibody production as well as IL-10 secretion from T cells.22, 23, 24 MCT also offers a compatible mode of adsorption with second generation immunomodulators (i.e. TLR agonists). Monophosphoryl lipid A (MPL®) is a Toll-like receptor 4 (TLR4) agonist and a non-toxic derivative of Lipid A from lipopolysaccharide (LPS), a component of bacterial cell walls. The mechanism involved in MPL adjuvancy is becoming clearer, mainly from studies in mice, but also from human studies implicating pro-tolerogenic Treg and Th1 responses, indicating that it is suitable for shorter course AIT.25, 26, 27, 28, 29
Pollinex Quattro Grass (PQ Grass) is available as named-patient allergoid product covering relevant grass species using a 13-grass mix.30,31 PQ Grass offers short courses of injections that has been in use throughout Europe since 1999.32 The formulation is built on established pharmaceutical principles and consists of a modified allergen MCT adsorbate and MPL to provide an adjuvant system as a means by which immune activation could be better focused in directing the desired balance of Th1 immune responses.33,34
Pollinex Quattro is listed in the current European Academy of Allergy and Clinical Immunology (EAACI) AIT guidelines with grade IA recommendation. Up until now, a wide range of studies have been performed to investigate the clinical effects of PQ Grass using the current available cumulative dose of 5100 SU (standardized units), consisting of 4 weekly pre-seasonal subcutaneous injections.17,32,35 Two recent studies with PQ Grass have shown significant and long-lasting improvements.36,37
To support development of PQ Grass for full registration, guidelines for clinical development of products for AIT require a study to establish a dose response relationship for clinical efficacy using provocation tests.38 CPT is a standardized and reliable provocation test that can be used for that purpose.39
Earlier dose response studies using CPT with Pollinex Quattro Birch (PQ Birch), differing only in the allergoid from PQ Grass, demonstrated a 50% greater reduction of the total symptom score (TSS) after a 5.5-fold increase in the cumulative dose (from 5100 SU to 27300 SU) without compromising safety.40
For this PQ Grass dose response study, three higher injection strengths of 2700 SU, 6000 SU and 8000 SU (per 1.0 mL), were developed. These were used to administer cumulative allergoid doses of 5100 SU, 14400 SU, 27600 SU or 35600 with the same short course of six weekly injections.
We describe the results from a dose finding study in which PQ Grass was evaluated in patients with SAR due to grass pollen, using CPT to evaluate the dose response relationship of TSS, and to establish an optimal therapeutic dose of PQ Grass for future Phase III studies.
Methods
Study design
A multicentre, randomized, double-blind, placebo-controlled, parallel group study [EudraCT number 2017-000333-31] with 5 treatment arms was conducted in Europe (Austria, Germany, and Poland) prior to the grass pollen season (between September 2017 and April 2018). The study patients were randomized 1:1:1:1:1 to receive 6 injections of placebo; 2 placebo and 4 active PQ Grass to achieve a cumulative dose of 5100 SU; 6 injections of PQ Grass to achieve respective cumulative doses of 14400 SU, 27600 SU and 35600 SU.
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the responsible competent and ethical bodies in each participating country. All patients provided written informed consent before any study activity.
The primary efficacy endpoint was the post-treatment TSS following Culture-Independent Assessment of the Conjunctival Provocation Test (CIA-CPT©).41 CPT was performed by testing increasing concentrations of allergen following the method proposed by Möller et al.,42 and adapted by Radcliffe et al.,43 and Riechelmann et al.,44 using grass pollen allergen extract. TSS was assessed at screening to determine patient eligibility, at randomization to establish the baseline TSS, and at the post-treatment visit (4 weeks after the last injection) to obtain the post-treatment TSS. CPT was performed until a TSS of ≥6 was reached following adjustment for the reference eye score at screening and baseline. The post-treatment CPT started with the concentration eliciting a positive CPT at baseline. The secondary efficacy endpoints included the change from baseline to post-treatment TSS following CPT, the number of additional allergen concentration steps required to elicit a positive CPT post-treatment, and the change in total immunoglobulin (Ig) E, specific IgE, IgG4, and specific IgE/total IgE ratio between screening and post-treatment (evaluated by a central laboratory by ImmunoCAP test).
Study patients
Eligible patients were male or female (of non-childbearing potential), 18–50 years in age, with a history of moderate to severe SAR due to grass pollen exposure requiring anti-allergic treatment for symptom control for at least 2 consecutive seasons prior to the study. Other main inclusion criteria included a positive skin prick test for grass pollen and histamine (wheals longest diameter ≥ 3 mm), a negative skin prick test to the negative control (wheals diameter = 0), a positive CPT at the first two visits (TSS ≥ 6), grass-specific IgE and Phl p 1/5-specific IgE class ≥ 2 at screening, and a forced expiratory volume in 1 s (FEV1) ≥ 80% of predicted, with a FEV1/forced vital capacity (FVC) ratio ≥ 70%.
Key exclusion criteria included a positive skin prick test (SPT) (wheal ≥ 3 mm) to another allergen with associated allergy concurrent to the study (Supplementary Fig. 1), and history of immunological disorders or other major diseases or a previous AIT to grass pollen in the last 5 years.
CPT allergen provocation test
CPT using grass pollen allergen extract (containing Dactylis glomerata, Festuca pratensis, Poa pratensis, Lolium perenne, Phleum pratense)39 was supplied by Laboratorios LETI S.L, at concentrations of 0.3, 1.0, 3.0 and 10 Histamine Equivalent Potency units (HEP)/mL. Additionally, the original reconstituted allergen solution of 30 HEP/mL was used at the post-treatment visit if required. Aqueous diluent served as the negative control.
Four eye symptoms after challenge with the allergen or negative control were recorded as patient and investigator reported outcomes (mixed patient-reported outcomes measures using a severity rating scale as follows: 0 = absent, 1 = mild, 2 = moderate and 3 = severe). Patients independently scored the eye symptoms of itching and irritation, followed by co-assessment of eye tearing in conjunction with the investigator and scoring of eye redness by the investigator independent of the patient. This questionnaire was administered 10 minutes after application of negative control or allergen.
The CPT was only conducted on patients with no visible and/or reported eye symptoms or complaints on the day of the assessment and no current antihistamine treatment.
Study medications and treatment schedules
The study sponsor provided all study medication. PQ Grass or placebo were administered as 6 1.0 mL subcutaneous injections at 7-day intervals over a period of approximately 31–41 days at concentrations of 0 (placebo), 300, 800, 900, 2000, 2700, 6000 and 8000 SU per 1.0 mL to deliver cumulative doses of 0, 5100, 14400, 27600, and 35600 SU. The treatment arm (5100 SU) consisting of 4 active injections started with 2 placebo injections for a total of 6 weekly (7 days, ±1) 1.0 mL injections, maintaining the blind for treatment arms with 6 active injections and the overall study. Active injections contained 50 μg/1.0 mL of MPL adjuvant, 2% (w/v) L-tyrosine (MCT); placebo contained no MPL adjuvant. Treatment was to be completed at least 3 weeks before the predetermined start of the local grass pollen season. The start of the grass pollen season was estimated for each of the pollen stations participating in the study (with a pollen station assigned to one or more neighboring clinical sites). For each of these pollen stations, the beginning of the grass pollen season was predicted using historical pollen data. The start date was estimated as the first date reaching at least 1% of the cumulative pollen load in the previous grass pollen season(s).
Statistical analysis
The following analysis sets were defined for statistical analysis. The Full Analysis Set (FAS) consisted of all patients who received at least 1 injection of study drug and followed the intention-to-treat principle. The modified Full Analysis Set (mFAS), consisted of all patients who received the full cumulative dose to which they were randomised and had non-missing values for post-treatment TSS. The Per Protocol Set (PPS) was a subset of the mFAS and excluded all patients with major protocol deviations that affected the evaluation of the primary endpoint of the study. mFAS was the primary analysis set; the FAS and PPS were used for sensitivity analyses. Safety and tolerability analyses were performed on the safety set (SAF) consisting of all patients who received at least 1 dose of study medication.
Primary efficacy analysis was performed using the Multiple Comparison Procedure and Modelling (MCP-Mod) methodology to evaluate a dose response relationship using the placebo (0 SU), and the 5100, 14400, 27600, and 35600 SU treatment arms as dose levels. A candidate set of 3 dose response models was predefined as: a maximum possible effect for the agonist (Emax) model, a logistic model, and a linear in log-dose model. For each of the dose response models, the null hypothesis of no dose response relationship was tested against a one-sided alternative using a multiple contrast test with significance level α/2 = 0.025. The test statistics for the multiple contrast test were calculated using an analysis of covariance (ANCOVA) model which included the baseline TSS as a covariate. By combining the single test statistics into a multivariate test statistic and comparing this to a multivariate t-distribution, the single tests were multiplicity-adjusted. No further adjustment of significance level was required.
For the secondary efficacy analysis, the change from baseline to post-treatment TSS following CPT, calculated as "post-treatment TSS - baseline TS", was analyzed exactly as the primary efficacy endpoint. Immunological measurements (total IgE, specific IgE and IgG4, specific IgE/total IgE ratio) and their changes between screening and post-treatment, calculated as "Igx post-treatment – Igx at screening", were analyzed descriptively. Additionally, the change from baseline in Igx measurements was analyzed using an ANCOVA model including the treatment group and the baseline measurement as covariates.
Safety analysis
All adverse events (AE) analyses were based on the Medical Dictionary for Regulatory Activities (MedDRA) system organ class, and MedDRA preferred terms. All AEs, severe AEs, adverse drug reactions (ADRs), severe ADRs, serious AEs (SAEs), serious ADRs, adverse reaction complexes (ARCs) and AEs leading to premature discontinuation from treatment or the study were recorded and assessed within each treatment group.
Results
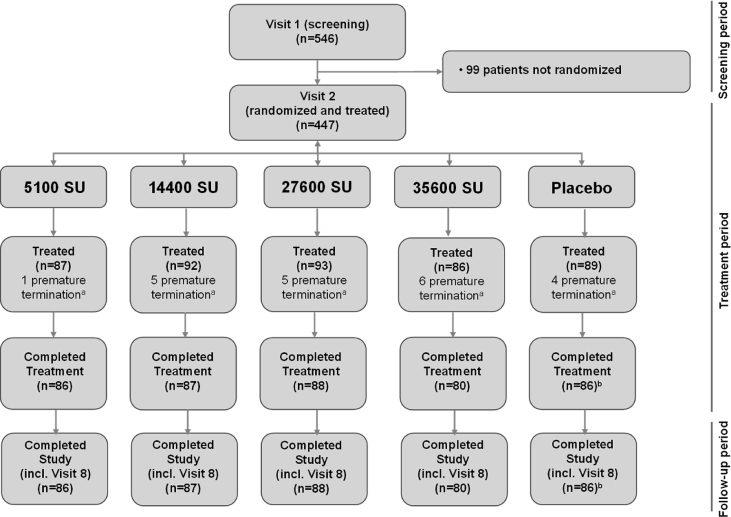
In this study, 546 patients were enrolled and screened, and 447 were randomized to receive study medication: 89, 87, 92, 93 and 86 patients received placebo, 5100 SU, 14400 SU, 27600 SU, and 35600 SU, respectively (Fig. 1). Compliance to treatment was high, with 427 patients (95.5%) completing treatment with all six injections. In total 21 (4.7%) patients discontinued their treatment before the end of the study, 13 (2.9%) due to AEs with similar incidences per treatment group (Fig. 1).
Fig. 1.
Patient disposition during the study amongst the treatment groups. a: Terminated during treatment; b: One patient was lost to follow up after the last injection. Abbreviation: SU: Standardised units
Demographic and baseline data were similar among the treatment groups in the MFAS population (426 patients), of which 234 (54.9%) were male and the mean (±SD) age was 30.7 years (±8.6) (Table 1). All patients had allergic rhinitis or conjunctivitis, with the vast majority having both, and had received anti-allergic treatment in the previous two grass pollen seasons (Table 2). Past and current asthma was reported in 106 (23.7%) patients overall.
Table 1.
Demographic data of patients who completed the study
| Total (N = 426) | Placebo (N = 85) | PQ Grass dose group |
||||
|---|---|---|---|---|---|---|
| 5100 SU (N = 86) | 14400 SU (N = 87) | 27600 SU (N = 88) | 35600 SU (N = 80) | |||
| Gender, n (%) | ||||||
| Female | 192 (45.1%) | 39 (45.9%) | 43 (50.0%) | 40 (46.0%) | 33 (37.5%) | 37 (46.3%) |
| Male | 234 (54.9%) | 46 (54.1%) | 43 (50.0%) | 47 (54.0%) | 55 (62.5%) | 43 (53.8%) |
| Country, n (%) | ||||||
| Austria | 19 (4.5%) | 3 (3.5%) | 5 (5.8%) | 4 (4.6%) | 4 (4.5%) | 3 (3.8%) |
| Germany | 266 (62.4%) | 51 (60.0%) | 54 (62.8%) | 56 (64.4%) | 55 (62.5%) | 50 (62.5%) |
| Poland | 141 (33.1%) | 31 (36.5%) | 27 (31.4%) | 27 (31.0%) | 29 (33.0%) | 27 (33.8%) |
| Age, years n | ||||||
| Mean | 426 | 85 | 86 | 87 | 88 | 80 |
| SD | 30.7 | 31.6 | 29.6 | 31.9 | 30.1 | 30.0 |
| 95% CI [%] | 8.6 [29.8; 31.5] | 9.3 [29.6; 33.6] | 8.5 [27.8; 31.4] | 9.1 [29.9; 33.8] | 8.1 [28.4; 31.8] | 8.1 [28.2; 31.8] |
| Race, n (%) | ||||||
| White | 416 (97.7%) | 83 (97.6%) | 86 (100.0%) | 85 (97.7%) | 84 (95.5%) | 78 (97.5%) |
| Ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 421 (98.8%) | 85 (100.0%) | 86 (100.0%) | 85 (97.7%) | 87 (98.9%) | 78 (97.5%) |
Abbreviations: SD: Standard deviation; SU: Standardized units; CI: Confidence interval (two-sided confidence interval based on normal approximation); 100%: Total number of patients in the corresponding treatment group
Table 2.
Patients suffering from grass pollen allergy and use of anti-allergic treatment
| Total (N = 426) |
Placebo (N = 85) |
PQ Grass dose group |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5100 SU (N = 86) |
14400 SU (N = 87) |
27600 SU (N = 88) |
35600 SU (N = 80) |
|||||||||
| Pat. N | Pat. % | Pat. n | Pat. % | Pat. n | Pat. % | Pat. n | Pat. % | Pat. n | Pat. % | Pat. n | Pat. % | |
| Allergic conjunctivitis | 402 | (94.4%) | 81 | (95.3%) | 83 | (96.5%) | 82 | (94.3%) | 81 | (92.0%) | 75 | (93.8%) |
| Anti-allergic treatment | 400 | (93.9%) | 81 | (95.3%) | 82 | (95.3%) | 81 | (93.1%) | 81 | (92.0%) | 75 | (93.8%) |
| Allergic rhinitis | 425 | (99.8%) | 85 | (100.0%) | 86 | (100.0%) | 86 | (98.9%) | 88 | (100.0%) | 80 | (100.0%) |
| Anti-allergic treatment | 424 | (99.5%) | 85 | (100.0%) | 86 | (100.0%) | 86 | (98.9%) | 87 | (98.9%) | 80 | (100.0%) |
| Allergic asthma | 81 | (19.0%) | 14 | (16.5%) | 18 | (20.9%) | 18 | (20.7%) | 14 | (15.9%) | 17 | (21.3%) |
| Anti-allergic treatment | 71 | (16.7%) | 14 | (16.5%) | 16 | (18.6%) | 15 | (17.2%) | 12 | (13.6%) | 14 | (17.5%) |
| Allergic urticaria | 16 | (3.8%) | 4 | (4.7%) | 5 | (5.8%) | 2 | (2.3%) | 1 | (1.1%) | 4 | (5.0%) |
| Anti-allergic treatment | 12 | (2.8%) | 3 | (3.5%) | 4 | (4.7%) | 2 | (2.3%) | 1 | (1.1%) | 2 | (2.5%) |
Abbreviations: Pat: Patients; SU: Standardized units
PQ grass primary efficacy endpoint
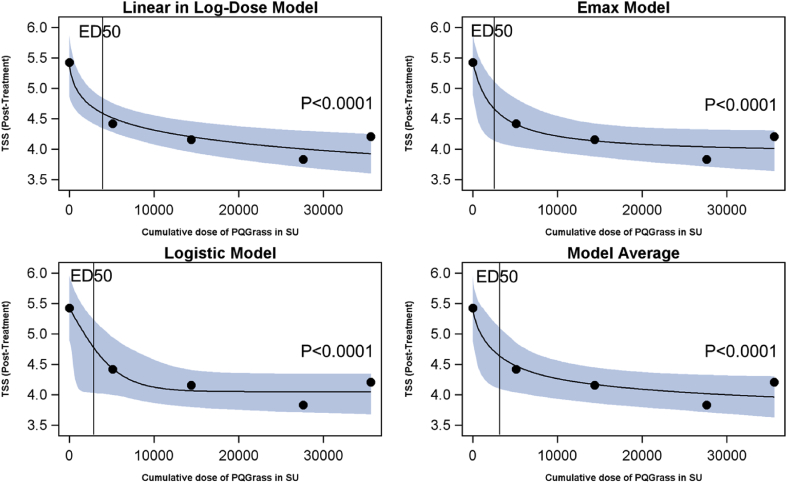
Each of the cumulative doses from 5100 SU to 35600 SU showed a reduction in TSS at 4 weeks after last treatment compared to placebo, with post-treatment mean TSS for placebo of 5.4 (SD 2.5) and post-treatment TSS ranging from 4.4 (SD 2.5, 5100 SU) to 4.0 (SD 2.5, 35600 SU) points compared to placebo. This resulted in a highly statistically significant dose response for all 3 candidate models (p < 0.0001; Figure), showing consistent modelling results and similar fits of the dose response relationship (Fig. 2). Based on the average dose response model of the three candidate models, the reduction in TSS relative to placebo increased significantly with the cumulative PQ Grass dose; this strong dose response relationship was confirmed in various sensitivity analyses performed (e.g. PPS and using various imputation strategies for missing values of the post-treatment TSS). The dose reaching at least 50% of the full CPT effect size over placebo (ED50) was approximately 2900 SU.
Fig. 2.
Primary analysis results of the post-treatment Total Symptom Score as measured during conjunctival provocation testing. Abbreviations: ED50: The dose reaching at least 50% of the full CPT effect size over placebo; Emax: The maximum possible effect for the agonist; SU: Standardized unit; TSS: Total symptom score
Based on the on the average dose response model, the 27600 SU and 35600 SU doses were close to reaching a plateau of the monotonic dose response curve for TSS. Furthermore, the individual mean changes between baseline and post-treatment TSS values reached statistical significance compared to placebo for all doses evaluated including the currently available cumulative dosage of 5100 SU (p = 0.009).
PQ grass secondary efficacy endpoints
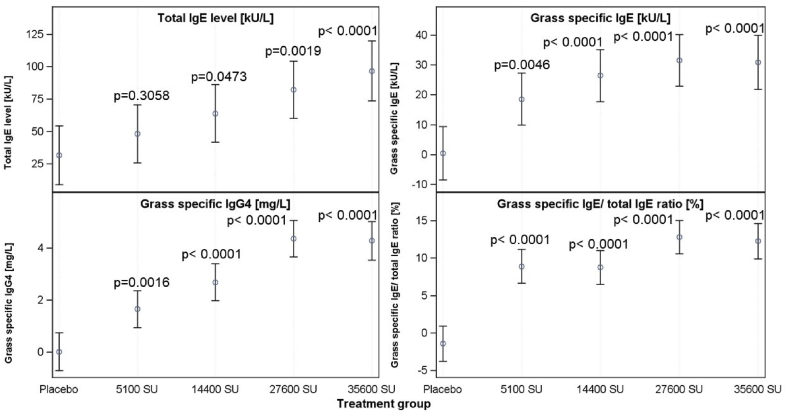
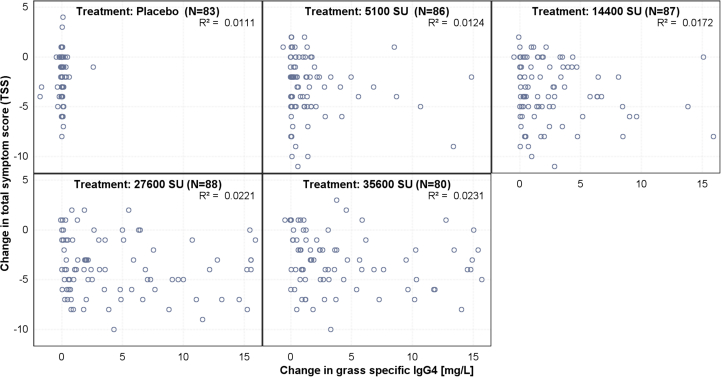
A similar dose response could be observed for the other immunological variables such as total IgE, grass-specific IgG4, and IgE/total IgE ratio. Immunoglobulin levels were significantly higher in all treatment groups following PQ Grass administration compared with placebo, and the levels of IgE and IgG increased with increasing PQ Grass doses, plateauing between the 27600 SU and 35600 SU (Fig. 3).
Fig. 3.
Change in immunoglobulin levels in relation to the dosage of PQ Grass (along with 95% CI). Abbreviations: kU/L: Kilounits per litre; SU: Standardized units
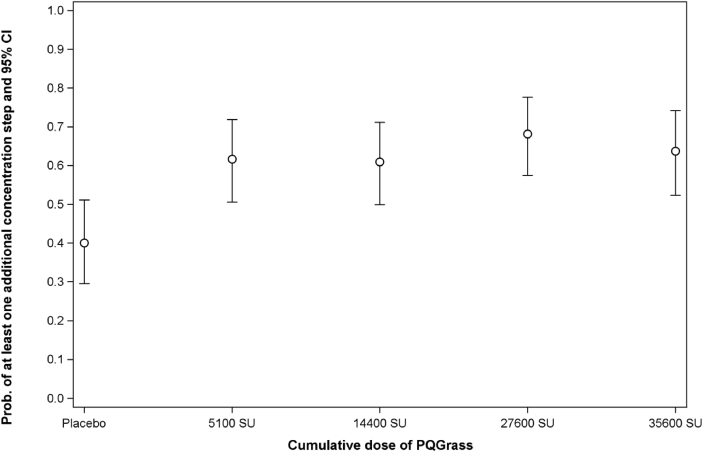
Furthermore, about 65% of patients in the active treatment groups needed at least 1 additional concentration step to elicit a positive CPT post-treatment compared with 40% of patients in the placebo group (Fig. 4).
Fig. 4.
Probabilities for at least one additional concentration step of allergen to elicit a Conjunctival Provocation Test response (along with 95% CI). Abbreviations: CI: Confidence interval (two-sided Clopper-Pearson confidence interval); SU: Standardized units
Safety
No SAEs occurred in this study. A summary of patients with treatment-emergent adverse events (TEAEs) across all dose groups is presented in Table 3. The percentage of patients suffering from local reactions was highest in the 27600 SU group (694 events in 81 [87%] patients). However, their occurrence was not markedly higher than those found in the other active groups.
Table 3.
Overall summary of treatment-emergent adverse events (Safety Set)
| Placebo (N = 166) |
PQ Grass dose group |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5100 SU (N = 301) |
14400 SU (N = 319) |
27600 SU (N = 347) |
35600 SU (N = 315) |
||||||||||||
| Pat. n | Pat. % | Ev. n | Pat. n | Pat. % | Ev. n | Pat. n | Pat. % | Ev. n | Pat. n | Pat. % | Ev. n | Pat. n | Pat. % | Ev. n | |
| Any local AE | 35 | 39.30% | 99 | 71 | 81.60% | 424 | 75 | 81.50% | 573 | 81 | 87.10% | 694 | 73 | 84.90% | 608 |
| Any local AE within 24 h of injection | 33 | 37.10% | 93 | 70 | 80.50% | 408 | 74 | 80.40% | 552 | 81 | 87.10% | 669 | 73 | 84.90% | 594 |
| Any systemic AE | 4 | 4.50% | 7 | 5 | 5.70% | 11 | 4 | 4.30% | 8 | 7 | 7.50% | 16 | 6 | 7.00% | 8 |
| Any systemic AE within 24 h of injection | 3 | 3.40% | 5 | 3 | 3.40% | 9 | 4 | 4.30% | 6 | 6 | 6.50% | 9 | 6 | 7.00% | 7 |
| Any severe AE | 0 | 0.00% | 0 | 3 | 3.40% | 3 | 4 | 4.30% | 6 | 4 | 4.30% | 8 | 2 | 2.30% | 2 |
| Any AE leading to study drug discontinuation | 1 | 1.10% | 1 | 1 | 1.10% | 5 | 5 | 5.40% | 10 | 3 | 3.20% | 19 | 5 | 5.80% | 7 |
| Patients with at least one TEAE | 53 | 59.60% | 161 | 76 | 87.40% | 484 | 78 | 84.80% | 626 | 84 | 90.30% | 762 | 76 | 88.40% | 655 |
| Patients with at least one TEADR | 37 | 41.60% | 106 | 72 | 82.80% | 435 | 75 | 81.50% | 583 | 81 | 87.10% | 714 | 74 | 86.00% | 615 |
Abbreviations: AE: Adverse event; Ev: Events; n: Number of events; N: Number of patients; SU: Standardized units; TEADR: Treatment-emergent adverse drug reaction; TEAE: Treatment-emergent adverse event
Overall, 15 patients (13 in the 3 higher dose group, and 1 each in the 5100 SU and placebo groups) had at least 1 TEAE that led to discontinuation of study drug (7 patients after the second injection, 3 patients after the third injection, 2 patients after the first and fifth injection, respectively, and 1 patient after the fourth injection). TEAEs of severe intensity were reported in 13 patients: 3 (3.4%), 4 (4.3%), 4 (4.3%) and 2 (2.3%) in the 5100 SU, 14400 SU, 27600 SU, and 35600 SU groups, respectively. For 8 of these 13 patients the severe local TEAEs were considered related to the study treatment and were experienced by 2 patients after the first, second and sixth injection, respectively, and by 1 patient after the third and the fifth injection. Systemic AEs were reported in 26 patients across the treatment groups within and after 24 hours of the injection.
Discussion
This Phase II clinical trial studied the dose response of cumulative doses ranging from 5100 SU to 35600 SU of PQ Grass, using TSS captured after CPT as the primary variable, one of the primary endpoints recommended in the guidance from the European Medicines Agency (EMA) and recommended by the EAACI.38,41 Selecting the optimal dose in general is particularly important because failure to do so has been associated with high failure rates in pivotal Phase III studies in the absence of adequate dose range finding studies.45 The doses of PQ Grass were selected in accordance with the EMA standards for allergen immunotherapy dose selection.13,41,46 The CPT was administered to patients outside the pollen season to avoid the influence of environmental allergens.30,47
TSS captured via CPT is shown to provide a reliable method for dose selection in AIT,39,48,49 and successfully implemented in two earlier PQ Birch AIT studies,40 and was applied to a population of 447 grass-allergic patients. The primary statistical analysis used to evaluate the dose response was recommended by the EMA, meeting the International Conference on Harmonisation E4 requirements.50 The cumulative PQ Grass doses evaluated were 2-, 5- and 7-fold above the currently available 5100 SU dose. The 2 highest doses, 27600 and 35600 SU, were close to the plateau of a monotonic dose response curve and were identified as suitable for further investigation in a pivotal Phase III study. All cumulative doses, including the 27600 and 35600 SU doses, were well tolerated and did not show any safety signals.
The dose response obtained from the different cumulative doses versus placebo was statistically significant (p < 0.0001) and confirmed in the sensitivity analyses. Like the PQ Birch dose response studies,40 the reduction in TSS relative to placebo increased with the cumulative PQ Grass dose. The decrease in post-treatment TSS after CPT compared to placebo reached statistical significance for the currently available cumulative dose of 5100 SU with the ED50 estimated at approximately half of this dose (2900 SU). Moreover, these results also show that the treatment effect compared to placebo is increased by approximately 50% with the 27600 SU and 35600 SU doses, which were both close to reaching a plateau of the dose response curve for TSS and were selected for further investigation in pivotal Phase III studies.
Over the last decade, the methods of CPT assessment have further improved. Recent advances in CPT methodology include the development of more standardised scoring systems such as the Total Ocular Symptom Score and the Riechelmann adaptation of the Gronemeyer score system.51 Importantly, the Riechelmann score was found to correlate with clinical symptoms even in patients having predominantly nasal symptoms of allergic disease, which has helped to validate CPT.44 Moreover, CPTs show a high level of reproducibility, and despite differences in techniques and pollen allergen sources, there is a consistent range for ocular allergy symptom elicitation using CPT.52 The standardization and reproducibility of the CPT method combined with the highly sensitive statistical analysis method has now been established across several studies as an effective means of identifying an optimal dose. The applied CPT methodology was initially evaluated in a pilot study39 and two previous PQ Birch studies have shown the robustness of this method of analysis in which mean TSS scores showed overlap on the dose response curve at similar doses.40 In addition, other studies have successfully used CPT methodology to assess the dose response relationship to support selection of the optimum dose for further development.53,54
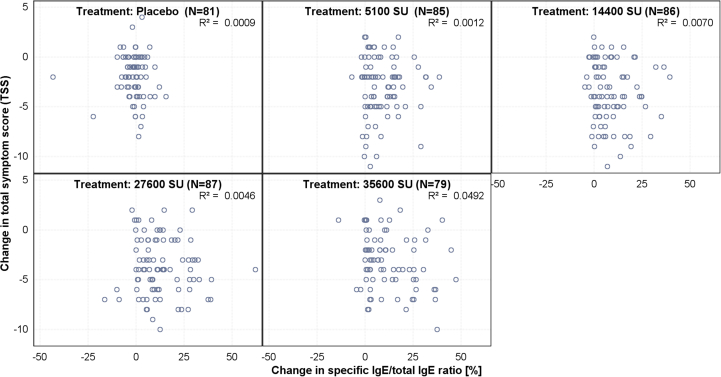
The considered optimal dose should be guided by both immunological and clinical dose response curves. Additional analyses of secondary endpoint immunoglobulin data revealed no clear correlation with the TSS after CPT (Supplementary Figs. 2–3). However, the observed results exhibited a clear step-wise increase in all the immunoglobulin domains tested, approaching a plateau with increasing strength, consistent with the dose response seen from TSS. The overall level of significance for each cumulative dose related to the increase in grass-specific IgG4 and grass-specific IgE/Total IgE ration (%) were highly significant in each case. It is a well-known phenomenon that an initial increase in IgE is followed by a gradual decline in allergen-specific IgE levels over several years.55 However, although increases in grass-specific IgE/Total IgE ratio have been described in many AIT studies so far, a dose dependent effect to the cumulative allergen given is a new finding. Interestingly, it has been suggested that early priming of Th2 cells by high allergen exposure is important for successful AIT.56 Furthermore, the strong dose response observed for IgG4 is notable and supports the potency of PQ Grass to induce allergen-specific IgG antibodies, which play a significant role in allergen-specific tolerance. Moreover, the absence of a correlation between allergen-specific immunoglobulins and clinical outcomes provides further evidence for similar observations made to date, while these results clearly demonstrate that the dose of AIT is independent of the absence of this relationship.
Interpretation of the clinical outcomes in this study should also take into consideration the characteristics in using MCT and MPL as an adjuvant system in the PQ Grass formulation.4,5,14 The significance of this may in part be in its contribution to the quality of the immune response.
As expected, the majority of TEAEs were mild local injection site reactions. All dose regimens were well tolerated, and AEs were mainly mild and similar across all active doses. Consequently, it is important to highlight that the use of a biodegradable depot (MCT) exhibits strong adsorptive power with allergen extracts that may facilitate consistent slow release (depot) properties across the doses investigated. Moreover, MCT used in formulations such as PQ Grass exhibits a 48-hour half-life at the injection site and hence offers compatibility with a rapid dose administration schedule.
In conclusion, after a short course of 6 injections with allergoid grass SCIT treatment with the adjuvant system MCT + MPL (PQ Grass), a highly statistically significant dose response was demonstrated between post-treatment TSS measured after CPT and dose of PQ Grass. The dose response curve decreased monotonously and showed very similar curves for each of the 3 pre-specified dose response models. The primary result was consistent with those from the secondary endpoint data. Moreover, an acceptable safety profile was illustrated across all active treatment groups. Therefore, this study was adequately designed to establish an optimal dose for PQ Grass using TSS measured during CPT as a primary endpoint. Both the cumulative 27600 SU and 35600 SU doses were close to reaching a plateau of the monotonic dose response curve for TSS and showed a similarly optimal benefit/risk profile. Either dose may, therefore, be selected for further investigation in pivotal Phase III studies.
Ethics approval
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the responsible competent and ethical bodies in each participating country. All patients provided written informed consent before any study activity.
Author contributions
All authors participated in the conception, design and implementation of the study. All authors were involved in the interpretation of analysed data and the decision to submit for publication. Medical writing support was provided by Bilal Bham and Hussein Hijazi of Bham Pharma Ltd, UK.
Funding
This clinical trial study was entirely funding by Allergy Therapeutics (UK) plc.
Data availability
The data that support the findings of this study are available from the corresponding author, Dr. MH, upon reasonable request.
Registration
This study is registered with the EU Clinical Trials register EudraCT number 2017-000333-31 (PQGrass205).
Declaration of competing interest
PJ. de Kam, A. Wade, K. Kluehr, J. Raab, D. Lee, M.F. Kramer, T. Higenbottam, M.D Heath, and M.A Skinner are full-time employees of Allergy Therapeutics (UK) Ltd. K. Gunawardena is a consultant working for Allergy Therapeutics (UK) Ltd. D. Wessiepe reported fees from Allergy Therapeutics Plc. to her employer Metronomia, during the conduct of the study. Dr. Pfaar reported grants and/or personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Biomay, Nuvo, Circassia, ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Novartis Pharma MEDA Pharma, Anergis S.A., Mobile Chamber Experts (a GA2LEN Partner), Pohl-Boskamp, Indoor Biotechnologies, and GlaxoSmithKline, outside of the submitted work. Dr. Zielen, reports personal fees from Aimmune, during the conduct of the study; grants and personal fees from bene-Arzneimittel GmbH, grants and personal fees from Biotest GmbH, grants from Vifor Pharma Deutschland GmbH, grants from ALK Arzneimittel, personal fees from Novartis GmbH, personal fees from Böhringer Ingelheim, personal fees from Lofarma GmbH, personal fees from IMS HEALTH GmbH & Co. OHG, personal fees from GSK, personal fees from Stallergen, personal fees from Procter and Gamble, personal fees from Allergopharma GmbH, personal fees from Allergy Therapeutics, outside the submitted work. P. Kuna has received lecture fees from Adamed, Allergopharma, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Chiesi, FAES, Novartis, and Polpharma W. Aberer has not conflict-of-Interest regarding this publication/study. L. Klimek has received research grants from Allergy Therapeutics/Bencard, Great Britain/Germany; ALK-Abelló, Denmark; Allergopharma, Germany; ASIT Biotech, Belgium; Bionorica, Germany; Biomay, Austria, Boehringer Ingelheim, Germany, Circassia, USA; Stallergenes, France; Cytos, Switzerland; Curalogic, Denmark; HAL, Netherlands; Hartington, Spain; Lofarma, Italy; MEDA/Mylan, Sweden/USA; Novartis, Switzerland, Leti, Spain; ROXALL, Germany; GlaxoSmithKline (GSK), Great Britain; Sanofi, France and/or has served on the speaker's bureau or was consulting for the above mentioned pharmaceutical companies. S. Lassmann has received grants and non-financial support from Allergy Therapeutics, during the conduct of the study.
Acknowledgments
The study was entirely funded by Allergy Therapeutics. We acknowledge the commitment and dedication of the patients who enrolled in the study and wish to thank them along with the project management and the team who supported all the work. The study was only possible due to dedicated colleagues, who recruited patients, and their staff, who managed the tests. The authors would like to thank Bham Pharma for their medical writing help with this manuscript, which was financed by Allergy Therapeutics.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100075.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Fig. S2.
Fig. S3.
References
- 1.Davies J.M. Grass pollen allergens globally: the contribution of subtropical grasses to burden of allergic respiratory diseases. Clin Exp Allergy. 2014;44:790–801. doi: 10.1111/cea.12317. [DOI] [PubMed] [Google Scholar]
- 2.Newson R.B., van Ree R., Forsberg B. Geographical variation in the prevalence of sensitization to common aeroallergens in adults: the GA(2) LEN survey. Allergy. 2014;69:643–651. doi: 10.1111/all.12397. [DOI] [PubMed] [Google Scholar]
- 3.Salo P.M., Arbes S.J., Jr., Jaramillo R. Prevalence of allergic sensitization in the United States: results from the national health and nutrition examination survey (NHANES) 2005-2006. J Allergy Clin Immunol. 2014;134:350–359. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimek L., Pfaar O., Bousquet J., Senti G., Kundig T. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897–906. doi: 10.1080/1744666X.2017.1333423. [DOI] [PubMed] [Google Scholar]
- 5.Klimek L., Schmidt-Weber C.B., Kramer M.F., Skinner M.A., Heath M.D. Clinical use of adjuvants in allergen-immunotherapy. Expert Rev Clin Immunol. 2017;13:599–610. doi: 10.1080/1744666X.2017.1292133. [DOI] [PubMed] [Google Scholar]
- 6.Baena-Cagnani C.E., Canonica G.W., Zaky Helal M. The international survey on the management of allergic rhinitis by physicians and patients (ISMAR) World Allergy Organ J. 2015;8:10. doi: 10.1186/s40413-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Cauwenberge P., Bachert C., Passalacqua G. Consensus statement on the treatment of allergic rhinitis. European academy of allergology and clinical Immunology. Allergy. 2000;55:116–134. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 8.Braido F., Arcadipane F., Marugo F., Hayashi M., Pawankar R. Allergic rhinitis: current options and future perspectives. Curr Opin Allergy Clin Immunol. 2014;14:168–176. doi: 10.1097/ACI.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 9.Greiner A.N., Hellings P.W., Rotiroti G., Scadding G.K. Allergic rhinitis. Lancet. 2011;378:2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 10.Krzych-Falta E., Namyslowski A., Samolinski B. Dilemmas associated with local allergic rhinitis. Postepy Dermatol Alergol. 2018;35:243–245. doi: 10.5114/ada.2018.76215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noon L. Prophylactic inoculation against hay fever. The Lancet. 1911;177:1572–1573. [Google Scholar]
- 12.Calderon M.A., Alves B., Jacobson M., Hurwitz B., Sheikh A., Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001936.pub2. CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderon M.A., Larenas D., Kleine-Tebbe J. European Academy of Allergy and Clinical Immunology task force report on 'dose-response relationship in allergen-specific immunotherapy. Allergy. 2011;66:1345–1359. doi: 10.1111/j.1398-9995.2011.02669.x. [DOI] [PubMed] [Google Scholar]
- 14.Bell A.J., Heath M.D., Hewings S.J., Skinner M.A. The adsorption of allergoids and 3-O-desacyl-4'-monophosphoryl lipid A (MPL(R)) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J Inorg Biochem. 2015;152:147–153. doi: 10.1016/j.jinorgbio.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein D.I., Wanner M., Borish L., Liss G.M. Immunotherapy Committee AAoAA, Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113:1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Hsu N.M., Reisacher W.R. A comparison of attrition rates in patients undergoing sublingual immunotherapy vs subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2012;2:280–284. doi: 10.1002/alr.21037. [DOI] [PubMed] [Google Scholar]
- 17.Rosewich M., Lee D., Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Hum Vaccines Immunother. 2013;9:1523–1531. doi: 10.4161/hv.24631. [DOI] [PubMed] [Google Scholar]
- 18.Bousquet J., Lockey R., Malling H.J. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization. American academy of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81:401–405. doi: 10.1016/s1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 19.Pfaar O., Cazan D., Klimek L., Larenas-Linnemann D., Calderon M.A. Adjuvants for immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12:648–657. doi: 10.1097/ACI.0b013e32835a11d6. [DOI] [PubMed] [Google Scholar]
- 20.Baldrick P., Richardson D., Wheeler A.W. Review of L-tyrosine confirming its safe human use as an adjuvant. J Appl Toxicol. 2002;22:333–344. doi: 10.1002/jat.869. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler A.W., Moran D.M., Robins B.E., Driscoll A. l-Tyrosine as an immunological adjuvant. Int Arch Allergy Appl Immunol. 1982;69:113–119. doi: 10.1159/000233157. [DOI] [PubMed] [Google Scholar]
- 22.Cabral-Miranda G., Heath M.D., Gomes A.C. vol. 5. Vaccines; Basel: 2017. (Microcrystalline tyrosine (MCT((R))): A Depot Adjuvant in Licensed Allergy Immunotherapy Offers New Opportunities in Malaria). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabral-Miranda G., Heath M.D., Mohsen M.O. vol. 5. Vaccines; Basel: 2017. (Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium Berghei/vivax). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roger A., Depreux N., Jurgens Y. A novel microcrystalline tyrosine-adsorbed, mite-allergoid subcutaneous immunotherapy: 1-year follow-up report. Immunotherapy. 2016;8:1169–1174. doi: 10.2217/imt-2016-0068. [DOI] [PubMed] [Google Scholar]
- 25.Casella C.R., Mitchell T.C. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg M., Subbarao B. Immune responses of systemic and mucosal lymphoid organs to Pnu-Imune vaccine as a function of age and the efficacy of monophosphoryl lipid A as an adjuvant. Infect Immun. 1992;60:2329–2336. doi: 10.1128/iai.60.6.2329-2336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore A., McCarthy L., Mills K.H. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine. 1999;17:2517–2527. doi: 10.1016/s0264-410x(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 28.Mothes N., Heinzkill M., Drachenberg K.J. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–1208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler A.W., Marshall J.S., Ulrich J.T. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol. 2001;126:135–139. doi: 10.1159/000049504. [DOI] [PubMed] [Google Scholar]
- 30.Kmenta M., Bastl K., Berger U. The grass pollen season 2015: a proof of concept multi-approach study in three different European cities. World Allergy Organ J. 2017;10:31. doi: 10.1186/s40413-017-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kmenta M., Bastl K., Kramer M.F. The grass pollen season 2014 in Vienna: a pilot study combining phenology, aerobiology and symptom data. Sci Total Environ. 2016;566–567:1614–1620. doi: 10.1016/j.scitotenv.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 32.Drachenberg K.J., Wheeler A.W., Stuebner P., Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 33.von Baehr V., Hermes A., von Baehr R. Allergoid-specific T-cell reaction as a measure of the immunological response to specific immunotherapy (SIT) with a Th1-adjuvanted allergy vaccine. J Investig Allergol Clin Immunol. 2005;15:234–241. [PubMed] [Google Scholar]
- 34.Leuthard D.S., Duda A., Freiberger S.N. Microcrystalline tyrosine and aluminum as adjuvants in allergen-specific immunotherapy protect from IgE-mediated reactivity in mouse models and act independently of inflammasome and TLR signaling. J Immunol. 2018;200:3151–3159. doi: 10.4049/jimmunol.1800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DuBuske L.M., Frew A.J., Horak F. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:466. doi: 10.2500/108854111798840203. [DOI] [PubMed] [Google Scholar]
- 36.Rabe UA J., Benke E., Erdmann A. Long-term efficacy of specific subcutaneous, short-term MPL adjuvant immunotherapy over three treatment and three follow-up years, as measured by quality of life. Allergo Journal International. 2017;26:147–154. [Google Scholar]
- 37.Zielen S., Gabrielpillai J., Herrmann E., Schulze J., Schubert R., Rosewich M. Long-term effect of monophosphoryl lipid A adjuvanted specific immunotherapy in patients with grass pollen allergy. Immunotherapy. 2018;10:529–536. doi: 10.2217/imt-2018-0004. [DOI] [PubMed] [Google Scholar]
- 38.Agency E.M., editor. CHMP/EWP/18504/2006. European Medicines Agency; London: 2008. Committee for medicinal products for human use CaEWP, (EWP). Guideline ON the clinical development OF products for specific immunotherapy for the treatment OF allergic diseases. [Google Scholar]
- 39.Pfaar O., Classen D.P., Astvatsatourov A., Klimek L., Mosges R. Reliability of a new symptom score in a titrated quantitative conjunctival provocation test supported by an objective photodocumentation. Int Arch Allergy Immunol. 2018;176:215–224. doi: 10.1159/000487884. [DOI] [PubMed] [Google Scholar]
- 40.Worm M., Higenbottam T., Pfaar O. Randomized controlled trials define shape of dose response for Pollinex Quattro Birch allergoid immunotherapy. Allergy. 2018;73:1812–1822. doi: 10.1111/all.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaar O., Demoly P., Gerth van Wijk R. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 42.Moller C., Dreborg S., Ferdousi H.A. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 43.Radcliffe M.J., Lewith G.T., Prescott P., Church M.K., Holgate S.T. Do skin prick and conjunctival provocation tests predict symptom severity in seasonal allergic rhinoconjunctivitis? Clin Exp Allergy. 2006;36:1488–1493. doi: 10.1111/j.1365-2222.2006.02594.x. [DOI] [PubMed] [Google Scholar]
- 44.Riechelmann H., Epple B., Gropper G. Comparison of conjunctival and nasal provocation test in allergic rhinitis to house dust mite. Int Arch Allergy Immunol. 2003;130:51–59. doi: 10.1159/000068369. [DOI] [PubMed] [Google Scholar]
- 45.Hay M., Thomas D.W., Craighead J.L., Economides C., Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 46.Pfaar O., Bachert C., Bufe A. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k guideline of the German society for allergology and clinical Immunology (DGAKI), the society for pediatric allergy and environmental medicine (GPA), the medical association of German allergologists (AeDA), the Austrian society for allergy and Immunology (OGAI), the Swiss society for allergy and Immunology (SGAI), the German society of dermatology (DDG), the German society of oto- rhino-laryngology, head and neck surgery (DGHNO-KHC), the German society of pediatrics and adolescent medicine (DGKJ), the society for pediatric pneumology (GPP), the German respiratory society (DGP), the German association of ENT surgeons (BV-HNO), the professional federation of paediatricians and youth doctors (BVKJ), the federal association of pulmonologists (BDP) and the German dermatologists association (BVDD) Allergo J Int. 2014;23:282–319. doi: 10.1007/s40629-014-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tovey E.R., Liu-Brennan D., Garden F.L., Oliver B.G., Perzanowski M.S., Marks G.B. Time-based measurement of personal mite allergen bioaerosol exposure over 24 hour periods. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153414. e0153414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kother J., Mandl A., Allekotte S. Early nonreactivity in the conjunctival provocation test predicts beneficial outcome of sublingual immunotherapy. Clin Transl Allergy. 2018;8:28. doi: 10.1186/s13601-018-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroder J., Mosges R. Conjunctival provocation tests: prediction of seasonal allergy. Curr Opin Allergy Clin Immunol. 2018;18:393–397. doi: 10.1097/ACI.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 50.Qualification E.M.A. Committee for Medicinal Products for Human Use (CHMP); 2014. Opinion of MCP-Mod as an efficient statistical methodology for model-based design and analysis of Phase II dose finding studies under model uncertainty; pp. 1–7. [Google Scholar]
- 51.Fauquert J.L., Jedrzejczak-Czechowicz M., Rondon C. Conjunctival allergen provocation test : guidelines for daily practice. Allergy. 2017;72:43–54. doi: 10.1111/all.12986. [DOI] [PubMed] [Google Scholar]
- 52.Prince A., Norris M.R., Bielory L. Seasonal ocular allergy and pollen counts. Curr Opin Allergy Clin Immunol. 2018;18:387–392. doi: 10.1097/ACI.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 53.Mosges R., Kasche E.M., Raskopf E. A randomized, double-blind, placebo-controlled, dose-finding trial with Lolium perenne peptide immunotherapy. Allergy. 2018;73:896–904. doi: 10.1111/all.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosges R., Rohdenburg C., Eichel A. Dose-finding study of carbamylated monomeric allergoid tablets in grass-allergic rhinoconjunctivitis patients. Immunotherapy. 2017;9:1225–1238. doi: 10.2217/imt-2017-0058. [DOI] [PubMed] [Google Scholar]
- 55.Durham S.R., Till S.J. Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998;102:157–164. doi: 10.1016/s0091-6749(98)70079-x. [DOI] [PubMed] [Google Scholar]
- 56.Shamji M.H., Durham S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140:1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Dr. MH, upon reasonable request.